 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The COVID-19 pandemic has claimed over six million lives and caused significant morbidities globally. The development and use of COVID-19 vaccines is a key strategy in ending this. There is a general public hesitancy on vaccine uptake, including pregnant women who are at high risk of severe forms of the disease and death when infected with the virus. To determine the magnitude of hesitancy toward COVID-19 vaccines and the associated factors among pregnant women attending public antenatal clinics in Dar es Salaam. This was a cross-sectional analytical study conducted among 896 pregnant women attending antenatal clinics at public health facilities in Dar es Salaam. A structured interviewer-based questionnaire, in an electronic form, was used. The analysis was done by a multivariable linear regression model using STATA 16 to obtain factors associated with vaccine hesitancy, and P < .05 was considered significant. The proportion of pregnant women with vaccine hesitancy was 45%. Hesitancy was higher among unemployed pregnant women (AOR 2.16 (95% CI 1.36–3.42) and the self-employed group (AOR 1.62 (95% CI 1.07–2.44). It was also higher among pregnant women with poor attitudes to COVID-19 vaccines (AOR 2.44 (95% CI 1.75–3.39) and women who had low perceived benefits of the vaccines (AOR 2.57 (95% CI 1.83–3.60). COVID-19 vaccine-targeted interventions should aim at the provision of knowledge on COVID-19 and the COVID-19 vaccine and address poor attitudes and perceptions that pregnant women have on these vaccines.
KEYWORDS:
Introduction
The coronavirus 2019 (COVID-19) pandemic has caused enormous loss of life and morbidities, with over six million deaths globally.Citation1 Pregnant women are at increased risk of developing severe disease and admissions to the intensive care unit (ICU) and mechanical ventilation compared to non-pregnant women.Citation2–5 Pregnant women in their third trimester who are obese, are >35 years or have comorbidity conditions are at higher risk of severe and critical disease with increased risk of pre-eclampsia, gestational diabetes, cesarean section delivery, and maternal deaths.Citation2,Citation6–9
Several literature have documented higher rates of iatrogenic preterm birth, smaller gestation babies, stillbirths, and increased admission to neonatal intensive care units among neonates of pregnant women infected with COVID-19.Citation2,Citation4,Citation10 Despite the increased mortality and morbidity, there are no increased cases of congenital anomalies or vertical transmission reported from the infection to date.Citation2,Citation5,Citation6 Evidence suggests that recovery from COVID-19 has been associated with cardiac function impairmentCitation11 and survivors still suffer from either neurological, physical, and/or psychological sequela 2 years post-infection.Citation12 Moreover, there is a higher risk of developing new onset type 1 diabetes mellitus in children who are infected with COVID-19Citation13
There is no cure for COVID-19 infection and vaccines are considered the most promising approach for curbing the pandemic and are being vigorously pursued.Citation14 Several vaccines are available since 2021, and all do not contain an active virus and cannot cause an infection.Citation15 Available data from systemic reviews and meta-analysis show that vaccination during pregnancy reduced the risk of infection by 60% and ICU admissions by 82%, and it also reduced the risks of stillbirth and preterm birth no harmful effects on pregnant women or the fetus and new-born have been shown from the systemic reviewsCitation15,Citation16 and there is evidence of passive immunity against SARS-CoV-2 in new-borns after maternal vaccination with mRNA vaccines.Citation17
It is now recommended by most health public authorities to vaccinate pregnant women to prevent the severity and consequences of COVID-19.Citation18–22 These recommendations are based on increasingly reassuring data regarding the safety and efficacy of COVID-19 vaccines during pregnancy, as well as data that pregnancy itself is associated with an increased risk of severe infection.Citation19,Citation20,Citation23,Citation24 The benefits of the COVID-19 vaccine in pregnant and non-pregnant women extend beyond reduced morbidity and mortality, to improved mental health status, reduced health system costs, and a return to individual and family economic activities that were paralyzed during the COVID-19 pandemic.Citation19,Citation25
The WHO Strategic Advisory Group of Experts on the vaccine (SAGE) defines Vaccine hesitancy as “a delay in acceptance or refusal of vaccination despite the availability of vaccination services.”Citation26 Unfortunately, several systemic reviews have reported global COVID-19 vaccine hesitancy among pregnant womenCitation27–32 and this has been influenced mainly by the socio-demographic characteristics of respondents,Citation33–36 their obstetrics history,Citation37 and their poor knowledge and attitude toward COVID and COVID-19 vaccine.Citation30,Citation34,Citation37,Citation38
In Tanzania, the first COVID-19 case was reported in March 2020,Citation39 and the COVID-19 vaccine was authorized to be used from July 2021.Citation40 The initial vaccination mandate gave priority to frontline healthcare workers, and groups that were at high risk of severe morbidities and deaths that included those ≥50 years, chronic/underlying medical conditions such as hypertension, diabetes, and respiratory conditions. In August 2021, the Ministry of Health recommended vaccination for pregnant women to be offered when the benefits outweigh the potential risks. The decision to vaccinate is based on individual choice, personal values, and understanding of the benefits of protecting against the risk of infection and/or morbidity and mortality of COVID-19Citation40
Vaccine hesitancy hinders efforts to control the spread of COVID-19 infection and this prolongs the public health crisis, leading to a higher number of severe cases and higher mortality rates.Citation41,Citation42 Increased cases and hospitalizations overwhelm the fragile healthcare system of low-income countries and reduce the capacity to handle other health issues. Tanzania as a lower-middle-income country can further be affected economically with the lockdowns, restrictions, and reduced economic activities that are associated with COVID-19 waves. Overall, COVID-19 vaccine uptake in Tanzania is low (<10% of population) as of April 2022Citation43 and the magnitude of hesitancy to vaccinate especially among pregnant women in Tanzania is not known yet. Hence, the study was aimed at determining the vaccine hesitancy magnitude toward COVID-19 vaccines and its associated factors among pregnant women.
The health behavior model has several components: perceived susceptibility to infection and severe infection, perceived benefits and risks, social norms, cues to action, self-efficacy, and health behaviors have been used in several studies to find the reason for the COVID-19 vaccine hesitancy.Citation35–44–Citation46 Similarly, it has been used in this study as it provides a structured framework for understanding why people make the decisions they do regarding their health and it offers a way to systematically analyze and predict behavior based on psychosocial, social, and environmental factors.
Materials and methods
Study design, setting, and population
This cross-sectional analytic study was done at four public health facilities of Dar es Salaam at four different levels: primary health center, district hospital, regional and tertiary-level hospital; Buguruni health center, Mnazi Mmoja district hospital, Amana Regional hospital, and Muhimbili National Hospital, respectively. Dar es Salaam is an important economic city with a population of over six millionCitation47 people, which made it to be the most affected region during the COVID-19 pandemic in Tanzania.Citation48 All studied facilities provide antenatal care services from Monday to Friday and COVID-19 vaccines are also provided at the same health facility but not at the antenatal clinic.
Sample size
Sample size was calculated using the Kelsey formula for a cross-sectional analytical study;
n = is the sample size in each study group of pregnant women,
p1 – proportion of exposed, p2 – proportion of unexposed
r- ratio of exposed to non-exposed where r is 1:1.
p1 – p2 – clinically meaningful difference in the vaccine hesitancy proportion between the two groups, p- is the average of p1 and p2
p (1-p) is the variance of the anticipated proportion difference
- corresponds to two-tailed significance level (1.96 for α = 5%) and zβ - corresponds to a power of 80%
The sample size calculation was based on the following assumption;
The exposure is good attitude toward COVID-19 vaccine. The proportion of unexposed with outcome was taken to be 5%, and the proportion of exposed with outcome was 10% (odds ratio-2.1).Citation49 The estimated sample size was computed using OpenEpi online calculator and was found to be n = 443; therefore, 2n = 886. Loss to follow up is assumed to be 10%, which was added to the total sample size. Therefore, a minimum of 984 pregnant women were required for this study. The Kelsey formula was used to provide adequate sample size in order to obtain factors associated.
Sampling technique
The sample size was divided among the four hospitals by stratified sampling using probability proportion to size (PPS). Based on ANC attendees booked for the past 6 months at each facility, the minimum number expected per facility was extrapolated.
Sampling fraction = n/Nx100, where by n = sample size (984) and N = total number of minimum participants expected to be recruited during the study period (1924)
Therefore, sampling fraction = 51.1%
Systematic random sampling technique was used at each facility. Sampling interval was determined by dividing the expected number of ANC attendees into the sample size (1924/984) which gave a sampling interval of 2. The first case was selected using lottery method, then every second pregnant woman coming to ANC service was interviewed until the total sample size was reached.
Inclusion criteria
We included all pregnant women at the age of ≥18 who were attending antenatal clinics at the selected health facilities
Exclusion criteria
Pregnant women who were sick or unable to communicate
Data collection procedure
Data was collected from 17th October to 25th November 2022 by the main author and eight research assistants who were recent graduates from the Muhimbili University of Health and Allied Sciences. The research assistants were trained in sampling, data collection tools, and procedures. Using the antenatal registry book, the first participant was identified by lottery method, then every second attendee was recruited after being asked for a written consent. Recruitment and questionnaire filling was done after they had received services or in case of long queues as they waited for the services. No incentives were provided to the participants for their participation in this study.
Data collection tool
An interviewer-administered structured questionnaire in the local language – Kiswahili (Appendix 1B) was used to collect the data. The questionnaire was in an electronic Red Cap and was pretested and piloted in 20 pregnant women attending antenatal care in a nonparticipating health facility. The questionnaire was adapted from various studiesCitation34,Citation37,Citation38,Citation45,Citation46 and was divided into the following parts:
Socio-demographic and obstetric factors part with age (years), marital status, education level, occupation/employment, income, having household members who are at risk due to age of comorbid conditions, gravidity, parity, gestation age, and perceived pregnancy risk.
Knowledge of COVID-19 infection and COVID-19 vaccine.
Questions on perceptions, attitudes, and trust using the Health Belief Model constructs
COVID-19 and vaccine hesitancy status
Data management and analysis
Responses to all questions in the Red Cap were made mandatory before submitting the questionnaire to avoid incomplete filling and missing data. The electronically filled questionnaires in the Red Cap were automatically transferred to a local server that was password protected. The data was downloaded by the data manager as an excel file that was used in STATA 16 for analysis.
Covid-19 vaccine hesitancy was assessed with a Likert scale question. A response of very unlikely/somewhat unlikely/not sure to get the vaccine when it is available was used to define women with vaccine hesitancy and was subsequently used to find the proportion of pregnant women with COVID-19 vaccine hesitancy.
The level of knowledge on the COVID-19 vaccine was determined using a set of seven questions, and a score of ≥70% or above was considered good knowledge. Similarly, a score of ≥70% of the items under the attitude and perception sections was used to define the good attitude and high perception, respectively. High-risk pregnancies were defined as pregnancies with an existing medical and/or obstetric condition as reported by the participants or documented on their antenatal card.
The dependent variable which was vaccine hesitancy and the independent variables which were socio-demographic and obstetrics characteristics, knowledge, and attitude toward COVID-19 vaccine and behavioural/perception toward vaccination were summarized using frequencies and percentages. Measures of association were calculated using cross-tabulation and binary logistic regression, and multivariable logistic regression was used to find factors associated at p-value <.05. The independent variables used in multiple linear regression were the one in linear relationship with dependent variable, with p value <.2 in bivariate analysis and with no multicollinearity
Ethical issues
The study was reviewed and received an ethical permit from MUHAS Institutional Review Board with Ref No: MUHAS-REC-09-2022–1364. Permission to conduct research was sought from the Ministry of Health, the President’s Office Regional Administration, and Local Government (PO-RALG), and individual health facilities’ administration. Written informed consent was sought from the pregnant women after the purpose of the study and their right to withdraw from the interview was explained. Privacy and confidentiality were respected throughout the research. Those who wished to vaccinate the same day were directed to the vaccination area.
Results
A total of 3876 (new and repeated) pregnant women attended ANC in the four public health facilities during the study duration. The sample included 968 women who met inclusion criteria, however, 72 refused to participate, and we remained with 896 who were analyzed. The response rate was 93%
As shown in , Majority of the pregnant women (81.4%) in the study were between 20 and 34 years of age, nearly half had a secondary level of education, and were self-employed and 86.7% were married. A high proportion of women (89.2%) had low-risk pregnancies (no existing medical and/or obstetric condition as reported by the participants or documented on their antenatal card) and 53.8% were in their third trimester of pregnancy.
Table 1. Socio-demographic and obstetrics characteristics of pregnant women attending antenatal clinics at public health facilities of Dar-es-Salaam.
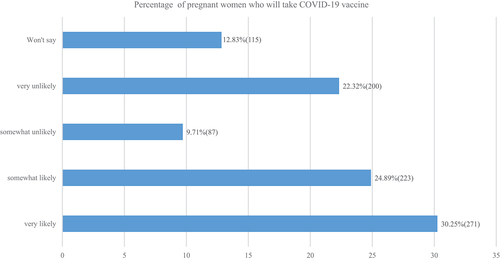
The proportion of pregnant women who had vaccine hesitancy was found to be 44.9% (402/896) as shown in . This was computed from number of women who were somewhat unlikely to vaccinate, very unlikely to vaccinate and those who did not reveal their response. As shown in , almost a quarter (200/896) reported that it was very unlikely for them to vaccinate which represents a stronger level of hesitance and 12.8% (115/896) were not ready to reveal their response but these are considered to have hesitancy as they do delay vaccination coverage as per WHO-SAGE definitionCitation36
Figure 1. COVID-19 vaccine hesitancy and acceptance as indicated by the likelihood of the pregnant women to take the vaccine when available.
As shown in , 36.8% of the pregnant women interviewed believed that very few people in the community would take the COVID-19 vaccine though over half believed that their close family, friends, community, and religious leaders would want them to get the vaccine. Only 35.8% of the pregnant women were very likely to get the vaccine if it was recommended by a doctor or nurse and only 10.3% reported to have been vaccinated during pregnancy, though most of them (63.6%) reported that it was not difficult at all to access it. About a third of pregnant women were not sure if they could trust the vaccine or if the vaccine was safe. More than 94.0% reported never to have been diagnosed with COVID-19, caring for a close family member/having a family member who died of a suspected COVID-19 infection.
Table 2. Perceived social norms, action efficacy, divine will, and COVID-19 status.
Among women who had poor attitudes toward COVID-19 vaccines, 24.2% revealed that they did not trust the vaccines, 18.7% believed that they did not need vaccines because they are healthy and at low risk, and 16.3% believed that even if they get COVID-19 infection they will not became seriously ill as shown in .
Table 3. Attitudes of pregnant women toward COVID-19 vaccines.
More than 40% of women as shown in had low perceptions on susceptibility and severity of COVID-19 infection. They were not worried about their likelihood of getting infection or dying from the infection. With regards to perceived benefits of COVID-19 vaccines, majority of women (>50%) perceived that the vaccine will decrease their risk of getting the infection and its complications. On perceived barriers, 71.1% of women were concerned with the efficacy of the vaccines and about 30% were worried of side effects of the vaccines and had concerns that they may receive fake or faulty vaccines.
Table 4. Perceptions of pregnant women toward COVID-19 vaccines.
As shown in , the variables that were controlled for in the logistic model were education level, age, marital status, occupation, knowledge, attitude, perceived susceptibility, and perceived benefits of vaccine as these were the variables that showed association in the bivariate analysis and had no multicollinearity. Unemployed pregnant women were twice as likely to have vaccine hesitancy compared to employed women (AOR 2.16 (95% CI 1.36–3.42) P-value < .001), and similarly pregnant women with poor attitudes to COVID-19 vaccines had two times the odds of vaccine hesitancy than women with a good attitude (AOR 2.44 (95% CI 1.75–3.39) P-value < .001). Pregnant women who had low perceived benefits of vaccines were twice as likely to have vaccine hesitancy as those who perceived the vaccines to be beneficial (AOR 2.57 (95% CI 1.83–3.60) P-value < .001).
Table 5. Factors associated with COVID-19 vaccine hesitancy among pregnant women using a binary logistic regression model.
Discussion
The study showed that nearly half of the pregnant women attending ANC in public facilities of Dar es Salaam showed COVID-19 vaccine hesitancy during pregnancy. The hesitancy was mainly influenced by individual attitudes and perceptions toward vaccination and perceived susceptibility and severity of COVID-19 infection.
In this study, almost half of the pregnant women attending public health facilities had COVID-19 vaccine hesitancy. This is a concerning finding especially when there is evidence of severe morbidity, death, and hospitalization for this group and increased adverse events for their fetuses. This result coincides with several systemic reviews that have been conducted worldwide.Citation32,Citation34,Citation37,Citation44,Citation50,Citation51 It is also worth noting that certain vaccines like Tetanus Toxoid (TT) have been recommended for pregnant women for many years in Tanzania and have a strong safety record; however, 50.3% of women were reported to have either one or no TT vaccination in the analysis of data from the 2015/2016 Tanzania demographic and health survey.Citation52 Pregnant women with lower education levels were noted to have less vaccine hesitancy compared to higher education levels. Similar findings were reported in KenyaCitation53 and Singapore.Citation33 This is inconsistent with studies that reported that higher than primary education improves acceptance of being vaccinatedCitation45 as well as increases in intention to be vaccinated against COVID-19 than their counterparts.Citation54 A possible explanation for this is that women who are highly educated are better informed about the lack of safety data on the vaccine.
The study revealed that pregnant women who were unemployed and self-employed were more likely to have vaccine hesitancy than employed women. Comparable findings were reported in other studies from various regions.Citation55–57 Strong recommendations on vaccinations in Tanzania have been given to people who were employed to prevent further workplace outbreaks and to prevent infection among workers and their customers. The high hesitancy could also be due to the fact that individuals who work primarily outside or in uncrowded conditions may feel less at risk of contracting COVID-19; however, these women are still at risk from exposure through their working husbands.
There is a concern on whether parity, pregnancy trimester, and pregnancy risk affect the intention to be vaccinated. Pregnant women in their first trimester with low parity and low-risk pregnancy are expected to have a higher hesitancy to vaccinate due to fear of teratogenicity, concerns about fetal safety, and perception of low risk of COVID-19 infection acquisition, respectively. There is limited literature that has reported associations of these obstetric factors to vaccine hesitancy. Our study found no significant association between these obstetric factors and vaccination hesitancy, though it was noted that women in their first pregnancy, third trimester and with a low-risk pregnancy had higher hesitancy.
Our findings are in line with previous literature that revealed a lack of or poor knowledge of the COVID-19 vaccine in the pregnant population influences the decision of not vaccinating during pregnancy.Citation34,Citation37,Citation38 The majority of pregnant women in the study were not aware of any guidelines on vaccination during pregnancy and were not sure of vaccine effects on their health, and pregnancy and this could explain why they were hesitant to vaccinate.
Pregnant women who had a poor attitude to the COVID-19 vaccine were found to have more hesitancy toward vaccination similar to other studies that found a positive attitude toward COVID-19 vaccination increased the chance of accepting the vaccine.Citation30,Citation58 The majority disclosed primary reasons for hesitancy to receive the COVID-19 vaccine were; not trusting the vaccine, believing they do not need the vaccine because they are healthy and at low risk for infection, and they do not need a COVID-19 vaccine because even if they get infected, they will not become seriously ill.
Pregnant women who had heightened perceptions of increased susceptibility to COVID-19 and high perceived severity of the disease during pregnancy showed less hesitancy toward being vaccinated than those without added risk perception. The findings were similar to other studies in the general population as well as in the pregnant population.Citation28,Citation33,Citation34 The majority were worried about their likelihood of getting the infection, being at higher risk, and they were concerned that they could die of COVID-19 infection.
Participants with low perceived benefits to vaccination were found to have high hesitancy toward vaccination. Many women did not believe vaccination would reduce their chances of having the disease or decrease complications in case they acquired an infection after vaccination. Though the perceived barrier did not have a statistically significant influence on hesitancy, it is worth noting that the majority of pregnant women (71.1%) were concerned with the efficacy of the vaccine and more than a quarter were worried about side effects, of receiving a faulty/fake vaccine and that the vaccine was too rushed to be manufactured. There are well-publicized events that showed long-term mistreatment and experiments that have been done to Africans that have created fear toward western medicine.Citation59 These are important concerns to be addressed while counseling pregnant women about vaccination.
Understanding the reasons for COVID-19 vaccine hesitancy among pregnant women in Tanzania provides valuable insights and informs strategies to address the issue. Identifying the knowledge, attitude, and perceptions of pregnant women on COVID-19 vaccine and specific types of misinformation they have can help develop targeted communication campaigns. This could involve addressing the rumors about vaccine safety, efficacy, or potential side effects through clear, accurate, and accessible information. Understanding factors such as perceived accountability can guide efforts to rebuild trust and strengthen confidence in the vaccination process.
Strength and limitation
To our knowledge, this is the first multicentre study done to assess COVID-19 vaccine hesitancy in pregnant women and factors associated with Tanzania from different levels of public health facilities. As the study was done among the urban population in Dar es salaam city, there may be differences between women living in the rural or semi-urban area. However, the city was the most affected, and we believe has received more attention such that residents are more likely to embrace vaccine than in the rural set-up.
The study might have been affected by a social desirability bias, as participants may have responded to questions in a manner that is viewed favorably by others, though they ensured confidentiality and received non-judgmental responses from the data collectors.
Conclusion
Nearly half of the pregnant women in public health facilities had COVID-19 vaccine hesitancy, and it was highly influenced by individual poor attitudes and perceptions toward both, the disease and the vaccine. Hesitancy was also higher among the unemployed and self-employed women.
Authors’ contributions
Z. H. Y.: Principal investigator, study design, data collection, data analysis, and manuscript preparation (Lead). F. A. A.: Participated in study design, data analysis, and critical review of the manuscript. F. A. and A. A.: Participated in study design and critical review of the manuscript. All authors read and approved the final manuscript
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author at the reasonable request
Ethical approval and consent to participate
The ethical clearance for the study was obtained from the Muhimbili University of Health and Allied Science (MUHAS) research and publication committee with Ref No MUHAS-REC-09-2022–1364. All participants signed a written consent form.
Implication policy
Successful vaccination coverage can be achieved by sharing information on COVID-19 and its vaccine and addressing myths and misinformation about the vaccine. Moreover, there is a need to maintain transparency regarding the efficacy and shortcomings of vaccines to get public trust and confidence in the vaccine.
Abbreviations
| ACOG | = | American College of Obstetricians and Gynaecologists |
| ANC | = | Ante-Natal Care |
| CDC | = | Centers for Disease Control and prevention |
| MNH | = | Muhimbili National Hospital |
| MUHAS | = | The Muhimbili University of Health and Allied Sciences |
| RCOG | = | Royal College of Obstetricians and Gynaecologists |
| SAGE | = | Strategic Advisory Group of Expert |
| WHO | = | World Health Organization |
Supplemental Material
Download PDF (359.4 KB)Acknowledgments
The authors would like to acknowledge the AMNE SALIM COVID-19 Research fund for funding the study, MUHAS Research and publication team for their continuous support, the Ministry of Health, President’s Office Regional Administration and Local Government, health facilities administrators for providing permission to conduct research, Regional Health Management Team (RHMT) members for their guidance and support during data collection and lastly we appreciate the support provided by pregnant women who were willing to participate in the study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplemental data
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2269777.
Additional information
Funding
References
- WHO COVID-19 dashboard. COVID-19 updates [Internet]. World Health Organization; 2023 [Accessed 2023 Sep 13]. https://covid19.who.int/.
- Allotey J, Fernandez S, Bonet M, Stallings E, Yap M, Kew T, Zhou D, Coomar D, Sheikh J, Lawson H, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ [Internet]. 2020 Sep 1; m3320. doi:10.1136/bmj.m3320.
- Ellington S, Strid P, Tong VT, Woodworth K, Galang RR, Zambrano LD, Ahabedian J, Anderson K, Gilboa SM. Characteristics of women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status—United States, January 22–June 7, 2020. Obstet Gynecol Surv [Internet]. 2020 Nov;75(11):664–10. doi:10.1097/01.ogx.0000721400.07132.fc.
- Peretz-Machluf R, Hirsh-Yechezkel G, Zaslavsky-Paltiel I, Farhi A, Avisar N, Lerner-Geva L, Meyer R, Tsur A, Yinon Y. Obstetric and neonatal outcomes following COVID-19 vaccination in pregnancy. JCM [Internet]. 2022 Apr 30;11(9):2540. doi:10.3390/jcm11092540.
- Cruz-Lemini M, Ferriols Perez E, de la Cruz Conty M, Caño Aguilar A, Encinas Pardilla M, Prats Rodríguez P, Muner Hernando M, Forcen Acebal L, Pintado Recarte P, Medina Mallen M, et al. Obstetric outcomes of SARS-CoV-2 infection in asymptomatic pregnant women. Viruses [Internet]. 2021 Jan 15;13(1):112. doi:10.3390/v13010112.
- DeBolt CA, Bianco A, Limaye MA, Silverstein J, Penfield CA, Roman AS, Rosenberg HM, Ferrara L, Lambert C, Khoury R, et al. Pregnant women with severe or critical coronavirus disease 2019 have increased composite morbidity compared with nonpregnant matched controls. Am J Obstet Gynecol [Internet]. 2021 May;224(5):.e510.1–.e2. doi:10.1016/j.ajog.2020.11.022.
- Pathirathna ML, Samarasekara BPP, Dasanayake TS, Saravanakumar P, Weerasekara I. Adverse perinatal outcomes in COVID-19 infected pregnant women: a systematic review and meta-analysis. Healthcare [Internet]. 2022 Jan 20;10(2):203. doi:10.3390/healthcare10020203.
- Leduc D, Senikas V, Lalonde AB, Leduc D, Ballerman C, Biringer A, Delaney M, Duperron L, Girard I, Jones D, et al. CLINICAL PRACTICE OBSTETRICS COMMITTEE. Active management of the third stage of labour: prevention and treatment of postpartum hemorrhage. J Obstet Gynaecol Can [Internet]. 2009;31(10):980–93. http://www.ncbi.nlm.nih.gov/pubmed/19941729.
- Wei SQ, Bilodeau-Bertrand M, Liu S, Auger N. The impact of COVID-19 on pregnancy outcomes: a systematic review and meta-analysis. Can Med Assoc J [Internet]. 2021 Apr 19; 193(16):E540–8. doi:10.1503/cmaj.202604.
- Jamieson DJ, Rasmussen SA. An update on COVID-19 and pregnancy. Am J Obstet Gynecol [Internet]. 2022 Feb;226(2):177–86. doi:10.1016/j.ajog.2021.08.054.
- Rahmati M, Koyanagi A, Banitalebi E, Yon DK, Lee SW, Il Shin J, Smith L. The effect of SARS-CoV-2 infection on cardiac function in post-COVID-19 survivors: a systematic review and meta-analysis. J Med Virol [Internet]. 2023;95(1). doi:10.1002/jmv.28325.
- Rahmati M, Yon DK, Lee SW, Soysal P, Koyanagi A, Jacob L, Li Y, Park JM, Kim YW, Shin JI, et al. New‐onset neurodegenerative diseases as long‐term sequelae of SARS‐CoV‐2 infection: a systematic review and meta‐analysis. J Med Virol [Internet]. 2023 Jul 2;95(7). doi:10.1002/jmv.28909.
- Rahmati M, Yon DK, Lee SW, Udeh R, McEvoy M, Kim MS, Gyasi RM, Oh H, López Sánchez GF, Jacob L, et al. New‐onset type 1 diabetes in children and adolescents as postacute sequelae of SARS‐CoV‐2 infection: a systematic review and meta‐analysis of cohort studies. J Med Virol [Internet]. 2023 Jun;95(6). doi:10.1002/jmv.28833.
- WHO. DRAFT landscape of COVID-19 candidate vaccines. 2020 Dec [Accessed 2021 Oct]. https://www.who.int/publications/m/item/draft/landscape-of-covid-19-candidate-vaccines.
- Hagrass AI, Almadhoon HW, Al M, Almaghary BK, Nourelden AZ, Fathallah AH, Hasan MT, Mohammed YA, Al-Nabahin AO, Wafi DS, et al. Maternal and neonatal safety outcomes after SAR ‑ CoV ‑ 2 vaccination during pregnancy: a systematic review and meta ‑ analysis. BMC Pregnancy Childbirth [Internet]. 2022;22:1–19. doi:https://doi.org/10.1186/s12884-022-04884-9.
- Rahmati M, Yon DK, Lee SW, Butler L, Koyanagi A, Jacob L, Shin JI, Smith L. Effects of COVID-19 vaccination during pregnancy on SARS-CoV-2 infection and maternal and neonatal outcomes: a systematic review and meta-analysis. Rev Med Virol [Internet]. 2023 May;33(3):e2434. doi:10.1002/rmv.2434.
- Nir O, Schwartz A, Toussia-Cohen S, Leibovitch L, Strauss T, Asraf K, Doolman R, Sharabi S, Cohen C, Lustig Y, et al. Maternal-neonatal transfer of SARS-CoV-2 immunoglobulin G antibodies among parturient women treated with BNT162b2 messenger RNA vaccine during pregnancy. Am J Obstet Gynecol MFM [Internet]. 2022 Jan;4(1):100492. doi:10.1016/j.ajogmf.2021.100492.
- EMA. COVID-19 vaccine safety update [Internet]. 2021 Jan:1–4. https://www.ema.europa.eu/en/documents/covid-19-vaccine-safety-update/covid-19-vaccine-safety-update-comirnaty-january-2021_en.pdf.
- Sarwal Y, Sarwal T, Sarwal R. Prioritizing pregnant women for COVID-19 vaccination [Internet]. Int J Gynecol Obstet [Internet]. 2021;155:57–63. doi:10.1002/ijgo.13816.
- Goodman T Update on WHO Interim recommendations on COVID-19 vaccination of pregnant and lactating women [ PowerPoint presentation]. World Heal Organ; 2021.
- CDC. COVID-19 vaccination during pregnancy. 2022. https://www.cdc.gov/coronavirus/2019-ncov/downloads/vaccines/covid-19-vaccination-during-pregnancy.pdf.
- AGOTA. Statement on COVID-19 vaccination of pregnant and lactating women. 2021;1–5.
- Lipkind HS, Vazquez-Benitez G, DeSilva M, Vesco KK, Ackerman-Banks C, Zhu J, Boyce TG, Daley MF, Fuller CC, Getahun D, et al. Receipt of COVID-19 vaccine during pregnancy and preterm or small-for-gestational-age at birth — eight Integrated health care organizations, United States, December 15, 2020–July 22, 2021. MMWR Morb Mortal Wkly Rep [Internet]. 2022 Jan 7;71(1):26–30. doi:10.15585/mmwr.mm7101e1.
- Shimabukuro TT, Kim SY, Myers TR, Moro PL, Oduyebo T, Panagiotakopoulos L, Marquez PL, Olson CK, Liu R, Chang KT, et al. Preliminary findings of mRNA covid-19 vaccine safety in pregnant persons. N Engl J Med [Internet]. 2021 Jun 17;384(24):2273–82. doi:10.1056/NEJMoa2104983.
- Naqvi S, Naqvi F, Saleem S, Thorsten VR, Figueroa L, Mazariegos M, Garces A, Patel A, Das P, Kavi A, et al. Health care in pregnancy during the COVID-19 pandemic and pregnancy outcomes in six low- and-middle-income countries: evidence from a prospective, observational registry of the global network for women’s and children’s health. BJOG An Int J Obstet Gynaecol [Internet]. 2022 Jul 20;129(8):1298–307. doi:10.1111/1471-0528.17175.
- MacDonald NE. Vaccine hesitancy: definition, scope and determinants. Vaccine [Internet]. 2015 Aug;33(34):4161–4. doi:10.1016/j.vaccine.2015.04.036.
- Domek GJ, O’Leary ST, Bull S, Bronsert M, Contreras-Roldan IL, Bolaños Ventura GA, Kempe A, Asturias EJ. Measuring vaccine hesitancy: field testing the WHO SAGE working group on vaccine hesitancy survey tool in Guatemala. Vaccine. 2018 Aug;36(35):5273–81. doi:10.1016/j.vaccine.2018.07.046.
- Biswas MR, Alzubaidi MS, Shah U, Abd-Alrazaq AA, Shah Z. A scoping review to find out worldwide COVID-19 vaccine hesitancy and its underlying determinants. Vaccines. 2021 Oct 25;9(11):1243. doi:10.3390/vaccines9111243.
- Nguyen LH, Hoang MT, Nguyen LD, Ninh LT, Nguyen HTT, Nguyen AD, Vu LG, Vu GT, Doan LP, Latkin CA, et al. Acceptance and willingness to pay for COVID‐19 vaccines among pregnant women in Vietnam. Trop Med Int Heal [Internet]. 2021 Oct 23;26(10):1303–13. doi:10.1111/tmi.13666.
- Marzo RR, Ahmad A, Islam MS, Essar MY, Heidler P, King I, Thiyagarajan A, Jermsittiparsert K, Songwathana K, Younus DA, et al. Perceived COVID-19 vaccine effectiveness, acceptance, and drivers of vaccination decision-making among the general adult population: a global survey of 20 countries. Van Weyenbergh J, editor. PLoS Negl Trop Dis [Internet]. 2022 Jan 28;16(1):e0010103. doi:http://dx.doi.org/10.1371/journal.pntd.0010103.
- Piltch-Loeb R, Silver DR, Kim Y, Norris H, McNeill E, Abramson DM. Determinants of the COVID-19 vaccine hesitancy spectrum. Rosenbaum JE, editor. PLoS One [Internet]. 2022 Jun 1;17(6):e0267734. doi:10.1371/journal.pone.0267734.
- Hossain MB, Alam Z, Islam S, Sultan S, Faysal M, Rima S, Hossain MA, Al Mamun A. Health Belief, planned behavior, or psychological antecedents: what predicts COVID-19 vaccine hesitancy better among the Bangladeshi adults? This study aimed to determine the prevalence and investigate the constellations of psychological determinants. 2021.
- Jayagobi P, Ong C, Thai YK, Lim CC, Jiun SM, Koon KL, Wai KC, Chan JK, Mathur M, Chien CM. Perceptions and acceptance of COVID-19 vaccine among pregnant and lactating women in Singapore: a cross-sectional study. 2021;19.
- Mose A, Yeshaneh A. COVID-19 vaccine acceptance and its associated factors among pregnant women attending antenatal care clinic in Southwest Ethiopia: Institutional-based cross-sectional study. Int J Gen Med [Internet]. 2021 Jun;14:2385–95.doi:10.2147/IJGM.S314346.
- Kalam MA, Davis TP, Shano S, Uddin MN, Islam MA, Kanwagi R, Islam A, Hassan MM, Larson HJ. Exploring the behavioral determinants of COVID-19 vaccine acceptance among an urban population in Bangladesh: implications for behavior change interventions. Metwally AM, editor. PLoS One [Internet]. 2021 Aug 23;16(8):e0256496. doi:10.1371/journal.pone.0256496.
- Khairat S, Zou B, Adler-Milstein J. Factors and reasons associated with low COVID-19 vaccine uptake among highly hesitant communities in the US. Am J Infect Control. 2022;50(3):262–7. doi:10.1016/j.ajic.2021.12.013.
- Goncu Ayhan S, Oluklu D, Atalay A, Menekse Beser D, Tanacan A, Moraloglu Tekin O, Sahin D. COVID‐19 vaccine acceptance in pregnant women. Int J Gynecol Obstet [Internet]. 2021 Aug;154(2):291–6. doi:10.1002/ijgo.13713.
- Skjefte M, Ngirbabul M, Akeju O, Escudero D, Hernandez-Diaz S, Wyszynski DF, Wu JW. COVID-19 vaccine acceptance among pregnant women and mothers of young children: results of a survey in 16 countries. Eur J Epidemiol [Internet]. 2021;36(2):197–211. doi:10.1007/s10654-021-00728-6.
- Tarimo CS, Wu J. The first confirmed case of COVID-19 in Tanzania: recommendations based on lesson learned from China. Trop Med Health. 2020;48(1):2. doi:10.1186/s41182-020-00214-x.
- MoH. Guideline for COVID-19 vaccination. 2021 July;1–54. https:www.eahealth.org/sites/www.eahealth.org/files/content/attachments/2021-08-02/final/guidelines/For/COVID-19/Vaccine.
- de Miguel-Arribas A, Aleta A, Moreno Y. Impact of vaccine hesitancy on secondary COVID-19 outbreaks in the US: an age-structured SIR model. BMC Infect Dis [Internet]. 2022 Jun 1;22(1):511. doi:10.1186/s12879-022-07486-0.
- Olivera Mesa D, Hogan AB, Watson OJ, Charles GD, Hauck K, Ghani AC, Winskill P. Modelling the impact of vaccine hesitancy in prolonging the need for non-pharmaceutical interventions to control the COVID-19 pandemic. Commun Med [Internet]. 2022 Feb 10;2(1):14. doi:10.1038/s43856-022-00075-x.
- MoH. Weekly trend of COVID-19 situation report in Tanzania [Internet]. 2022 [accessed 2022 Jul 24]. 1–7. https://www.moh.go.tz/storage/app/uploads/public/629/5ce/837/6295ce8370a3d468651045.pdf.
- Davis TP, Yimam AK, Kalam MA, Tolossa AD, Kanwagi R, Bauler S, Kulathungam L, Larson H. Behavioural determinants of COVID-19-Vaccine acceptance in rural areas of six lower- and middle-income countries. Vaccines. 2022 Jan 29;10(2):214. doi:10.3390/vaccines10020214.
- Wong MCS, Wong ELY, Huang J, Cheung AWL, Law K, Chong MKC, Ng RWY, Lai CKC, Boon SS, Lau JTF, et al. Acceptance of the COVID-19 vaccine based on the health belief model: a population-based survey in Hong Kong. Vaccine. 2021 Feb;39(7):1148–56. doi:10.1016/j.vaccine.2020.12.083.
- Cai Z, Hu W, Zheng S, Wen X, Wu K. Cognition and behavior of COVID-19 vaccination based on the health Belief model: a cross-sectional study. Vaccines. 2022 Apr 1;10(4):544. doi:10.3390/vaccines10040544.
- Macrotrends. Tanzania Metro area population 1950-2023 [Internet]. Dar es Salaam; 2023. [accessed 2023 Jan 20]. https://www.macrotrends.net/cities/22894/dar-es-salaam/population.
- MoH. Weekly trend of COVID-19 confirmed cases in Tanzania. 2022 [Accessed 2022 Jun 20]. 27:1–7.https://www.moh.go.tz/storage/app/uploads/publics/623/bf6/9a3/623bf69a3d819268181372.
- Bishaw Z, Id A, Bogale TW, Bantie GM, Ayalew F, Tamir W. Factors associated with willingness to take COVID-19 vaccine among pregnant women at. PLoS One [Internet]. 2022;1–17. doi:10.1371/journal.pone.0276763.
- Solís Arce JS, Warren SS, Meriggi NF, Scacco A, McMurry N, Voors M, Syunyaev G, Malik AA, Aboutajdine S, Adeojo O, et al. COVID-19 vaccine acceptance and hesitancy in low- and middle-income countries. Nat Med [Internet]. 2021 Aug 16;27(8):1385–94. doi:10.1038/s41591-021-01454-y.
- Jayagobi PA, Ong C, Thai YK, Lim CCW, Jiun SM, Koon KL, Wai KC, Chan JK, Mathur M, Chien CM. Perceptions and acceptance of COVID-19 vaccine among pregnant and lactating women in Singapore: a cross-sectional study. medRxiv [Internet]. 2021;19. 2021.06.29.21259741. http://medrxiv.org/content/early/2021/07/04/2021.06.29.21259741.abstract.
- Maximillian biyemo vicent fabiola. Predictors for the uptake of tetanus toxoid vaccination during pregnancy among women of reproductive age in Tanzania. 2020.
- Osur JO, Chengo R, Muinga E, Kemboi J, Sidibe M, Rarieya M. Determinants of COVID ‑ 19 vaccine behaviour intentions among the youth in Kenya: a cross ‑ sectional study. Arch Public Heal [Internet]. 2022;80:1–13. doi:https://doi.org/10.1186/s13690-022-00904-4.
- Hailemariam S, Mekonnen B, Shifera N, Endalkachew B, Asnake M, Assefa A, Qanche Q. Predictors of pregnant women ’ s intention to vaccinate against coronavirus disease 2019: a facility-based cross-sectional study in southwest Ethiopia. SAGE Open Med. 2021;9:205031212110384. doi:10.1177/20503121211038454.
- King WC, Rubinstein M, Reinhart A, Mejia R. COVID-19 vaccine hesitancy January-May 2021 among 18 – 64 year old US adults by employment and occupation. Prev Med Rep [Internet]. 2021;24(August):101569. doi:10.1016/j.pmedr.2021.101569.
- Takahashi S, Takahashi N, Sasaki S, Nohara M, Kawachi I. SSM - Population health occupational disparities in COVID-19 vaccine hesitancy in Japan. SSM - Popul Heal [Internet]. 2022;19(July):101226. doi:10.1016/j.ssmph.2022.101226.
- Mahmoud M, Alanazi M, Albarrak MS, Aljarba K, Almutairi NG. The percentage of vaccine hesitancy among Married individuals in times of the COVID-19 pandemic: a cross sectional study in Riyadh city. Kingdom Saudi Arabia. 2022;2:20–6. doi:10.1159/000520681.
- Naqvi S, Saleem S, Naqvi F, Billah SM, Nielsen E, Fogleman E, Peres‐da‐Silva N, Figueroa L, Mazariegos M, Garces AL, et al. Knowledge, attitudes, and practices of pregnant women regarding COVID‐19 vaccination in pregnancy in 7 low‐ and middle‐income countries: an observational trial from the global network for women and children’s health research. BJOG An Int J Obstet Gynaecol [Internet]. 2022 Nov 5;129(12):2002–9. doi:10.1111/1471-0528.17226.
- H A. Why Africa fears western medicine. The New Work Times [Internet]. 2007;1–3. http://www.nytimes.com/2007/07/31/opinion/31washington.html?_r=1&oref=slogin.
