ABSTRACT
The Coronavirus Disease 2019 (COVID-19) pandemic has now persisted globally for four years, resulting in a staggering death toll of over 4 million individuals. The COVID-19 vaccine has emerged as a highly effective tool in controlling the spread of this virus. However, as the number of individuals receiving COVID-19. In this context, the investigation of adverse reactions related to COVID-19 vaccines holds paramount importance in relevant research. The purpose is to evaluate the current research status regarding adverse reactions associated with COVID-19 vaccines, offering insights for future research. A total of 3,746 articles were included in this analysis, and there has been a notable upward trajectory in the volume of published articles. The CiteSpace v6.1.R6, VOSviewer, SCImago Graphica, and Excel 2019 were employed to analyze and visualize the results. The institutions, countries, journals, authors, co-cited references, and keywords of these articles were analyzed. Furthermore, this study delves into the characteristics of articles on adverse reactions associated with COVID-19 vaccines. It was observed that the number of studies on COVID-19 vaccines has increased year by year since 2019 and witnessed a surge in output in 2021. The vast majority of studies have affirmed the overall safety of COVID-19 vaccines, with adverse reactions tending to be more concentrated in specific diseases. These findings provide valuable ideas for future research in this field and suggest the importance of strengthening international cooperation on adverse reactions to COVID-19 vaccines.
Introduction
The Coronavirus Disease 2019 (COVID-19), which emerged abruptly in Wuhan, China, in December 2019, has been circulating worldwide for four years. Patients infected with COVID-19 typically present with fever, dry cough, dyspnea, muscle soreness, fatigue, and imaging evidence of pneumonia. Additionally, some patients experience ground-glass lung changes, along with decreased numbers in lymphocytes, white blood cells, and platelets.Citation1,Citation2 COVID-19 is highly transmissible, rapidly spreading from person to person and achieving global reach in a short time. It can be transmitted not only through contact with mucosal secretions of infected patients, such as nasal passages, but also via the inhalation of droplets released during coughing.Citation1 As of February 2021, there have been more than 100 million confirmed cases and over 2 million deaths.Citation3 In the absence of modern medical management and vaccines, COVID-19 may pose a great threat to global public health, underscoring the urgent need to effectively contain its spread.Citation4
Vaccines indeed are the most effective and the best permanent solution to combat the pandemic.Citation5 Various types of COVID-19 vaccines have been developed and are currently available, including whole virus vaccines, subunit vaccines, and nucleic acid vaccines.Citation5 However, vaccine hesitancy remains a significant obstacle, driven by concerns about their safety.Citation6 Therefore, investigating adverse reactions to COVID-19 vaccines is also a vital focus of vaccine development. Most adverse reactions linked to COVID-19 vaccines are mild to moderate, with only a few cases being severe. Common local adverse events include pain, swelling, and redness at the injection site. Studies have demonstrated that the most reported injection site reactions and delayed large local reactions of all vaccine types are redness and erythema, which is usually self-limited, requires little therapeutic intervention. Serious adverse reactions are rare.Citation7,Citation8 In addition to local skin reactions, there have been reports of local adverse reactions affecting the eyesCitation9 and ears.Citation10 Systemic reactions encompass fever, fatigue, myalgia, and headache.Citation11 Cases involving adverse reactions in the nervous system,Citation12 blood system,Citation13,Citation14 immune system,Citation15 and multisystem inflammation syndromeCitation16 have also been documented following COVID-19 vaccine administration. In addition, vaccination may have adverse mental and psychological effects, with a small number of vaccinated individuals experiencing depression or anxiety.Citation17 Furthermore, there is evidence suggesting that these adverse mental states can impair the immune system’s response to vaccines, particularly among the elderly.Citation18 These adverse reactions have posed challenges for increasing vaccination rates.
So far, there is no bibliometric data on adverse reactions linked to COVID-19 vaccines. This absence underscores the necessity to systematically document adverse reactions stemming from various types of COVID-19 vaccines, evaluate their safety, and ultimately provide directions for the development and promotion of COVID-19 vaccines. Studying these adverse reactions can provide practical strategies to increase the coverage rate of COVID-19 vaccines and prevent the transmission of the virus at its source. Through bibliometrics, this study analyzed the development trend of research on adverse reaction induced by application of COVID-19 vaccines, predict research hotspots, and offer guidance for further research.
Methods
Data source and search
This study relied entirely on data from the Web of Science Core Collection (WoSCC), which should be issued from 1 January 2019 to 11 January 2023. The following retrieval strategies were employed: TS= ((corona* OR 2019-nCoV OR nCoV-19 OR SARS-CoV-2 OR SARS-CoV2 OR COVID*) AND (vacc* OR immuniz*) AND (adverse reaction* OR adverse effect* OR side effect*)) which retrieved on 11 January 2023. A total of 4,088 articles were retrieved, the language was further specified as English (excluding 68 documents), and the literature type was limited to “Article or Review” (excluding 274 documents). In addition, the CiteSpace v6.1.R7 was adopted to deduplicate the obtained data, yielding 3,746 documents (2,985 articles and 761 reviews).
Data analysis methods
CiteSpace is a valuable free software based on network analysis and visualization techniques to identify trends and patterns in scientific literature. Meanwhile, it facilitates the exploration of prevailing research hotspots, significant studies, outstanding researchers, institutions, and countries within a given field.Citation19 In this study, relevant files were downloaded from the Web of Science (WOS) and subsequently used Microsoft Excel 2019, Citespace v6.1.R6, and VOSviewer for comprehensive evaluation and analysis (). Citespace V6.1.R6 was adopted to visually explore the collaboration of authors, co-citation of authors, source, co-citation of journals, co-citation of references, and co-occurrence of keywords. For this purpose, the log-likelihood rate (LLR) served as a clustering algorithm. After that, the country/region and institution data exported from VOSviewer were integrated into SCImago Graphica to visualize the collaboration of countries/regions and institutions. The merged words are detailed in Table S1.
Results
General information and annual trend
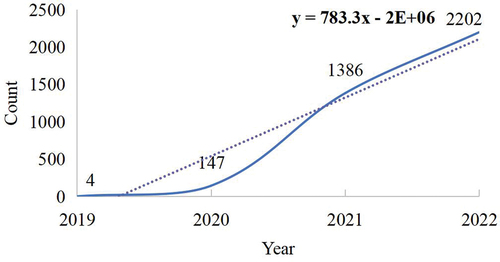
A total of 3,746 publications met the inclusion criteria, comprising 2,985 articles and 761 reviews (). We have used CiteSpace v6.1.R6, VOSviewer, SCImago Graphica, and Excel 2019 to analyze 3,746 papers visually, including research trends, national/regional cooperation, institutional cooperation, author citations, keyword co-occurrence, etc. indicates the number distribution of the selected documents according to the year of the publication. The number of documents from 2019 to 2022 were increasing yearly. The publication record reveals a trajectory of 4 documents reported in 2019, followed by 147 documents in 2020, 1,386 documents in 2021, 2,202 documents in 2022, and 7 documents as of January 11, 2023. These data suggest the remarkable upsurge in research articles in this field over the last four years. From 2019 to 2022, there has been a consistently substantial annual publication volume concerning the adverse reactions to COVID-19 vaccines, signifying the increasing attention in the academic community.
Distribution by countries/regions and institutions
The 3,746 publications come from 150 countries/regions and 355 institutions, as outlined in . The United States is the most productive country in terms of the number of publications (N = 855), followed by China (N = 484) and Italy (N = 339). Furthermore, the United States consistently maintains a leading position in centrality, reaching 0.21, matched only by the United Kingdom. This suggests that the United States plays a crucial role in the field of adverse reactions to COVID-19 vaccines. Besides, although China ranks second in terms of the number of publications, it possesses a relatively low betweenness centrality. In this regard, there is room for China to enhance its international cooperation in this field.
Table 1. Top 12 productive countries/regions and institutions.
To investigate international collaboration, Scimago was employed in this study to construct the visualization map. illustrates collaborations among countries/regions that have published more than 100 documents. In this representation, nodes symbolize countries/regions, and colors denote the clustering of countries. The thicker and darker the line, the closer the cooperation between the countries. suggests that the United States has established close cooperative relations with Germany, Canada, China, and the United Kingdom in this field. Simultaneously, China and the United Kingdom have also established a strong collaborative relationship.
Figure 3. Analysis of country/regions and institutions. (a) Scimago Graphica network visualization map of country/regions; (b) Scimago Graphica network visualization map of institutions.
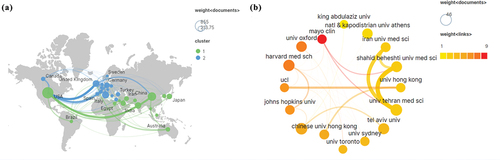
presents the institutions that have published more than 26 documents. The size of each node corresponds to the number of published papers by the institution, with larger nodes signifying a higher number of publications. The intensity of the red color of each node correlates with the extent of collaboration between the institution and other entities. The thickness of the lines connecting institutions is proportional to the number of institutional partnerships. Moreover, the figure unveils that the University of Tehran Medical Sciences, Iran University of Medical Sciences, and Shahid Beheshti University of Medical Sciences; as well as the University of Hong Kong and University College London, have engaged in more frequent collaborations. Conversely, the Mayo Clinic has published a large number of articles in partnership with other institutions but has collaborated less frequently with other institutions.
Authors and co-cited authors
CiteSpace was adopted to analyze the authors and co-cited authors of adverse reactions associated with COVID-19 vaccines. As given in , a total of 329 authors were searched to have published papers in this field, with 836 lines of cooperation.
Figure 4. Analysis of authors and co-cited authors. (a) CiteSpace network visualization map of authors; (b) CiteSpace network visualization map of co-cited authors.
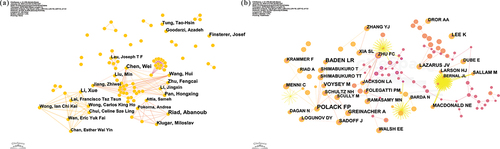
presents the top nine productive authors and co-cited authors on adverse reactions linked to COVID-19 vaccines. Eight authors have authored more than nine articles, and their contributions to the academic discourse on this subject have been significant. Among them, Riad, Abanoub, with 13 publications, holds the top position and has a prominent presence as a very large node in the graph. Chen, Wei (N = 11) and Li, Xue (N = 10) ranked second and third, forming notable nodes in the graph, too. Additionally, there is a tie for fourth place, with five authors each having the same number of publications. It means that not only have many authors published papers about adverse reactions to COVID-19 vaccines, but several have also contributed to multiple papers.
Table 2. Top nine productive authors and co-cited authors.
illustrates the network visualization map of co-cited authors, where authors with more co-references are usually represented by larger nodes. Notable examples include POLACK FP (936 citations), BADEN LR (595 citations), and VOYSEY M (374 citations). However, the top nine prolific authors in number of publications do not overlap with the top nine most commonly co-cited authors. This suggests that authors who publish more articles may not necessarily receive more citations.
Journals and co-cited journals
lists the 10 most productive and co-cited journals regarding adverse reactions to COVID-19 vaccines. Notably, the VACCINES (N = 399) has published a far greater number of articles than other journals and possessed an influencing factor (IF) of 4.961 in 2021 and a high IF in the top 10. On the other hand, FRONTIERS IN IMMUNOLOGY exhibits the highest IF of 8.786 among the 10 most productive journals but only ranks fifth in the number of papers published.
Table 3. Top 10 journals and co-cited journals.
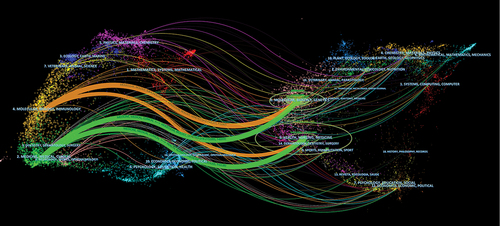
The network visualization map of the source and co-cited journal drawn by CiteSpace was visualized in . The citing journals are on the left side and the reference journals are arranged on the right side. The colored paths denote citation relationships. Four main reference routes that intersect with each other are observed from the figure, spanning from Medicine/Medical/Clinical or Molecular/Biology/Immunology to Molecular/Biology/Genetics or Health/Nursing/Medicine.
Co-cited references analysis
CiteSpace was employed to visually analyze the authors of co-cited references, and the results are depicted in . provides the co-cited articles, with that titled “Safety and Efficacy of the BNT162b2 mRNA Vaccine for Covid-19” (IF = 176.079) ranking first. This article has been co-cited 935 times, nearly twice as often as the second-ranked article. The second article “Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine” (IF = 176.079), has been co-cited 585 times. Notably, these two articles are published in the same journal, THE NEW ENGLAND JOURNAL OF MEDICINE. This suggests that this journal is highly regarded as the most authoritative in the study of adverse reactions to COVID-19 vaccines. In addition, the top 10 articles with the highest co-citation frequency all have a total citation frequency exceeding 150 and an IF of surpassing 100, indicating that they are all published in high-quality journals.
Figure 6. Analysis of reference. (a) CiteSpace network visualization map of references cluster timeline view; (b) Top 10 references with the strongest citation burst.

Table 4. Top 10 co-cited references.
According to the and Table S2, Cluster #0 (COVID-19 vaccine), Cluster #1 (COVID-19 vaccine hesitancy), and Cluster #7 (convalescent plasma) exhibit the brighter colors, indicating that they were formed earlier and research directions lasting longer. On the other hand, the remaining clusters, which are concentrated in 2021 and 2022, are relatively newly established research areas. In particular, Cluster #3 (cerebral venous sinus thrombosis) contains the top two co-cited references, suggesting it to be the most popular research hotspot.
demonstrates that most of the strongest citation bursts appeared in 2020 and 2021, except for the World Health Organization (WHO) appearing in 2022. The reference with the strongest burst is “Gautret P” with a burst strength of 10.78, which has attracted the greatest attention.
Keywords and co-occurring keywords
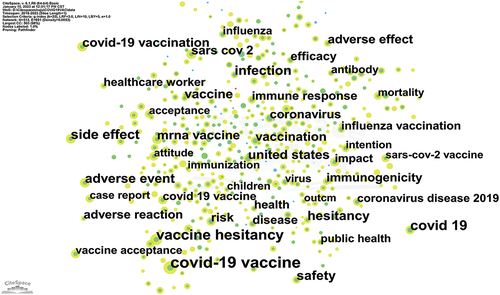
The knowledge map of keyword co-occurrence in the context of adverse reactions to COVID-19 vaccines was generated, as given in . It presents 42 keywords that appear at least 50 times in the literature. Additionally, lists the top 20 keywords, with 19 of them being used more than 100 times, indicating their frequent use. Notably, “COVID-19 vaccine” (N = 473) ranks first in terms of usage frequency, significantly surpassing other keywords. However, its centrality is 0. In addition, keywords ranked 3rd, 4th, 8th, 11th, 14th, and 16th also exhibit a centrality of 0. The keyword with the highest centrality is “vaccination,” ranked 12th. This observation suggests that the usage frequency of keywords is not closely related to centrality.
Table 5. Top 20 keywords.
Furthermore, a knowledge map of keyword co-occurrence clusters was generated by the CiteSpace ( and Table S3). Cluster #7 (covid-19 vaccine) is produced early and contains the keyword “covid-19 vaccine,” which ranks among the top 10 most cited keywords. However, it currently has fewer nodes, with just one in 2022, suggesting that it may no longer be a primary focus of research. Conversely, Cluster #1 (vaccine acceptance) incorporates one of the top 10 most cited keywords “vaccine hesitancy” and possesses a lot of nodes distributed in 2020, 2021, and 2022, suggesting that it may be a hot line of research.
Figure 8. Analysis of keywords. (a) CiteSpace network visualization map of keywords cluster timeline view; (b) Top 10 keywords with the strongest citation burst.

The hotspots and research frontiers over time was depicted in demonstrate that the burst typically start in 2019 or 2020 and generally conclude within two years. Notably, “replication” holds the strongest burst of 4.45, suggesting its prevalence.
Discussion
Analysis of basic information
The macroscopical application of bibliometrics in knowledge network holds an important role in evaluating and analyzing the research results and trends.Citation20 According to the WoSCC data from 2019 to 2023, a total of 329 authors from 150 countries and 355 institutions published 3,746 articles on adverse reactions associated with COVID-19 vaccines.
Since the outbreak of COVID-19 in 2019, the research and development of COVID-19 vaccines have attracted increasing attention, and a particular focus on adverse reactions. In 2021, Doroftei et al.Citation21 discussed the adverse reactions linked to various vaccines, but many of these vaccines have just been approved with incomplete available data. Concurrently, Kaur et al.Citation11 concluded that the COVID-19 vaccines did not exhibit serious adverse reactions. In 2023, Wang et al.Citation22 explored the prevalence of adverse reactions and demonstrated that it was similar to that of the general individuals with underlying diseases and those aged 60 years and older. These outcomes offer a scientific basis for the safety of vaccination in these populations. In this study, the studies focusing on adverse reactions to COVID-19 vaccines from 2019–2023 were searched. During the search process, it is observed that there are extremely increasing number of annual publications until 2023. This trend is likely attributed to the rising vaccination rates, which have led to increased public concern about adverse reactions to COVID-19 vaccines. This indicates that future public health policies are more inclined to research the adverse reactions of COVID-19 vaccines, to develop COVID-19 vaccines that are safer and efficient.
Among the top 12 productive countries/regions and institutions, three are from Iran, two are from the United States, and two are from China. What’s more, the centralities for the United States, the United Kingdom, Germany, and Saudi Arabia are all higher than 0.1, indicating the pivotal roles of these countries in the research network focusing on adverse reactions to COVID-19 vaccines. One possible explanation is that countries with different economic levels invest differently in health research, leading to research bias in the same field. In the meantime, due to the different reporting systems for vaccine adverse events in countries, the availability of vaccine adverse reactions varies for each country. The United States stands out as the foremost contributor, both in the highest number of published articles and the highest centrality, signifying its dominant position in the study about adverse reactions associated with COVID-19 vaccines. This may be because the United States experiences a developed economy and a good scientific research foundation, leading to stronger “transnational cooperation attribute” in their published papers. Although the number of published articles in China is second only to the United States, it maintains a centrality of 0, indicating a lower research quality compared to the United States. Nevertheless, the role of China in the study of adverse reactions to COVID-19 vaccines cannot be denied, but it still needs to consider strengthening international cooperation and publishing higher-quality articles in this field.
The primary authors contributing to the study of adverse reactions to COVID-19 vaccines are Riad, Abanoub, Chen, Wei, and Li, Xue, who occupy the top three positions in the number of published articles. However, none of the top 10 authors exhibit notably high centralities. reveals that Wang, Hui, who ranks fourth, and Jiang, Zhiwei, who is not in the top 9, engage in more cooperation with other authors. It prompts that the authors should strengthen international cooperation while increasing output. Among the top nine co-cited authors, Polack FP and Baden LR not only receive the highest number of citations but also actively engage in co-authorship with others. This emphasizes that strengthening cooperation can improve the quality of articles.
“VACCINES” emerges as the most significant among the top 10 journals, which published four times as many articles as the runner-up. The IF values of these top-10 journals range from 3.752 (“PLOS ONE”) to 8.786 (“FRONTIERS IN IMMUNOLOGY”), with most journals falling within 3–4. Moreover, 5 journals are placed in Q1, 4 are in Q2, and 1 is in Q3. This suggests that the authors prioritize publishing high-end articles and tend to choose the right journals. However, it is noteworthy that many articles find their way into the top journals. This inference is also supported by the fact that the top 2 co-cited journals are not in the top 10 journals. illustrates that citation journals and reference journals are always mutual, signifying a high level of academic communication about adverse reactions to COVID-19 vaccines.
Analysis of reference
One top 10 co-cited reference was a global survey on the potential acceptance of the COVID-19 vaccine, and the remaining nine were clinical trials on the safety and efficacy of different vaccines, such as mRNA 1273 SARS-CoV-2 vaccine, BNT162b2 mRNA COVID-19 vaccine, and ChAdOx1 nCov-19 vaccine. These studies reveal that most adverse reactions to COVID-19 vaccines are local adverse reactions, such as pain at the injection site, with rarely severe adverse reactions. These studies have been cited hundreds of times and yielded high IF values, indicating their reliable reference values for subsequent relevant studies. Meanwhile, the “Safety and Efficacy of the BNT162b2 mRNA Vaccine for Covid-19,” focusing on the efficacy and safety of BNT162b2, a nucleoside-modified RNA vaccine, exhibits a centrality of 0.23.Citation23 The results unveil that BNT162b2 could induce pain at the injection site, with rarely serious adverse reactions, which are similar to the mRNA 1273 SARS-CoV-2 vaccine. This underscores the significance attached to the clinical studies of each vaccine, with a particular emphasis on adverse reactions, which plays a crucial determining role in the wide application of the vaccines.
It is worth noting that Cluster #0 - Cluster #12 continues to the present (), indicating that they are the current hot pot on adverse reactions in COVID-19 vaccines. Cluster #5 (allergic reaction) and Cluster #11 (subacute thyroiditis) are adverse reactions to the COVID-19 vaccines. Since the introduction of the first COVID-19 vaccine, 13.2 billion people have been vaccinated, mainly with multiple doses of messenger RNA vaccine. After the COVID-19 vaccination, there were mild local and systemic adverse reactions, allergic reaction was the main one.Citation24 An increasing number of studies suggest a possible relationship between subacute thyroiditis and the SARS-CoV-2 vaccine. A prospective study evaluating cases of acute thyroiditis during COVID-19 showed that only 6 out of 64 cases were vaccine-related.Citation25 At the same time, 98 cases of subacute thyroiditis induced by SARS-CoV-2 vaccine have been recorded in English literature.Citation26 However, no clinical trials are evaluating the efficacy and safety of vaccines for this side effect, and further research may be needed in this area in the future. In addition, several new topics emerged and made their way into the top 12 topics. These topics include cancer patients, pregnant women, and rheumatic patients. The acceptance of COVID-19 vaccines varies among different cancer patients, especially among younger patients.Citation27,Citation28 Limited data are available on the safety of COVID-19 vaccines for pregnant women, as they were initially excluded from the Phase 19 clinical trials of the COVID-19 vaccines.Citation29 Rheumatic patients are considered to be at high risk of contracting COVID-19 due to their underlying conditions and the use of immunosuppressants, making them an urgent population in need of vaccine protection.Citation30
After comprehensively analyzing the top 10 co-cited references with the most vigorous citation burst and network visualization map of references cluster timeline view, we support the hotspots of future research that may focus on the evaluation of the effectiveness and safety of the COVID-19 vaccines for special populations, such as cancer patients, pregnant women, and rheumatic patients. In the meantime, further clinical trial evaluation will be conducted for vaccine-specific adverse reactions such as subacute thyroiditis.
Analysis of keywords
The majority of the top 20 keywords are COVID-19 vaccines and related hesitancy. Vaccine hesitancy, characterized by people refusing or delaying vaccinations despite vaccine availability,Citation31 varies among different groups.Citation32 Ranking 12th, “vaccination” is the most central of the top 20 keywords, indicating the critical role of COVID-19 vaccines in controlling the pandemic. Research on adverse reactions to COVID-19 vaccines may indeed contribute to reducing vaccine hesitancies. In addition, “myocarditis” and “systemic lupus erythematosus” are present in the top eight network visualization maps of keywords cluster timeline view (). Clinical evidence suggests that COVID-19 vaccines can indeed induce myocarditis.Citation33,Citation34 Most COVID-19 vaccine-associated myocarditis is associated with a second dose of mRNA vaccination, but most recover rapidly and serious complications are rare.Citation35 While adverse reactions to the COVID-19 vaccines are more common, but not more severe, in patients with systemic lupus erythematosus.Citation36 Nevertheless, patients with systemic lupus erythematosus have a higher risk of COVID-19 infection, hospitalization, severe illness, and death due to immunosuppressant use and other factors.Citation37 These patients are excluded from clinical trials,Citation38 so they represent a unique population for the study of adverse reactions to COVID-19 vaccines.
As depicted in , notably, topics such as monoclonal antibodies, interferons, and traditional Chinese medicine have garnered significant attention, demonstrating the diverse range of research on COVID-19 vaccines. This emphasizes the importance of studying the adverse reactions of various vaccine types. Of particular significance, adverse reactions, mainly myocarditis and some special disease groups, should also be studied more. These findings collectively indicate that the COVID-19 vaccines have been studied since early in the pandemic. As these vaccines are increasingly applied in clinical practice, there is a growing focus on understanding their adverse reactions, solidifying adverse reactions to COVID-19 vaccines as a prominent research hotspot.
It can be seen that there should increase in the evaluation of the safety and effectiveness of the COVID-19 vaccines used by special populations and the implementation of some targeted public health interventions to solve vaccine hesitancy, especially the special population, COVID-19 vaccine uptake, to improve the vaccination rate of the population. At the same time, increase the research on some adverse reactions of the COVID-19 vaccine, such as myocarditis, and reduce the corresponding adverse reactions.
Limitations
This study has some limitations. To begin, to increase the reliability of the publications in this study, the data only came from the WoSCC database, indicating a certain lack of data sources. Secondly, the language used is limited to English, and literature other than English is excluded. Finally, our analysis was selected from 2019-01-01 to 2023-01-11. However, with the research progress, publications are constantly increasing, so future research should add new publications.
Conclusion
This study marks the first bibliometric analysis of adverse reactions to COVID-19 vaccines. While research on COVID-19 vaccines has increased year by year since the outbreak of COVID-19 in 2019, studies specifically investigating adverse reactions to these vaccines began gaining prominence in 2021. Using the bibliometric method, this study searched the literature from January 1, 2019 to January 11, 2023, and evaluated the current research status of adverse reactions to COVID-19 vaccines. This study encompasses various aspects, including the number of publications, authors, countries and institutions, journals, references, keywords, and more. The United States and China have made the most important contribution in this regard. On the other hand, the journal VACCINES exhibits the highest number of published papers. Polack FP is the most influential author. The top three authors (Riad, Abanoub, Chen, Wei and Li, Xue) all carefully studied the specific adverse reactions of different COVID-19 vaccines and concluded that the vaccines demonstrate a good safety profile. In recent years, research on adverse reactions linked to COVID-19 vaccines has focused more on specific diseases, exploring potential complications (such as myocarditis) and targeting special population groups (such as lupus erythematosus).
In this study, bibliometrics were employed to investigate the adverse reactions of COVID-19 vaccines. This approach can allow for an objective assessment of the safety of COVID-19 vaccines and offer insights into the potential direction of COVID-19 vaccines. In this field, reducing adverse reactions to COVID-19 vaccines and enhancing its safety are important measures to weaken people’s concerns about vaccine injection and increase the coverage rate of COVID-19 vaccines. Consequently, it is an important part of the anti-epidemic work.
When considering the future research direction of adverse reactions to COVID-19 vaccines, it is imperative to focus on two key aspects. On the one hand, it is recommended to strengthen international cooperation and academic exchanges. On the other hand, investigation of adverse reactions of patients with specific diseases after vaccination is of high clinical significance to facilitate the implementation of the research results.
Authors’ contributions
Xuan Yang and Mingcong Chen contributed equally to this work. Mingyi Zhao had the idea for the study. Mingyi Zhao, Xuan Yang and Mingcong Chen selected studies for inclusion and extraction of data. The Mingcong Chen and Xuan Yang values were analyzed statistically. Xuan Yang and Mingcong Chen explain these data. Xuan Yang wrote the first draft. Mingcong Chen and Mingyi Zhao have critically revised the important intellectual content of the paper. All authors have read and approved the content of the manuscript.
Supplemental Material
Download PDF (115.2 KB)Acknowledgments
The authors thank all participants who volunteered to participate in this study, including the Third Xiangya Hospital of Central South University, Changsha Medical College, and other units and individuals, for scientific and intellectual opportunities. At the same time, we would like to express our gratitude to the Wisdom Accumulation and Talent Cultivation Project of the Third xiangya hospital of Central South University for its financial support for this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used and analyzed during the current article are available from the corresponding author on reasonable request.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2270194.
Additional information
Funding
References
- Chilamakuri R, Agarwal S. COVID-19: characteristics and therapeutics. Cells. 2021;10(2):206. doi:10.3390/cells10020206.
- Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of Coronavirus disease 2019 (COVID-19) a review. JAMA J Am Med Assoc. 2020;324:782–11.
- Khan M, Adil SF, Alkhathlan HZ, Tahir MN, Saif S, Khan M, Khan ST. COVID-19: a global challenge with old history, epidemiology and progress so far. Molecules. 2021;26(1):39. doi:10.3390/molecules26010039.
- To KK-W, Sridhar S, Chiu KH-Y, Hung D-L-L, Li X, Hung IF-N, Tam AR, Chung TW-H, Chan JF-W, Zhang AJ-X, et al. Lessons learned 1 year after SARS-CoV-2 emergence leading to COVID-19 pandemic. Emerg Microbes Infect. 2021;10(1):507–35. doi:10.1080/22221751.2021.1898291.
- Chen W-H, Strych U, Hotez PJ, Bottazzi ME. The SARS-CoV-2 vaccine pipeline: an overview. Curr Trop Med Rep. 2020;7(2):61–4. doi:10.1007/s40475-020-00201-6.
- Troiano G, Nardi A. Vaccine hesitancy in the era of COVID-19. Public Health. 2021;194:245–51. doi:10.1016/j.puhe.2021.02.025.
- Qaderi K, Golezar MH, Mardani A, Mallah MA, Moradi B, Kavoussi H, Shamsabadi A, Golezar S. Cutaneous adverse reactions of COVID-19 vaccines: a systematic review. Dermatol Ther. 2022;35(5):11. doi:10.1111/dth.15391.
- Gambichler T, Boms S, Susok L, Dickel H, Finis C, Abu Rached N, Barras M, Stuecker M, Kasakovski D. Cutaneous findings following COVID-19 vaccination: review of world literature and own experience. J Euro Acad Dermatol Venereol. 2022;36:172–80.
- Pang KP, Pan LJ, Guo H, Wu XY. Case report: associated ocular adverse reactions with inactivated COVID-19 vaccine in China. Front Med. 2022;8:6. doi:10.3389/fmed.2021.823346.
- Parrino D, Frosolini A, Gallo C, De Siati RD, Spinato G, de Filippis C. Tinnitus following COVID-19 vaccination: report of three cases. Int J Audiol. 2022;61(6):526–9. doi:10.1080/14992027.2021.1931969.
- Kaur RJ, Dutta S, Bhardwaj P, Charan J, Dhingra S, Mitra P, Singh K, Yadav D, Sharma P, Misra S. Adverse events reported from COVID-19 vaccine trials: a systematic review. Ind J Clin Biochem. 2021;36(4):427–39. doi:10.1007/s12291-021-00968-z.
- Zlotnik Y, Gadoth A, Abu-Salameh I, Horev A, Novoa R, Ifergane G. Case report: anti-LGI1 encephalitis following COVID-19 vaccination. Front Immunol. 2022;12:12. doi:10.3389/fimmu.2021.813487.
- Lai KY, Au SY, Fong KM, Choi PYI, Eichinger S, Warkentin TE, Greinacher A. Thrombotic thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;385:1.
- Fiorillo G, Pancetti S, Cortese A, Toso F, Manara S, Costanzo A, Borroni RG. Leukocytoclastic vasculitis (cutaneous small-vessel vasculitis) after COVID-19 vaccination. J Autoimmun. 2022;127:127. doi:10.1016/j.jaut.2021.102783.
- Rela M, Jothimani D, Vij M, Rajakumar A, Rammohan A. Auto-immune hepatitis following COVID vaccination. J Autoimmun. 2021;123:123. doi:10.1016/j.jaut.2021.102688.
- Park JW, Yu SN, Chang SH, Ahn YH, Jeon MH. Multisystem inflammatory syndrome in an adult after COVID-19 vaccination: a Case report and literature review. J Korean Med Sci. 2021;36(45). doi:10.3346/jkms.2021.36.e312.
- Al-Obaidy LM, Attash HM, Al-Qazaz HK. Depression, anxiety and stress after COVID-19 vaccination: a retrospective cross-sectional study among health care providers. Pharm Pract (Granada). 2022;20:3. doi:10.18549/PharmPract.2022.3.2689.
- Madison AA, Shrout MR, Renna ME, Kiecolt-Glaser JK. Psychological and behavioral predictors of vaccine efficacy: considerations for COVID-19. Respect Psychol Sci. 2021;16(2):191–203. doi:10.1177/1745691621989243.
- Guzmán MV, Chen C. CiteSpace: A CiteSpace: A Practical Guide for Mapping Scientific Literature. Hauppauge (NY): Nova Science; 2016. p.169. CiteSpace: una guía práctica para el mapeo de la literatura científica. Investigación bibliotecológica. 2017;31(spe):293–295.
- Blakeman K. Bibliometrics in a digital age: help or hindrance. Sci Prog. 2018;101(3):293–310. doi:10.3184/003685018X15337564592469.
- Doroftei B, Ciobica A, Ilie O-D, Maftei R, Ilea C. Mini-review discussing the reliability and efficiency of COVID-19 vaccines. Diagnostics. 2021;11(4):579. doi:10.3390/diagnostics11040579.
- Wang Y, Zhang Y, Zhang M, Zhang X, Li H, Wang Y, Wang W, Ji J, Wu L, Zheng D. The prevalence of adverse reactions among individuals with three-dose COVID-19 vaccination. J Infect Public Health. 2023;16(1):125–32. doi:10.1016/j.jiph.2022.12.004.
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Perez Marc G, Moreira ED, Zerbini C, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603–15.
- Blumenthal KG, Greenhawt M, Phillips EJ, Agmon-Levin N, Golden DBK, Shaker M. An update in COVID-19 vaccine reactions in 2023: progress and understanding. J Allergy Clin Immunol Pract. 2023. doi:10.1016/j.jaip.2023.06.057.
- Bahcecioglu AB, Karahan ZC, Aydogan BI, Kalkan IA, Azap A, Erdogan MF. Subacute thyroiditis during the COVID-19 pandemic: a prospective study. J Endocrinol Invest. 2022;45(4):865–74. doi:10.1007/s40618-021-01718-x.
- Sendur SN, Oguz SH, Unluturk U. COVID-19 vaccination and thyroiditis. Best Pract Res Clin Endoc Metab. 2023;37(4):23. doi:10.1016/j.beem.2023.101759.
- Revon-Riviere G, Ninove L, Min V, Rome A, Coze C, Verschuur A, de Lamballerie X, Andre N. The BNT162b2 mRNA COVID-19 vaccine in adolescents and young adults with cancer: a monocentric experience. Eur J Cancer. 2021;154:30–4. doi:10.1016/j.ejca.2021.06.002.
- Couderc A-L, Ninove L, Nouguerede E, Rey D, Rebroin M, Daumas A, Tomasini P, Greillier L, Salas S, Duffaud F, et al. Acceptance, efficacy, and safety of COVID-19 vaccination in older patients with cancer. J Geriatr Oncol. 2022;13(6):850–5. doi:10.1016/j.jgo.2022.05.002.
- Rasmussen SA, Kelley CF, Horton JP, Jamieson DJ. Coronavirus disease 2019 (COVID-19) vaccines and pregnancy what obstetricians need to know. Obstet Gynecol. 2021;137(3):408–14. doi:10.1097/AOG.0000000000004290.
- Santosa A, Xu C, Arkachaisri T, Kong KO, Lateef A, Lee TH, Leong KH, Low AHL, Sriranganathan MK, Tan TC, et al. Recommendations for COVID-19 vaccination in people with rheumatic disease: developed by the Singapore chapter of rheumatologists. Int J Rheum Dis. 2021;24(6):746–57. doi:10.1111/1756-185X.14107.
- Dorman C, Perera A, Condon C, Chau C, Qian J, Kalk K, DiazDeleon D. Factors associated with willingness to be vaccinated against COVID-19 in a large convenience sample. J Community Health. 2021;46(5):1013–19. doi:10.1007/s10900-021-00987-0.
- Yasmin F, Najeeb H, Moeed A, Naeem U, Asghar MS, Chughtai NU, Yousaf Z, Seboka BT, Ullah I, Lin C-Y, et al. COVID-19 vaccine hesitancy in the United States: a systematic review. Front Public Health. 2021;9:9. doi:10.3389/fpubh.2021.770985.
- Nassar M, Nso N, Gonzalez C, Lakhdar S, Alshamam M, Elshafey M, Abdalazeem Y, Nyein A, Punzalan B, Durrance RJ, et al. COVID-19 vaccine-induced myocarditis: case report with literature review. Diabetes Metab Syndr. 2021;15(5):102205.
- Gargano JW, Wallace M, Hadler SC, Langley G, Su JR, Oster ME, Broder KR, Gee J, Weintraub E, Shimabukuro T, et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the advisory committee on immunization practices - United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70:977–82.
- Salah HM, Mehta JL. COVID-19 vaccine and myocarditis. Am J Cardiol. 2021;157:146–8. doi:10.1016/j.amjcard.2021.07.009.
- Bartels LE, Ammitzboll C, Andersen JB, Vils SR, Mistegaard CE, Johannsen AD, Hermansen M-LF, Thomsen MK, Erikstrup C, Hauge E-M, et al. Local and systemic reactogenicity of COVID-19 vaccine BNT162b2 in patients with systemic lupus erythematosus and rheumatoid arthritis. Rheumatol Int. 2021;41(11):1925–31. doi:10.1007/s00296-021-04972-7.
- Mehta P, Gasparyan AY, Zimba O, Kitas GD. Systemic lupus erythematosus in the light of the COVID-19 pandemic: infection, vaccination, and impact on disease management. Clin Rheumatol. 2022;41(9):2893–910. doi:10.1007/s10067-022-06227-7.
- Tang W, Askanase AD, Khalili L, Merrill JT. SARS-CoV-2 vaccines in patients with SLE. Lupus Sci Med. 2021;8(1):e000479. doi:10.1136/lupus-2021-000479.