ABSTRACT
CAR-T cell therapy, a novel therapeutic approach that has attracted much attention in the field of cancer treatment at present, has become the subject of many studies and has shown great potential in the treatment of hematological malignancies, such as leukemia and lymphoma. This study aims to analyze the characteristics of articles published on CAR-T cell therapy in the lymphoma field and explore the existing hotspots and frontiers. The relevant articles published from 2013 to 2022 were retrieved from the Web of Science Core Collection. CiteSpace, VOSviewer, Bibliometric online analysis platform, Microsoft Excel, and R software were used for bibliometric analysis and visualization. The number of publications related to the research has been increasing year by year, including 1023 articles and 760 reviews from 62 countries and regions, 2092 institutions, 1040 journals, and 8727 authors. The United States, China, and Germany are the main publishing countries in this research field. The top 10 institutions are all from the United States, the journal with the highest impact factor is BLOOD, the author with the most publications is Frederick L Locke, and the most influential author is Carl H June. The top three keywords are “Lymphoma,” “Immunotherapy,” and “Therapy.” “Maude (2014)” is the most cited and strongest burstiness reference over the past decade. This study provides a comprehensive bibliometric analysis of CAR-T cell therapy in lymphoma, which can help researchers understand the current research hotspots in this field, explore potential research directions, and identify future development trends.
Introduction
The chimeric antigen receptor (CAR)-T cell therapy is a treatment method that involves modifying the patient’s own T-cells to possess stronger anti-tumor activity and the ability to recognize and attack tumor cells.Citation1 Currently, this treatment has made tremendous breakthroughs in the research and treatment of malignant tumors, especially in hematological malignancies, and has developed rapidly in recent years, bringing new hope to patients. In 1989, Gross et al. first proposed the concept of CAR in their research.Citation2 CAR is a recombinant receptor for antigens that redirects the specificity and function of T lymphocytes and other immune cells in a single molecule.Citation3 The general premise of using it for cancer immunotherapy is to generate tumor-targeting T cells rapidly, bypassing obstacles of the active immune response and increasing dynamics.Citation4,Citation5 Cellular therapy based on CAR, including CAR-T, CAR-NK, and CAR-Treg, is considered a versatile therapeutic approach that can target almost any tumor-associated cell surface antigen.Citation6–10 CAR-T therapy has completely transformed the treatment of certain hematological malignancies, namely acute lymphoblastic leukemia and diffuse large B-cell lymphoma.Citation11 At present, there are two kinds of therapies based on CAR-T cells, namely, Tisagenlecleucell and Axiacobatagene Cilloleucell, which have been approved by the Food and Drug Administration (FDA).Citation12 In addition, there is excellent research potential for treating autoimmune diseases, cardiac fibrosis, and chronic hepatitis B or C virus infections.Citation13–19 Therefore, it is necessary to conduct in-depth research on the application of CAR-T cells in the field of lymphoma.
As a hot topic in the field, the number of studies and publications related to CAR-T cell therapy for lymphoma has been increasing yearly. For those researchers who are just starting to delve into this field, especially nonprofessionals who are interested in this area, it is difficult to quickly grasp and summarize relevant knowledge within a short period of time. Bibliometric analysis can effectively summarize and analyze these large amounts of literature and complex data. There is currently no bibliometric study on CAR-T cell therapy in the field of lymphoma. In this study, the bibliometric analysis and knowledge map analysis were combined for the first time to analyze the evolution process, research hot spots, development trends, and emerging topics comprehensively and objectively in this field.
Bibliometrics is a useful method for describing the development trends in a research field, which enables quantitative and statistical analysis of publications in a specific field, and accurately identifies the most representative studies.Citation20–22 In addition, we conducted an analysis of annual publication output, country, institution, journal, authorship, citation, and keywords. By displaying a large amount of data in the form of knowledge maps, researchers can comprehensively analyze the development of specific topics and understand cutting-edge trends more intuitively.Citation23
This study analyzed publications related to CAR-T cell therapy in the field of lymphoma using CiteSpace, VOSviewer, R, RStudio, Microsoft Excel, and the Bibliometric online analysis platform and constructed a scientific knowledge map. Our study aims to provide an overview, summary, and highlight emerging topics in these publications to explore the evolution and development trends of research hotspots in the field and to assist researchers in providing new clues and ideas for future research.
Materials and methods
Data sources and search strategy
Web of Science Core Collection is a significant academic information database, with its core collection from 1985. This includes the Science Citation Index Expanded (SCIE), Social Sciences Citation Index (SSCI), and Arts and Humanities Citation Index (A&HCI), among others. The two main advantages of the WoSCC are reference tracking and citation reporting. In addition to supporting the retrieval of leading academic journals, books, and citation networks, it also employs a rigorous selection mechanism based on bibliometrics’ Bradford’s law,Citation24 only including critical academic journals and major international academic conferences across all subject areas. The selection process is neutral and unbiased and has been validated for over half a century. To avoid potential biases due to daily database updates, we chose to conduct all searches on the same day.
The search strategy is shown in . TS= (CAR-T Cell Therapy OR CAR T-Cell Therapy OR CAR T Cell Therapy OR CAR T-Cell Therapies OR Chimeric Antigen Receptor Therapy OR Chimeric Antigen Receptor T-cell Therapy) AND TS=(Lymphoma) refined by WEB OF SCIENCE INDEX (Web of Science Core Collection. SCI) AND DOCUMENT TYPES (ARTICLE OR REVIEW) AND LANGUAGES (ENGLISH), and the time span of 2013–2022.
Data collection and analysis
We obtained raw data from the WoSCC database and performed initial download and validation. All complete records and referenced citations for WoSCC are collected and downloaded in a text or tab-delimited file format. Data cleaning is carried out on the downloaded data through CiteSpace to remove duplicate data. Then the data was imported into Microsoft Excel (version 16.70), CiteSpace (version 6.2.R2; https://CiteSpace.podia.com), VOSviewer (version 1.6.18; https://www.vosviewer.com), Bibliometric Online Analysis Platform (https://bibliometric.com), R (version 4.2.3; https://cran.r-project.org/) and RStudio (version 4.2.3; https://rstudio.com/) for systematic analysis.
We analyzed the included publications by annual publication count, country and region, institution, journal, author, citation, and keywords and attempted to extract their characteristics to obtain descriptive results. CiteSpace is a citation visualization and analysis software tool that can analyze cooperation networks between countries, authors, and institutions, revealing their co-occurrence and centrality.Citation25 In addition, CiteSpace can perform systematic analyses of cited and co-cited-by references. VOSviewer is a software program used to construct and visualize bibliometric maps.Citation26 It can be used to construct author or journal networks based on collaborative data, to construct keyword networks based on co-occurrence data, and to visualize scientific landscapes through network/overlap/density models using Linlog/modularity methods.Citation26 In addition, the BiblioShiny package can convert data downloaded from SCOPUS, Web of Science, Cochrane Database of Systematic Reviews (CDSR), and RISmed PubMed/Medline databases.Citation27 We used the R programming language and the RStudio platform to run the R Bibliometrix and Biblioshiny packages, enabling their normal usage within the R environment. This tool contains a complete bibliometric analysis process, which can meet the needs of bibliometric research and achieve knowledge and social structure mining related to the literature.Citation27 The Bibliometrics Online Analysis Platform is an online platform that can be used to analyze collaborative networks between countries or regions. Microsoft Excel is a fundamental tool used for importing and categorizing data and creating tables.
Results
Distribution of publications by year
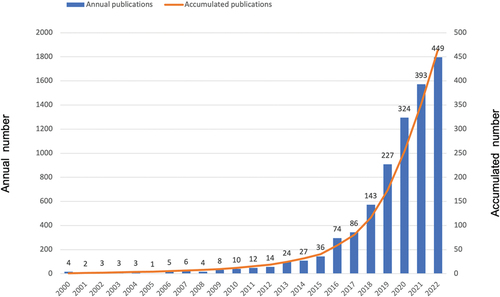
In the literature records retrieved from WoSCC, a total of 1,858 publications meeting the inclusion criteria were identified between 2000 and 2022, consisting of 1,081 articles (58.18%) and 777 reviews (41.82%). The first publication on CAR-T Cell Therapy in the field of lymphoma was published in 2000, titled “In vitro adenoviral vector p53-mediated transduction and killing correlates with expression of coxsackie-adenovirus receptor and alpha(v)beta (5) integrin in SUDHL-1 cells derived from anaplastic large-cell lymphoma.” displays the distribution of publications for each year between 2000 and 2022. Research has found that the number of publications in this field has exhibited a fluctuating upward trend over the past 23 years, roughly presenting a period of stability (2000–2009), a stage of slow development (2010–2014), and a phase of rapid growth (2015–2022). However, our focus is on the research findings of the past decade. Between 2013 and 2022, a total of 1,783 publications met the inclusion criteria, including 1,023 articles (57.38%) and 760 reviews (42.62%). The annual publication output has undergone a significant qualitative leap, with a cumulative total of over 1800 publications. Indicating that the research on CAR-T cell therapy is becoming a popular research direction, which has attracted certain attention from the academic community. In summary, the increasing number of annual publications over time directly reflects the research trends of CAR-T cell therapy in the field of lymphoma at different stages, indicating specific stages of research.
Countries/regions and institutions
Between 2013 and 2022, a total of 62 countries/regions and 2,092 institutions contributed to the publications of CAR-T cell therapy in the field of lymphoma. presents the top 10 countries/regions ranked by the number of publications. Our main evaluation criteria include the number of publications, citation frequency, centrality, and half-life. The country with the highest number of published articles is the United States (n = 1011, 56.70%), followed by China (n = 352, 19.74%) and Germany (n = 177, 9.93%). Among these countries, the United States leads in the number of published articles and total citation counts (55652), exceeding the combined publications and citation counts of the 2nd through 10th-ranked countries. Moreover, among them, the United States has a high centrality (0.44) and half-life (6.5), indicating that the United States plays a vital bridging role in international cooperation, and its publications have high persistence and influence. illustrates the current status of research publications related to CAR-T cell therapy in the field of lymphoma for the top 10 countries in recent years. The overall trend of publication output has shown a relatively sharp increase, with the United States ranking first in the steady growth of annual publication output, followed closely by China. The international collaboration visualization map presents a more intuitive display of scientific cooperation among countries/regions, where the cooperation between China and the United States is the closest, followed by Germany, France, and Italy. However, research collaboration among other countries is relatively scattered ().
Figure 3. (a) The current status of publication volume on CAR-T cell therapy based on lymphoma research from 2013 to 2022 in the main countries is analyzed. (b) Scientific collaboration networks among all countries/regions. The size of each colored block represents the number of publications originating from that country/region, and the connections between countries indicate collaboration relationships.
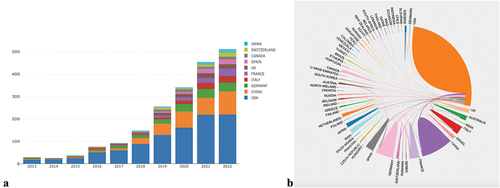
Table 1. Top 10 countries and regions by number of publications.
presents the top 10 institutions ranked by publication count. As for the leading research institutions, Memorial Sloan-Kettering Cancer Center ranks first in productivity, with 124 publications cited 8,233 times. However, The University of Texas MD Anderson Cancer Center has the most citations (13,437) and the highest total link strength (378). In addition, among the top 10 institutions with the most publications, 7 institutions were cited more than 5,000 times, while among all institutions with publications related to this topic, 15 institutions were cited more than 5,000 times. From a geographical perspective, the top 10 institutions are all from the United States, which further indicates the dominant position of the United States in this research.
Table 2. Top 10 institutions by number of publications.
Journals
From 2013 to 2022, a total of 1,040 journals published relevant articles, and 7,324 journals were co-cited. The details of the top 10 journals by publication count and the top 10 co-citation journals by citation count are listed in . Impact Factor (IF) is a quantitative indicator used to evaluate the importance of a journal, representing the total citation frequency (https://clarivate.com/webofsciencegroup/essays/impact-factor/). Journal Citation Reports (JCR) is the most commonly used international standard for journal ranking. It is published by Clarivate Analytics and classifies journals into different subject categories based on their impact factor, which is used to divide them into four quartiles. Among them, FRONTIERS IN IMMUNOLOGY published the most articles (n = 93), followed by CANCERS (n = 70) and BLOOD (n = 54). Among the top 10 ranked journals, there are four journals with over 50 published articles and seven journals located in the Q1 JCR area. provides a more intuitive display of the publication output of the top 10 journals, where the size and color depth of the circles represent the publication volume of the journals. In addition, among these journals, TRANSPLANTATION AND CELLULAR THERAPY changed its name in 2021, and thus its official impact factor and category have not yet been announced. The IF of these journals ranges from 2.822 to 25.669, with the highest IF belonging to BLOOD (IF = 25.669). The three most frequently cited journals were BLOOD (n = 17465), JOURNAL OF CLINICAL ONCOLOGY (n = 7167), and NEW ENGLAND JOURNAL OF MEDICINE (n = 7089). Among the top 10 co-cited journals, four journals were cited over 3,000 times, with BLOOD having far more citations (17,465) than the other journals. Among these journals, three have an IF greater than 50. Among them, the journal with the highest IF isNEW ENGLAND JOURNAL OF MEDICINE(IF = 176.082). The Three-Fields Plot graph can comprehensively analyze the relationship between different bibliometric indicators and construct a comprehensive network map of the indicators. shows an innovative three-field plot on the basis of a Sankey diagram, showing how productive countries (left), the most relevant sources (middle), and frequent author keywords (right) were correlated.Citation28 It is evident that the majority of articles on immunotherapy published in FRONTIERS IN IMMUNOLOGY are mainly authored by scholars from China, the United States, Germany, Spain, and Italy. Similarly, American scholars have made significant contributions to CAR-T cell therapy, with most of the publications appearing in FRONTIERS IN IMMUNOLOGY, CANCERS, TRANSPLANTATION, AND CELLULAR THERAPY, JOURNAL FOR IMMUNOTHERAPY OF CANCER, and FRONTIERS IN ONCOLOGY. Overall, the United States has published a large number of articles in the top 10 journals, with FRONTIERS IN IMMUNOLOGY performing best in CAR-T cell therapy in the field of lymphoma.
Figure 4. (a) The main sources of publications and the number of publications. (b) Three-fields plot. Network relationships among countries, sources, and authors. (c) Double image overlay of journals. Image parameters: a: 1; source circle size: 40; target circle size: 5; captured center point (radius): 0. The citing journals are located on the left, and the cited journals are located on the right. The colored paths (one orange and two green reference paths) represent the citation relationships.
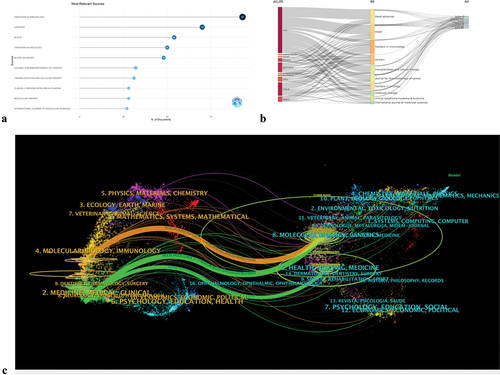
Table 3. Top 10 journals and co-cited journals.
The double-overlay of journals in can effectively display the distribution of journals and the relationship between journals and cited journals (the colored paths represent the cited relationships).Citation29 The labels on the map describe the various research areas covered by all the journals. The citing journals are shown on the left side of the map, while the cited journals are shown on the right side. The reference paths are indicated by different colored lines, each line starting from the beginning of a citation and ending at the end of the citation. There are basically three main citation pathways on the existing map. It indicates that articles published in journals of “Molecular, Biology, and Genetics” and “Health, Nursing, and Medicine” are frequently cited by journals of “Molecular, Biology, and Immunology” and “Medicine, Healthcare, and Clinical.”
Authors
According to a collaborative analysis by the authors of VOSviewer, a total of 8,727 authors published related literature between 2013 and 2022, of which 283 authors reached the threshold (threshold settings: the minimum number of documents for an author is 5; the minimum number of citations for an author is 0). presents detailed information on the top 10 most productive authors and co-cited authors in terms of citation rankings, including primary authors, number of publications, citation counts, total link strength, and H-index. The top three authors with the most published papers are Frederick L Locke (n = 32), Sattva S Neelapu (n = 25), and Jianfeng Zhou (n = 25). However, the author’s influence in a scientific field is more dependent on the number of citations.Citation30–32 The most frequently cited author is Carl H June (8188), followed by Renier J Brentjens (3401) and Frederick L Locke (2737). The co-cited authors refer to two or more authors who are simultaneously cited by another article, forming a co-citation relationship. The analysis shows that James N Kochenderfer has the highest citation frequency with 1392 total citations, followed by Sattva S Neelapu (1333) and Stephen J Schuster (1261). Among these indices, the total link strength reflects the degree of association with other researchers, while the H-index provides an evaluation parameter for the importance and impact of a researcher’s cumulative research contributions.Citation33 Among the top 10 most productive authors, the author with the highest total link strength is Frederick L Locke (192), while among the top 10 authors with the most co-citations, the author with the highest total link strength is James N Kochenderfer (65344). The top three authors with the highest H-index are Carl H June (118), David G Maloney (59), and Miguel-Angel Perales (55). Visual analysis of revealed the collaboration network among the authors and the collaboration network among co-cited authors. The collaboration networks among authors were divided into seven clusters based on their degree of interconnectedness, represented by seven different colors (red, orange, yellow, green, light blue, blue, purple) (); the collaboration networks among co-cited authors were divided into three clusters, represented by three different colors (red, green, blue) (). It can be observed that there is a stable co-occurrence relationship among the most productive authors (Frederick L. Locke, Carl H. June, David G. Maloney) in the list, and authors in the same cluster have active collaborations. The visual map with overlaid years () demonstrates that the yellow cluster was the most active in 2022, while some light blue clusters showed weak connectivity with other clusters. illustrates the affiliation relationship between the corresponding authors and the top 10 countries ranked by publication volume, revealing that American scholars have published more articles and hold a leading position in this research field. In summary, analyzing the relationships between authors and co-cited authors can help to identify the core authors and key collaborations in the field.
Figure 5. Author collaborations. (a) Network visualization mapping of author collaborations. (b) Network visualization mapping of co-cited authors. (C) visualization of the density of author collaborations. (d) Density visualization mapping of co-cited authors. (e) Temporal overlay visualization mapping of author partnerships. (f) The higher the number of publications, the longer the bar; MCP indicates the number of coauthored articles with authors from other countries; SCP indicates the number of coauthored articles with authors of the same nationality.
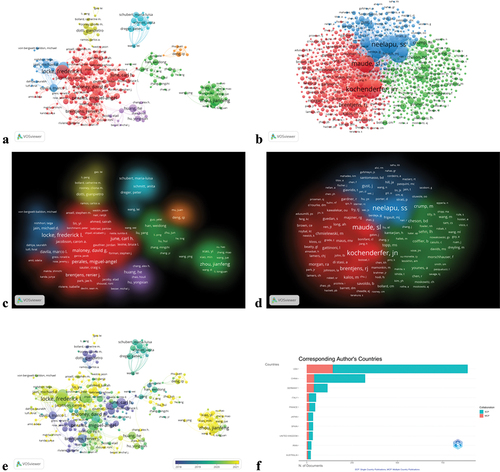
Table 4. Top 10 authors and co-cited authors.
Citations
Citation analysis is considered an important method for assessing the impact of publications. In addition, analyzing highly cited articles can help identify research hotspots. presents detailed information on the top 10 most cited articles in this research field, including the title, first author, journal, publication year, and citation count. This helps to sort specialized literature in specific fields and identify the time points when certain projects have appeared in the past. The most frequently cited article is by Shannon L. Maude et al., published in the New England Journal of Medicine, which has been cited 3,380 times. It can be observed that “CAR-T Cell Therapy,” “Chimeric antigen receptor,” “CAR-T cell immunotherapy,” “Diffuse Large B-Cell Lymphoma,” and “CD19 chimeric antigen receptors” repeatedly appeared in most of the cited literature, indicating that CAR-T cell therapy has received widespread attention in the field of lymphoma research.
Table 5. Top 10 co-cited references.
Co-citation analysis, as a research method for measuring the degree of association between articles, refers to the situation where two or more articles are cited by one or more papers simultaneously, and the two articles are considered to have a co-citation relationship. Literature co-citation analysis is suitable for studying and revealing the internal connections and patterns in the literature. The co-citation network was visualized using knowledge structure analysis in the BiblioShiny package, and a co-citation network graph was generated (). From the visual analysis of the co-citation network in this study, we know that it is roughly divided into two clusters, which are red and blue. The size of the circles in the figure represents the frequency of co-citation, and the more lines between the literature, the more citations. The different colors represent different research directions.
Figure 6. (a) Visualization of the co-citation analysis network, divided into two clusters in red and blue. (b) Clusters of references. A total of seven clusters were classified. (c) Top 20 references with the strongest citation bursts. The blue bars indicate references that have been published; the red bars indicate citation bursts.
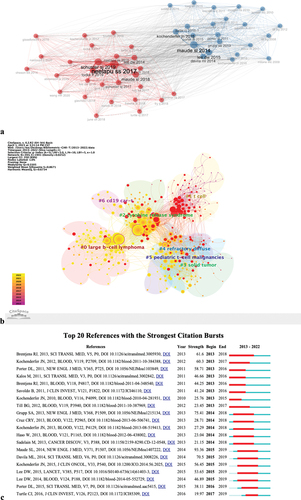
According to the logarithmic likelihood ratio (LLR) clustering of references in CiteSpace (), a total of seven clusters were identified, with a modularity degree Q of 0.5505 and a weighted average silhouette S of 0.8671. It is generally believed that Q > 0.3 indicates a significant clustering structure, and S > 0.7 indicates convincing clusters with strong clustering effects and even network collaboration. As indicated by the label of cluster #2, cytokine release syndrome (CRS) is a hot research topic in the related field. It means that an excessive amount of T cells being activated simultaneously can lead to the immune system releasing a large number of cytokines, which can cause a severe systemic inflammatory response syndrome characterized by fever and multi-organ dysfunction. In addition, based on the year of the first appearance of each cluster, the research hotspots in the field can be visually identified according to the colors.
Citation burst references are references that have been cited frequently over a certain period resulting in a sudden increase in the number of citations. We obtained 124 highly cited references through CiteSpace analysis and selected the top 20 cited references (). The strongest citation burst indicates that a variable has undergone a significant change in a short period of time.
The reference with the strongest burstiness (strength = 93.36) is “Chimeric Antigen Receptor T Cells for Sustained Remissions in Leukemia”Citation34 (published in 2014). This indicates that it has had a significant impact on the research field. Furthermore, from a temporal perspective, the blue bars indicate that the cited references have already been published, while the red bars represent sudden bursts of citations. All of this suggests that future research on CAR-T cell therapy in the field of lymphoma will continue to receive attention.
Keywords
Keywords generally reflect the themes and research contents of an article and provide a highly condensed representation of its core contents. In this study, we merged different spellings of the same keyword (abbreviations, hyphenated words, etc.) into the same item and extracted a total of 4659 keywords from the titles and abstracts using VOSviewer. However, only 22 keywords appeared with a frequency exceeding 120 times (). The most frequently mentioned term was “Lymphoma” (n = 441), followed by “Immunotherapy” (n = 431), “Therapy” (n = 382), “Chimeric antigen receptor” (n = 326), and “Adoptive immunotherapy” (n = 219). Through keyword cloud analysis, a frequency count of the concentrated keywords in the literature is conducted, and highly frequent keywords are extracted and visually emphasized to form a keyword cloud. This enables researchers to quickly understand the research hotspots and directions in a certain field. To draw a keyword cloud using the BiblioShiny package, follow the path: Documents-WordCloud, as shown in . From the figure, it can be seen that the current main research focuses on therapy, lymphoma, chimeric antigen receptor, adoptive immunotherapy, and immunotherapy.
Figure 7. (a) Keyword-based word cloud map. (b) Trends topics based on annual distribution of keywords. (c) Timeline viewer of keywords. Footnote: Keywords were clustered into different items represented by six colors.
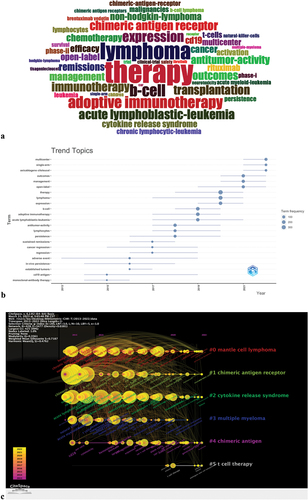
Table 6. The keywords for the CAR-T cell therapy research in the field of lymphoma (n ≥ 120).
analyzes the trends and themes of CAR-T cell therapy in the field of lymphoma based on years. The maximum number of words is restricted to five per year, and the time span is set to “2013–2022.” Therefore, 37 trending topics were obtained. The size of the circles in the figure represents the frequency of the appearance of the keywords. Keywords with higher frequency have larger circles, while keywords with lower frequency have smaller circles. Clearly, monoclonal-antibody therapy (6) and CD19 antigen (9) were the hottest topics in 2015. Therapy (369), lymphoma (281), expression (181), immunotherapy (160), and transplantation (159) were important research topics in 2020. “Multicenter” (105), “single arm” (52), “axicabtagene ciloleucel” (42), “chop” (20), and “Hodgkin” (18) are the hot topics of research in 2022.
We used CiteSpace to establish a timeline viewer for these keywords. The timeline chart clusters the keywords and adds a temporal dimension to the clustering of these keywords. It allows us to observe the temporal changes of a specific topic within a research field, which helps us to quickly understand the development and forefront of the field. Through , we can visually observe the evolution trajectory of key terms in this field, as well as the focus keywords of each stage. The annual rings of each node in the figure reflect the chronological order of each keyword, with larger annual rings indicating earlier appearances and smaller annual rings indicating later ones.
Discussion
Bibliometric analysis is a quantitative analytical method that can be used to evaluate the impact of research institutions, research fields, journals, authors, and articles. It can also be used to discover research hotspots, trends, and the influence of research results. As time goes by, bibliometrics is increasingly being used to obtain scientific achievements of specific academic fields during a certain period of time and to evaluate the trends and advances in various research areas.Citation35 CAR-T cell therapy is a novel immunotherapy approach that utilizes genetically modified T cells to attack and kill cancer cells, thereby improving treatment efficacy and reducing treatment risks. Here, we employed bibliometric analysis to examine the literature related to CAR-T cell therapy in the field of lymphoma, providing valuable insights into the current development and research frontiers of this field.
The first article on the research of CAR-T cell therapy in the field of lymphoma was published in 2000, but the number of articles published annually before 2013 was less than 20, indicating a slow development of CAR-T cell therapy research in the field of lymphoma between 2000 and 2012. During the 10-year period covered by this study, a statistical analysis of relevant publications published each year revealed an overall upward trend in the number of publications related to CAR-T cell therapy in the field of lymphoma. The peak was reached in 2022, which indicates that CAR-T cell therapy will continue to receive attention in this field in the future. At the same time, an analysis of the cooperation between countries and institutions shows that this research area has certain characteristics in terms of country and institutional distribution. The number of countries involved in the research field has been increasing worldwide, with the United States having the highest number of publications, followed by China and Germany. The United States is the country with the highest number of citations and the highest centrality. As one of the main cooperative bridges connecting research from various countries, its enthusiasm and authority are self-evident. In addition, all of the top 10 institutions are from the United States, and the institution with the highest publication output is the Memorial Sloan-Kettering Cancer Center. The University of Texas MD Anderson Cancer Center is the most frequently cited institution and carries substantial authority in the research field. The collaborative networks between countries and institutions indicate that research on CAR-T cell therapy in the field of lymphoma has drawn the attention of researchers worldwide. The participation of more countries/regions is crucial to the development of this research. The uneven research development in different countries or regions may be related to the diversity of multilateral cooperation, financial investment, and the emphasis on medical research.
We found that in the past decade, publications related to this research were published in 1040 journals, with an average of 1.71 articles per journal. The articles published in the journals of “Molecular, Biology, Immunology” and “Medicine, Healthcare, and Clinical” often cite the journals of “Molecular, Biology, Genetics” and “Health, Nursing, and Medicine.” presents the top 10 journals with the most published papers. Almost all journals in the fields of oncology, immunology, biology, or genetics have high IF (such as Molecular Therapy, Clinical Cancer Research, Blood, Cancer Research, etc.), but there is no comprehensive medical journal (such as The Lancet, New England Journal of Medicine, etc.) with a high IF. It is reported that any journal in a specific field represents the highest readership and citation rate within that field. The articles published in these specialized journals will attract more attention than those published in generalist journals. If authors deviate from these core journals, the impact of their articles in key fields may decrease, and the trend of publishing high-impact papers in specialized journals is becoming increasingly evident.Citation36 In addition, we found no significant correlation between the number of publications and the IF of the journals.
According to the results of authorship collaboration analysis and citation analysis, researchers with a high publication count or total citation count are largely affiliated with institutions with high citation counts. Citation analysis has been widely used to measure the quality and impact of scientific publications.Citation37,Citation38 Articles with high citation rates are considered the foundation or hotspot of research areas.Citation39 As shown in , Frederick L Locke is the author with the highest number of publications in this research field, Carl H June is the most frequently cited author, and James N Kochenderfer is the author with the highest co-citations. This suggests that they may serve as leaders and advocates to advance research on this topic, contributing to the exploration and development of this field. As shown in , the top 10 most cited publications were mainly authored by researchers who have published a large number of papers or have a high total citation count. Analysis of the top 10 cited papers can provide information on the scientific progress and research trends in CAR-T cell therapy in the field of lymphoma research. We believe that with the continuous development of CAR-T cell therapy in lymphoma research in the coming years, there will be more researchers dedicated to working in this field.
Frequently occurring keywords can reveal research hotspots, focal points of attention, and potential developments in a field. According to the results of keyword extraction and analysis, it is evident that high-frequency keywords reflect the research hotspots in the field of CAR-T cell therapy. The most frequently occurring terms are “Lymphoma,” followed by “Immunotherapy,” “Therapy,” “Chimeric antigen receptor,” and “Adoptive immunotherapy.” Further analysis of relevant publications and references in conjunction with keyword analysis reflects that CAR-T cell therapy research is mainly used for “Lymphoma” and “leukemia.” This is consistent with the research reflected in the “Top 10 Highly Cited Papers.”Citation40,Citation41 A timeline graph of keywords categorized by year can better reflect the research hotspots of CAR-T cell therapy in the field of lymphoma in recent years and even foreshadow future research directions. Therefore, analyzing and mining high-frequency keywords can assist researchers in better understanding the research dynamics and trends, as well as the core concepts and research hotspots in a field, providing valuable references and guidance for further studies.
Lymphoma is a common hematologic malignancy. They are traditionally divided into Hodgkin’s lymphoma (which accounts for about 10% of all lymphomas) and non-Hodgkin lymphoma.Citation42 Non-Hodgkin lymphoma represents a wide spectrum of illnesses that vary from the most indolent to the most aggressive malignancies. Based on the affected lymphocyte type (B cells, T cells, or natural killer cells) and the intrinsic characteristics of the cells, classification is performed.Citation42 B-cell lymphoma is the most common, with subtypes including Diffuse Large B-cell Lymphoma (DLBCL) and Follicular Lymphoma (FL), among others.Citation43 T-cell lymphomas are relatively uncommon, encompassing entities such as Peripheral T-cell lymphoma (PTCL) and Anaplastic Large Cell Lymphoma (ALCL).Citation44 NK/T-cell lymphomas originate from subgroups of natural killer (NK) cells or T cells.Citation44 They are typically associated with sites such as the nasal cavity and throat. Lymphoblastic leukemia/lymphoma is a disease characterized by both leukemia and lymphoma features, originating from lymphocytes.Citation45 Further classification is based on the growth rate of lymphomas. Some lymphomas, known as indolent lymphomas, exhibit slow growth and may not necessitate immediate treatment. Examples include FL and chronic lymphocytic leukemia (CLL).Citation42 On the contrary, aggressive lymphomas such as DLBCL and Burkitt lymphomaCitation42 exhibit rapid growth and necessitate timely intervention. Each subtype of lymphoma exhibits distinct clinical features, necessitating specific diagnostic approaches, and may potentially respond differently to treatment modalities.
Currently, CAR-T cell therapy has achieved some remarkable results in the field of lymphoma research. The advantage of CAR-T cell therapy lies in its ability to target cancer cell-specific antigens while avoiding damage to normal cells. The first CAR-T cell therapy, YESCARTA (axicabtagene ciloleucel), was approved by the United States FDA in 2017.Citation46 Currently, the FDA has granted approval for a total of six CAR-T cell therapies, targeting two antigens: BCMA and CD19. All of these therapies belong to the category of autologous CAR-T. Based on their respective target antigens, they can be further classified into two main groups: CD19-targeted CAR-T and non-CD19-targeted CAR-T.Citation47–50 CD19-targeted CAR-T cells are mainly used for the treatment of B-cell-related malignancies, such as acute lymphoblastic leukemia (ALL),Citation46 CLL,Citation51 relapsed or refractory B cell lymphoma,Citation52 myelodysplastic syndromes (MDS),Citation53 acute myeloid leukemia (AML)Citation53and B-NHL,Citation47 among others.Citation47,Citation50 Representative CD19-targeted CAR-T therapies include Yescarta, Kymriah, Breyanzi (lisocabtagene maraleucel), all of which have been approved by the United States FDA.Citation46,Citation47,Citation54 In 2017, the United States FDA approved Kymriah (tisagenlecleucel), a type of CAR-T cell therapy, for the treatment of B-ALL patients under the age of 25 with poor prognoses.Citation46 The therapy involves genetically modifying the patient’s own T cells to recognize and attack tumor cells. In a clinical trial of Kymriah, 80% of B-ALL patients achieved complete remission after receiving CAR-T cell therapy.Citation52 In addition, in 2018, the United States FDA approved Yescarta (axicabtagene ciloleucel), a CAR-T cell therapy, for the treatment of relapsed or refractory B-cell non-Hodgkin lymphoma in adults.Citation47 This therapy also involves modifying the patient’s own T cells to recognize and attack tumor cells by altering their genes. In a clinical trial targeting Yescarta, 51% of B-NHL patients achieved complete remission after receiving CAR-T cell therapy.Citation55 Non-CD19-targeted CAR-T can be used to treat some other types of tumors, such as solid tumors and patients with under-expressed CD19 in non-Hodgkin’s lymphoma.Citation56–58 Non-CD19-targeted CAR-T mainly targets another tumor-associated antigen (TAA), such as CAR-T cell therapy against CD20, CD22, CD30, BCMA, and other antigens that have entered the clinical trial stage.Citation59–62 Among them, CD19 and CD20 are common surface antigens in B-cell lymphoma,Citation63 CD22 is a surface antigen in acute lymphoblastic leukemia,Citation64 and CD30 and BCMA are associated with Hodgkin’s lymphoma and multiple myeloma.Citation65 In addition to CAR-T cell therapy targeting different antigens, there are new strategies being investigated in the field of lymphoma to enhance the safety and efficacy of CAR-T cell therapy, with the aim of bringing better treatment outcomes to lymphoma patients. For example, CAR-NK cell therapy involves the transduction of CAR genes into natural killer (NK) cells to enhance their tumor specificity and killing ability.Citation66 CAR-Macrophage cell therapy involves the transduction of CAR genes into macrophages to effectively eliminate tumor cells.Citation67 CAR-DC cell therapy, on the other hand, involves the transduction of CAR genes into dendritic cells to enhance their immune recognition and killing ability and activate the body’s immune response.Citation68 These novel CAR cell therapies, compared to CAR-T cell therapy, have lower toxicity, better controllability, and wider applicability. Although these novel CAR cell therapies are still in the laboratory and clinical trial stages, their continuous emergence and development bring new hope for cancer treatment.
Although CAR-T cell therapy has achieved significant efficacy in the treatment of some hematological malignancies, there are still some issues in its clinical application, such as adverse reactions after treatment and the durability of efficacy. Some studies have found that CAR-T cell therapy may induce cytokine release syndrome (CRS), a severe reaction caused by over-activation of the immune system, which can lead to death.Citation69,Citation70 To effectively prevent and manage CRS, various methods can be adopted, including the use of drugs, reducing the dose of CAR-T cells, selecting appropriate patients, and early intervention. CAR-T cell infusion may also cause T cell depletion, suppressive tumor microenvironment, and tumor overload.Citation71–73 In addition, the alpha diversity of the intestinal microbiota may change before and after CAR-T cell therapy, which may lead to the activation and regulation of immune responses and affect the efficacy and safety of CAR-T cell therapy.Citation74,Citation75 Therefore, monitoring changes in the intestinal microbiota is crucial. Future clinical applications of CAR-T cell therapy still need to be supplemented by continuously updated research.
CAR-T cell therapy has become an essential part of a series of hematological malignancies, and the indications of these therapies are still expanding to adapt to earlier treatment. The application of CAR-T cell therapy in hematological malignancies showed promising results that increase the application of CAR-T cell therapy in the treatment of solid tumors. In recent years, there has been a growing number of clinical trials involving CAR-T cell therapy for solid tumors. These trials encompass a range of malignancies including glioblastoma,Citation76 lung cancer,Citation77 hepatocellular carcinoma,Citation78 gastric cancer,Citation79 renal cell carcinoma,Citation80 prostate cancer,Citation81 central nervous system tumors, and neuroblastoma,Citation82 among others. The immunotherapy approach has yielded promising clinical outcomes. However, the development of CAR-T cell therapy in solid tumors is still difficult due to the lack of suitable targets and tumor heterogeneity, the inability of CAR-T cells to effectively infiltrate tumor tissues, and the complexity of the solid tumor microenvironment.Citation83–85 However, with the gradual discovery of specific targets and technological development, there is now a bit of hope for CAR-T cell therapy in solid tumors. It is expected that future research will bring real therapeutic hope.
This is the first bibliometric study on CAR-T cell therapy in the field of lymphoma, but some potential limitations should still be noted. Firstly, although CiteSpace and VOSviewer can be used for bibliometric analysis, they cannot completely replace systematic searches and are unable to combine multiple databases for analysis. Secondly, despite retrieving and downloading all data from the WoSCC database, it is still possible that some publications not included in this database may have been overlooked. In addition, bibliometrics primarily focuses on quantitative indicators such as the number of publications and citation frequency while neglecting the quality factors of publications. Therefore, some high-quality studies may be overwhelmed by low-quality studies, and some innovative studies may be overlooked due to inadequate citation, but we believe that this will not have a substantial impact on the overall trend. In summary, WoSCC is one of the most commonly used and academically recognized influential publication databases in bibliometric analysis. Therefore, visual analysis based on retrieved data can still assist researchers in gaining a comprehensive understanding of the research hotspots, evolution, and development trends of CAR-T cell therapy in the field of lymphoma.
Conclusion
CAR-T cell therapy is a novel cancer treatment technique that utilizes genetically modified immune T cells to recognize and attack cancer cells and has shown significant efficacy in the treatment of some hematologic malignancies. This study combines bibliometrics with scientific knowledge mapping to comprehensively and objectively analyze research on CAR-T cell therapy in the field of lymphoma. Research has shown that there is a growing interest worldwide in the field of CAR-T cell therapy, and over the past decade, the number of research papers on CAR-T cell therapy for lymphoma has rapidly increased. This trend is expected to continue in the coming years. The United States is the leading country in this field, with the highest number of publications. The top 10 institutions are all located in the United States, which greatly promotes the development of CAR-T cell therapy in the field of lymphoma. As one of the most active major participants, China is striving to narrow the gap with developed countries and has made significant contributions to the development of this field. The collaboration network between institutions and authors is tight and comprehensive. CAR-T cell therapy is a promising field of research that will benefit more cancer patients. This study provides a valuable reference for further research in the field of CAR-T cell therapy in lymphoma by summarizing the published research papers on this topic. These research data can assist researchers in better understanding the current research status and focus of the field, thereby discovering more novel hotspots and insights, thus promoting the development of the field and providing new ideas for clinical treatment.
CRediT authorship contribution statement
All authors contributed to the conceptualization and design of the study. Hui Zhou and Ling Xiao participated in the conceptualization and design of the study. Lijia Ou, Chang Su, and Liang Liang contributed to the conceptualization of the study, implementation of the research, and statistical analysis of the data. Qintong Duan, Yufeng Li, and Hui Zang collected and organized the data. Lijia Ou, Yizi He, Ruolan Zeng, and Yajun Li designed and produced the figures and tables. Lijia Ou completed the manuscript writing. All authors participated in the manuscript revision and read and approved the final manuscript for submission.
Acknowledgments
I am grateful for the academic assistance provided by Shengxiang Hu from the School of Computer Engineering and Science at Shanghai University, Danwei Zhao from the West China School of Stomatology at Sichuan University, and Zhigang Zhu from the Xiangya School of Stomatology at Central South University. Additionally, I thank my family for their understanding and support of my academic work.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Additional information
Funding
References
- Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11(4):69. doi:10.1038/s41408-021-00459-7.
- Gross G, Waks T, Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci U S A. 1989;86(24):10024–16. doi:10.1073/pnas.86.24.10024.
- Sadelain M, Brentjens R, Riviere I. The basic principles of chimeric antigen receptor (CAR) design. Cancer Discov. 2013;3(4):388–98. doi:10.1158/2159-8290.CD-12-0548.
- Sadelain M, Rivière I, Brentjens R. Targeting tumours with genetically enhanced T lymphocytes. Nat Rev Cancer. 2003;3(1):35–45. doi:10.1038/nrc971.
- Ho WY, Blattman JN, Dossett ML, Yee C, Greenberg PD. Adoptive immunotherapy: engineering T cell responses as biologic weapons for tumor mass destruction. Cancer Cell. 2003;3(5):431–7. doi:10.1016/S1535-6108(03)00113-2.
- Boardman DA, Philippeos C, Fruhwirth GO, Ibrahim MAA, Hannen RF, Cooper D, Marelli-Berg FM, Watt FM, Lechler RI, Maher J, et al. Expression of a chimeric antigen receptor specific for donor HLA class I enhances the potency of human regulatory T cells in preventing human skin transplant rejection. Am J Transplant. 2017;17(4):931–43. doi:10.1111/ajt.14185.
- Demaria O, Cornen S, Daëron M, Morel Y, Medzhitov R, Vivier E. Harnessing innate immunity in cancer therapy. Nature. 2019;574(7776):45–56. doi:10.1038/s41586-019-1593-5.
- Basar R, Daher M, Rezvani K. Next-generation cell therapies: the emerging role of CAR-NK cells. Hematology Am Soc Hematol Educ Program. 2020;2020(1):570–8. doi:10.1182/hematology.2020002547.
- Daher M, Rezvani K. Outlook for new CAR-based therapies with a focus on CAR NK cells: what lies beyond CAR-Engineered T cells in the race against cancer. Cancer Discov. 2021;11(1):45–58. doi:10.1158/2159-8290.CD-20-0556.
- Yuan Y, Fu Q, Zhang Y, Xu D, Wu Y, Qiu Q, Zhou W. CAR-based cell therapy: evaluation with bibliometrics and patent analysis. Hum Vaccin Immunother. 2021;17(11):4374–82. doi:10.1080/21645515.2021.1947100.
- Ahmad A. CAR-T cell therapy. Int J Mol Sci. 2020;21(12):4303. doi:10.3390/ijms21124303.
- Ahmad A, Uddin S, Steinhoff M. CAR-T cell therapies: an overview of clinical studies supporting their approved use against acute lymphoblastic leukemia and large B-cell lymphomas. Int J Mol Sci. 2020;21(11):3906. doi:10.3390/ijms21113906.
- Torikai H, Reik A, Liu PQ, Zhou Y, Zhang L, Maiti S, Huls H, Miller JC, Kebriaei P, Rabinovitch B, et al. A foundation for universal T-cell based immunotherapy: T cells engineered to express a CD19-specific chimeric-antigen-receptor and eliminate expression of endogenous TCR. Blood. 2012;119(24):5697–705. doi:10.1182/blood-2012-01-405365.
- Barrett DM, Singh N, Porter DL, Grupp SA, June CH. Chimeric antigen receptor therapy for cancer. Annu Rev Med. 2014;65(1):333–47. doi:10.1146/annurev-med-060512-150254.
- Ellebrecht CT, Bhoj VG, Nace A, Choi EJ, Mao X, Cho MJ, Di Zenzo G, Lanzavecchia A, Seykora JT, Cotsarelis G, et al. Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease. Science. 2016;353(6295):179–84. doi:10.1126/science.aaf6756.
- Aghajanian H, Kimura T, Rurik JG, Hancock AS, Leibowitz MS, Li L, Scholler J, Monslow J, Lo A, Han W, et al. Targeting cardiac fibrosis with engineered T cells. Nature. 2019;573(7774):430–3. doi:10.1038/s41586-019-1546-z.
- Mao Y, Zhao C, Zheng P, Zhang X, Xu J. Current status and future development of anti-HIV chimeric antigen receptor T-cell therapy. Immunotherapy. 2021;13(2):177–84. doi:10.2217/imt-2020-0199.
- Wang Y, Liu Y, Tan X, Pan B, Ge J, Qi K, Cheng H, Cao J, Shi M, Yan Z, et al. Safety and efficacy of chimeric antigen receptor (CAR)-T-cell therapy in persons with advanced B-cell cancers and hepatitis B virus-infection. Leukemia. 2020;34(10):2704–7. doi:10.1038/s41375-020-0936-4.
- Strati P, Nastoupil LJ, Fayad LE, Samaniego F, Adkins S, Neelapu SS. Safety of CAR T-cell therapy in patients with B-cell lymphoma and chronic hepatitis B or C virus infection. Blood. 2019;133(26):2800–2. doi:10.1182/blood.2019000888.
- Ellegaard O, Wallin JA. The bibliometric analysis of scholarly production: how great is the impact? Scientometrics. 2015;105(3):1809–31. doi:10.1007/s11192-015-1645-z.
- Lawson McLean A. Publication trends in transcranial magnetic stimulation: a 30-year panorama. Brain Stimul. 2019;12(3):619–27. doi:10.1016/j.brs.2019.01.002.
- Zheng K, Wang X. Publications on the association between cognitive function and pain from 2000 to 2018: a bibliometric analysis using CiteSpace. Med Sci Monit. 2019;25:8940–51. doi:10.12659/MSM.917742.
- Chen C. Searching for intellectual turning points: progressive knowledge domain visualization. Proc Natl Acad Sci U S A. 2004;101 Suppl 1(Suppl 1):5303–10. doi:10.1073/pnas.0307513100.
- Brookes BC. Bradford’s law and the bibliography of science. Nature. 1969;224(5223):953–6. doi:10.1038/224953a0.
- Chen C. CiteSpace II: detecting and visualizing emerging trends and transient patterns in scientific literature. J Am Soc Inf Sci. 2006;57(3):359–77. doi:10.1002/asi.20317.
- van Eck NJ, Waltman L. software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. 2010;84(2):523–38. doi:10.1007/s11192-009-0146-3.
- Ogunsakin RE, Ebenezer O, Ginindza TG. A bibliometric analysis of the literature on norovirus disease from 1991-2021. Int J Environ Res Public Health. 2022;19(5):2508. doi:10.3390/ijerph19052508.
- Munim ZH, Dushenko M, Jimenez VJ, Shakil MH, Imset M. Big data and artificial intelligence in the maritime industry: a bibliometric review and future research directions. Marit Policy Manag. 2020;47(5):577–97. doi:10.1080/03088839.2020.1788731.
- Chen C, Leydesdorff L. Patterns of connections and movements in dual-map overlays: a new method of publication portfolio analysis. J Assn Inf Sci Tec. 2014;65(2):334–51. doi:10.1002/asi.22968.
- Schilling C, Stoeckli SJ, Haerle SK, Broglie MA, Huber GF, Sorensen JA, Bakholdt V, Krogdahl A, von Buchwald C, Bilde A, et al. Sentinel European Node Trial (SENT): 3-year results of sentinel node biopsy in oral cancer. Eur J Cancer. 2015;51(18):2777–84. doi:10.1016/j.ejca.2015.08.023.
- Den Toom IJ, Heuveling DA, Flach GB, van Weert S, Karagozoglu KH, van Schie A, Bloemena E, Leemans CR, de Bree R. Sentinel node biopsy for early-stage oral cavity cancer: the VU University Medical center experience: sentinel node biopsy for early oral cancer. Head Neck. 2015;37(4):573–8. doi:10.1002/hed.23632.
- de Bree R, Takes RP, Castelijns JA, de Bree R, Medina JE, Stoeckli SJ, Mancuso AA, Hunt JL, Rodrigo JP, Triantafyllou A, et al. Advances in diagnostic modalities to detect occult lymph node metastases in head and neck squamous cell carcinoma: diagnostic modalities for detection of lymph node metastasis. Eisele DW, ed. Head Neck. 2015;37(12):1829–39. doi:10.1002/hed.23814.
- Hirsch JE. an index to quantify an individual’s scientific research output. Proc Natl Acad Sci U S A. 2005;102(46):16569–72. doi:10.1073/pnas.0507655102.
- Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–17. doi:10.1056/NEJMoa1407222.
- Ninkov A, Frank JR, Maggio LA. bibliometrics: methods for studying academic publishing. Perspect Med Educ. 2022;11(3):173–6. doi:10.1007/s40037-021-00695-4.
- Brudno JN, Kochenderfer JN. recent advances in CAR T-cell toxicity: mechanisms, manifestations and management. Blood Rev. 2019;34:45–55. doi:10.1016/j.blre.2018.11.002.
- West R, McIlwaine A. what do citation counts count for in the field of addiction? An empirical evaluation of citation counts and their link with peer ratings of quality: citations and quality. Addiction. 2002;97(5):501–4. doi:10.1046/j.1360-0443.2002.00104.x.
- Radicchi F, Fortunato S, Castellano C. Universality of citation distributions: toward an objective measure of scientific impact. Proc Natl Acad Sci U S A. 2008;105(45):17268–72. doi:10.1073/pnas.0806977105.
- Yoon SJ, Yoon DY, Ja Lim K, Moon JY, Hong SJ, Baek S, Yun EJ. The 100 top-cited articles focused on magnetic resonance: a bibliometric analysis. Acta radiol. 2019;60(6):710–5. doi:10.1177/0284185118795325.
- Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, June CH. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3(95):95ra73. doi:10.1126/scitranslmed.3002842.
- Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF, et al. Chimeric antigen receptor–modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368(16):1509–18. doi:10.1056/NEJMoa1215134.
- Armitage JO, Gascoyne RD, Lunning MA, Cavalli F. Non-Hodgkin lymphoma. Lancet. 2017;390(10091):298–310. doi:10.1016/S0140-6736(16)32407-2.
- Vaqué JP, Martínez N, Batlle-López A, Perez C, Montes-Moreno S, Sanchez-Beato M, Piris MA. B-cell lymphoma mutations: improving diagnostics and enabling targeted therapies. Haematologica. 2014;99(2):222–31. doi:10.3324/haematol.2013.096248.
- International T-cell lymphoma project. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. JCO. 2008;26(25):4124–30. doi:10.1200/JCO.2008.16.4558.
- Inaba H, Mullighan CG. Pediatric acute lymphoblastic leukemia. Haematologica. 2020;105(11):2524–39. doi:10.3324/haematol.2020.247031.
- Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, Bader P, Verneris MR, Stefanski HE, Myers GD, et al. tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439–48. doi:10.1056/NEJMoa1709866.
- Abramson JS. anti-CD19 CAR T-cell therapy for B-cell non-Hodgkin lymphoma. Transfus Med Rev. 2020;34(1):29–33. doi:10.1016/j.tmrv.2019.08.003.
- Ying Z, Huang XF, Xiang X, Liu Y, Kang X, Song Y, Guo X, Liu H, Ding N, Zhang T, et al. A safe and potent anti-CD19 CAR T cell therapy. Nat Med. 2019;25(6):947–53. doi:10.1038/s41591-019-0421-7.
- Danhof S, Hudecek M, Smith EL. CARs and other T cell therapies for MM: the clinical experience. Best Pract Res Clin Haematol. 2018;31(2):147–57. doi:10.1016/j.beha.2018.03.002.
- Grigor EJM, Fergusson D, Kekre N, Montroy J, Atkins H, Seftel MD, Daugaard M, Presseau J, Thavorn K, Hutton B, et al. Risks and benefits of chimeric antigen receptor T-cell (CAR-T) therapy in cancer: a systematic review and meta-analysis. Transfus Med Rev. 2019;33(2):98–110. doi:10.1016/j.tmrv.2019.01.005.
- Fraietta JA, Lacey SF, Orlando EJ, Pruteanu-Malinici I, Gohil M, Lundh S, Boesteanu AC, Wang Y, O’Connor RS, Hwang W-T, et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med. 2018;24(5):563–71. doi:10.1038/s41591-018-0010-1.
- Thudium Mueller K, Grupp SA, Maude SL, Levine JE, Pulsipher MA, Boyer MW, August KJ, Myers GD, Tam CS, Jaeger U, et al. Tisagenlecleucel immunogenicity in relapsed/refractory acute lymphoblastic leukemia and diffuse large B-cell lymphoma. Blood Adv. 2021;5(23):4980–91. doi:10.1182/bloodadvances.2020003844.
- Liu Y, Bewersdorf JP, Stahl M, Zeidan AM. Immunotherapy in acute myeloid leukemia and myelodysplastic syndromes: the dawn of a new era? Blood Rev. 2019;34:67–83. doi:10.1016/j.blre.2018.12.001.
- Westin JR, Kersten MJ, Salles G, Abramson JS, Schuster SJ, Locke FL, Andreadis C. Efficacy and safety of CD19 -directed CAR-T cell therapies in patients with relapsed/refractory aggressive B-cell lymphomas: observations from the JULIET , ZUMA -1, and TRANSCEND trials. Am J Hematol. 2021;96(10):1295–312. doi:10.1002/ajh.26301.
- Jacobson CA, Chavez JC, Sehgal AR, William BM, Munoz J, Salles G, Munshi PN, Casulo C, Maloney DG, de Vos S, et al. Axicabtagene ciloleucel in relapsed or refractory indolent non-Hodgkin lymphoma (ZUMA-5): a single-arm, multicentre, phase 2 trial. Lancet Oncol. 2022;23(1):91–103. doi:10.1016/S1470-2045(21)00591-X.
- Ma S, Li X, Wang X, Cheng L, Li Z, Zhang C, Ye Z, Qian Q. Current progress in CAR-T cell therapy for solid tumors. Int J Biol Sci. 2019;15(12):2548–60. doi:10.7150/ijbs.34213.
- Moghimi B, Muthugounder S, Jambon S, Tibbetts R, Hung L, Bassiri H, Hogarty MD, Barrett DM, Shimada H, Asgharzadeh S, et al. Preclinical assessment of the efficacy and specificity of GD2-B7H3 SynNotch CAR-T in metastatic neuroblastoma. Nat Commun. 2021;12(1):511. doi:10.1038/s41467-020-20785-x.
- Marofi F, Rahman HS, Achmad MH, Sergeevna KN, Suksatan W, Abdelbasset WK, Mikhailova MV, Shomali N, Yazdanifar M, Hassanzadeh A, et al. A deep insight into CAR-T cell therapy in non-Hodgkin lymphoma: application, opportunities, and future directions. Front Immunol. 2021;12:681984. doi:10.3389/fimmu.2021.681984.
- Hu Y, Zhou Y, Zhang M, Ge W, Li Y, Yang L, Wei G, Han L, Wang H, Yu S, et al. CRISPR/Cas9-engineered universal CD19/CD22 dual-targeted CAR-T cell therapy for relapsed/refractory B-cell acute lymphoblastic leukemia. Clin Cancer Res. 2021;27(10):2764–72. doi:10.1158/1078-0432.CCR-20-3863.
- Pavlasova G, Mraz M. the regulation and function of CD20: an “enigma” of B-cell biology and targeted therapy. Haematologica. 2020;105(6):1494–506. doi:10.3324/haematol.2019.243543.
- Pierce JMR, Mehta A. Diagnostic, prognostic and therapeutic role of CD30 in lymphoma. Expert Rev Hematol. 2017;10(1):29–37. doi:10.1080/17474086.2017.1270202.
- Mei H, Li C, Jiang H, Zhao X, Huang Z, Jin D, Guo T, Kou H, Liu L, Tang L, et al. A bispecific CAR-T cell therapy targeting BCMA and CD38 in relapsed or refractory multiple myeloma. J Hematol Oncol. 2021;14(1):161. doi:10.1186/s13045-021-01170-7.
- Shah NN, Johnson BD, Schneider D, Zhu F, Szabo A, Keever-Taylor CA, Krueger W, Worden AA, Kadan MJ, Yim S, et al. Bispecific anti-CD20, anti-CD19 CAR T cells for relapsed B cell malignancies: a phase 1 dose escalation and expansion trial. Nat Med. 2020;26(10):1569–75. doi:10.1038/s41591-020-1081-3.
- Fry TJ, Shah NN, Orentas RJ, Stetler-Stevenson M, Yuan CM, Ramakrishna S, Wolters P, Martin S, Delbrook C, Yates B, et al. CD22-Targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med. 2018;24(1):20–8. doi:10.1038/nm.4441.
- Jardin F. NFkB pathway and Hodgkin Lymphoma. Biomedicines. 2022;10(9):2153. doi:10.3390/biomedicines10092153.
- Gong Y, Klein Wolterink RGJ, Wang J, Bos GMJ, Germeraad WTV. Chimeric antigen receptor natural killer (CAR-NK) cell design and engineering for cancer therapy. J Hematol Oncol. 2021;14(1):73. doi:10.1186/s13045-021-01083-5.
- Pan K, Farrukh H, Chittepu VCSR, Xu H, Pan CX, Zhu Z. CAR race to cancer immunotherapy: from CAR T, CAR NK to CAR macrophage therapy. J Exp Clin Cancer Res. 2022;41(1):119. doi:10.1186/s13046-022-02327-z.
- Lai J, Mardiana S, House IG, Sek K, Henderson MA, Giuffrida L, Chen AXY, Todd KL, Petley EV, Chan JD, et al. Adoptive cellular therapy with T cells expressing the dendritic cell growth factor Flt3L drives epitope spreading and antitumor immunity. Nat Immunol. 2020;21(8):914–26. doi:10.1038/s41590-020-0676-7.
- Pensato U, Muccioli L, Cani I, Janigro D, Zinzani PL, Guarino M, Cortelli P, Bisulli F. Brain dysfunction in COVID-19 and CAR-T therapy: cytokine storm-associated encephalopathy. Ann Clin Transl Neurol. 2021;8(4):968–79. doi:10.1002/acn3.51348.
- Freyer CW, Porter DL. Cytokine release syndrome and neurotoxicity following CAR T-cell therapy for hematologic malignancies. J Allergy Clin Immunol. 2020;146(5):940–8. doi:10.1016/j.jaci.2020.07.025.
- Zhang C, Palashati H, Rong Z, Lin N, Shen L, Liu Y, Li S, Yu B, Yang W, Lu Z, et al. Pre-depletion of TRBC1+ T cells promotes the therapeutic efficacy of anti-TRBC1 CAR-T for T-cell malignancies. Mol Cancer. 2020;19(1):162. doi:10.1186/s12943-020-01282-7.
- Liu G, Rui W, Zhao X, Lin X. Enhancing CAR-T cell efficacy in solid tumors by targeting the tumor microenvironment. Cell Mol Immunol. 2021;18(5):1085–95. doi:10.1038/s41423-021-00655-2.
- Zebley CC, Youngblood B. Mechanisms of T cell exhaustion guiding next-generation immunotherapy. Trends Cancer. 2022;8(9):726–34. doi:10.1016/j.trecan.2022.04.004.
- Hu Y, Li J, Ni F, Yang Z, Gui X, Bao Z, Zhao H, Wei G, Wang Y, Zhang M, et al. CAR-T cell therapy-related cytokine release syndrome and therapeutic response is modulated by the gut microbiome in hematologic malignancies. Nat Commun. 2022;13(1):5313. doi:10.1038/s41467-022-32960-3.
- Smith M, Dai A, Ghilardi G, Amelsberg KV, Devlin SM, Pajarillo R, Slingerland JB, Beghi S, Herrera PS, Giardina P, et al. Gut microbiome correlates of response and toxicity following anti-CD19 CAR T cell therapy. Nat Med. 2022;28(4):713–23. doi:10.1038/s41591-022-01702-9.
- Ahmed N, Brawley V, Hegde M, Bielamowicz K, Kalra M, Landi D, Robertson C, Gray TL, Diouf O, Wakefield A, et al. HER2-Specific chimeric antigen receptor–modified virus-specific T cells for progressive glioblastoma. JAMA Oncol. 2017;3(8):1094–101. doi:10.1001/jamaoncol.2017.0184.
- Hu Z, Zheng X, Jiao D, Zhou Y, Sun R, Wang B, Tian Z, Wei H. LunX-CAR T cells as a targeted therapy for non-small cell lung cancer. Mol Ther Oncolytics. 2020;17:361–70. doi:10.1016/j.omto.2020.04.008.
- Batra SA, Rathi P, Guo L, Courtney AN, Fleurence J, Balzeau J, Shaik RS, Nguyen TP, Wu M-F, Bulsara S, et al. Glypican-3–specific CAR T cells coexpressing IL15 and IL21 have superior expansion and antitumor activity against hepatocellular carcinoma. Cancer Immunol Res. 2020;8(3):309–20. doi:10.1158/2326-6066.CIR-19-0293.
- Jiang H, Shi Z, Wang P, Wang C, Yang L, Du G, Zhang H, Shi B, Jia J, Li Q, et al. Claudin18.2-specific chimeric antigen receptor engineered T cells for the treatment of gastric cancer. JNCI J Nat Cancer Inst. 2019;111(4):409–18. doi:10.1093/jnci/djy134.
- Li H, Ding J, Lu M, Liu H, Miao Y, Li L, Wang G, Zheng J, Pei D, Zhang Q, et al. CAIX-specific CAR-T cells and sunitinib show synergistic effects against metastatic renal cancer models. J Immunother. 2020;43(1):16–28. doi:10.1097/CJI.0000000000000301.
- Gorchakov AA, Kulemzin SV, Kochneva GV, Taranin AV. challenges and prospects of chimeric antigen receptor T-cell therapy for metastatic prostate cancer. Eur Urol. 2020;77(3):299–308. doi:10.1016/j.eururo.2019.08.014.
- Safarzadeh Kozani P, Safarzadeh Kozani P, Ahmadi Najafabadi M, Yousefi F, Mirarefin SMJ, Rahbarizadeh F. recent advances in solid tumor CAR-T cell therapy: driving tumor cells from hero to zero? Front Immunol. 2022;13:795164. doi:10.3389/fimmu.2022.795164.
- Marofi F, Motavalli R, Safonov VA, Thangavelu L, Yumashev AV, Alexander M, Shomali N, Chartrand MS, Pathak Y, Jarahian M, et al. CAR T cells in solid tumors: challenges and opportunities. Stem Cell Res Ther. 2021;12(1):81. doi:10.1186/s13287-020-02128-1.
- Lancet Oncology T. The lancet oncology. CAR T-cell therapy for solid tumours. Lancet Oncol. 2021;22(7):893. doi:10.1016/S1470-2045(21)00353-3.
- Zhang Q, Ping J, Huang Z, Zhang X, Zhou J, Wang G, Liu S, Ma J. CAR-T cell therapy in cancer: tribulations and road ahead. J Immunol Res. 2020;2020:1–11. doi:10.1155/2020/1924379.