 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
A humanized monoclonal antibody h2E2 designed to bind cocaine with high affinity, specificity, and a long half-life (~7 d in rats) is being developed as a treatment for cocaine use disorder. We report here a pharmacokinetic (PK) study of h2E2 using male and female rats conducted under a Good Laboratory Practice (GLP) protocol over a dose range of 40 to 1200 mg/kg. The maximum concentration measured in rat plasma (Cmax) varied proportionately to the dose administered in both male and female rats. The terminal elimination half-lives (t1/2β) were not significantly different in male and female rats at all doses tested. Importantly, this study reports pharmacokinetics for a humanized monoclonal antibody at a dose never tested before. h2E2 has a high affinity for cocaine, whereas low or no affinity was demonstrated for cocaine metabolites (all except cocaethylene), endogenous monoamines, and methamphetamine. This demonstrates its specificity and a potential lack of interactions with physiological and endocrine systems. A review of the clinical signs in single-dose toxicity studies in rats revealed no effects on the central nervous, respiratory, or cardiovascular systems following single intravenous doses of 40 to 1200 mg/kg. This study predicts that this monoclonal antibody may be safe and effective in humans.
Introduction
Cocaine abuse is a major public health problem with over 20 million users based on a recent United Nations World Drug Report.Citation1 Cocaine abuse can lead to life-threatening consequences including tachydysrhythmia, severe hypertension, acute coronary syndrome, stroke, acute myocardial and renal failure, seizures, and hyperthermia.Citation2–4 After several decades of research, there is still no effective treatment.Citation5,Citation6 Many researchers have explored conventional pharmacological approaches that target dopaminergic neurotransmission in the brain. However, this approach has not resulted in any successful treatment outcomes, and there are no approved pharmacotherapies for cocaine use disorder.Citation7 An alternative approach, one that directly targets the cocaine molecule instead of a downstream target, has been explored as a potential treatment option. Utilizing immunotherapy, such as vaccines, to treat cocaine abuse has had some success. Immunotherapy involves the application of antibodies through active or passive immunization to target certain molecules such as cocaine, nicotine, and opioids, thereby sequestering them in the peripheral circulation and reducing their central effects underlying use disorders. Clinical trials involving vaccines (active immunization route) against cocaine have shown mixed efficacy.Citation8,Citation9 Of the two anti-cocaine vaccines that made it to clinical trials, one (TA-CD) failed to meet the clinical outcome of a Phase III trial and the other (dAD5GNE) is still undergoing clinical evaluation.Citation9,Citation10 The response to the vaccine is slow to develop and is unpredictable with regard to the levels of antibodies, their affinities and specificities and, therefore, efficacy differs widely between individuals.Citation9,Citation11 There are attempts underway to improve haptens and adjuvants used in preparing cocaine vaccines that have achieved success in animal studies.Citation12 To summarize, although the vaccine trials were able to show that the polyclonal anti-cocaine antibodies produced were able to bind cocaine and prevent its effects on the brain, they showed limited success because of the variability in antibody titers and efficacy between different individuals.Citation13,Citation14 Due to the limited success of vaccines, there is a lot of interest in the use of monoclonal antibodies (mAbs) for the treatment of drugs of abuse especially because there are no US Food & Drug Administration (FDA) approved medications for cocaine addiction yet. Our purpose was to develop an effective humanized monoclonal antibody that would provide a safe and effective treatment for cocaine use and abuse. A monoclonal antibody provides the best alternative to block cocaine from reaching the brain and acting on the dopaminergic neurotransmission.
We have developed a recombinant humanized anti-cocaine monoclonal antibody, known as h2E2, that has shown promising results in preclinical animal studies. The mAb, h2E2, has a high affinity and selectivity for cocaine over its inactive metabolites.Citation15 h2E2 has a high binding affinity for 3H-cocaine with Kd values of 4.1 to 16 nM demonstrated in different studies.Citation15,Citation16 An alternate fluorescence assay (with excitation at 295 nm) also resulted in virtually identical Kd values of 4.4 to 6 nM.Citation17 Previously published data has demonstrated that h2E2 prevents entry of cocaine and its active metabolite, cocaethylene, into the brains of mice.Citation15,Citation16 The binding of h2E2 to cocaine and cocaethylene sequesters them in blood, preventing their entry into the brain, thereby reducing all their effects in the central nervous system (CNS). It has been demonstrated in rats that self-administer cocaine that a single dose of h2E2 (120 mg/kg iv) increases the concentration of cocaine required to reinstate cocaine self-administration consistent with antagonizing cocaine-induced relapse.Citation18,Citation19 h2E2 has been shown to slow the elimination and distribution of cocaine in mice, although there was no evidence for cocaine entering the brain indicating no potential for cocaine re-intoxication.Citation20 Thus, the clinical use for h2E2 would be relapse prevention in patients with cocaine-use disorder.
In preparation for a first-in-human study, preclinical studies were carried out first in male rats and mice in our laboratory.Citation10,Citation14 This was then followed by this GLP study in male and female rats. Our previously published data from the mice study show that the terminal elimination half-life (t1/2β) of h2E2 in mice and rats is approximately 7.8–9 d, respectively, which is expected to translate to approximately 3 weeks in humans. This is based on the observation that the clearance rates of IgG, are typically one-third that of rats in humans but with a similar volume of distribution.Citation21,Citation22 This indicates that h2E2 would remain in the blood of people for a protracted period antagonizing cocaine’s toxic effects. h2E2 could aid in preventing relapse and because of its long half-life enhance therapeutic compliance.
All previously published data and preclinical studies used a formulation of h2E2 in phosphate-buffered saline (PBS), at a single dose (120 mg/kg) of h2E2, in male mice, and were performed in a non-GLP academic research laboratory. We report here an expanded pharmacokinetic study with a dose range of 40–1200 mg/kg of h2E2 in a formulation closest to the clinical formulation in rats under GLP conditions in an independent laboratory.
Studies were carried out to examine the potential cross-reactivity of h2E2 with monoamine neurotransmitters and other amine-based stimulants to demonstrate specificity for cocaine-mimetic compounds. In a GLP human tissue cross-reactivity study, h2E2 did not exhibit binding to any tissue. There was also no indication of potential alterations in CNS-mediated behaviors that would have indicated an interference with the endogenous neuronal systems in the single-dose toxicity studies (40–1200 mg/kg) conducted. The results from all the preclinical studies have suggested that h2E2 has promising characteristics that could translate to its successful development as a treatment option for people.
Results
In vivo studies
All animals in the main and recovery groups survived until scheduled necropsy at all doses tested. Administration of h2E2 to rats via a single intravenous infusion (60 min) at dose levels of 40, 120, and 360 mg/kg resulted in no adverse effects on clinical observations, body weights, food consumption, coagulation, or urinalysis. There were no macroscopic or microscopic alterations. Impacts were limited to non-adverse slightly higher neutrophil and monocyte counts and recoverable higher globulin and lower albumin/globulin ratios in the 40, 120, and 360 mg/kg group males and females, and non-adverse higher adrenal and liver weights in the 360 mg/kg group males at the recovery evaluation. In the higher dose study at 1200 mg/kg, there were no test article-related clinical observations or effects on body weights, body weight gains, food consumption, or organ weights, and there were no gross or microscopic findings. Test article-related changes to clinical pathology parameters were noted in the 1200 mg/kg group of males and females on Day 2. Hematology changes were limited to higher neutrophil counts. Coagulation changes included prolonged activated partial thromboplastin time and higher fibrinogen values. Clinical chemistry changes included higher alanine aminotransferase, creatinine, total protein, globulin, aspartate aminotransferase (males only), urea nitrogen (males only), and lower cholesterol, albumin/globulin ratio, and albumin (females only). Urinalysis parameter changes were limited to lower urine pH in females. All changes were resolved by Day 29 except the minimal to mild degeneration of the testis noted in two out of five male rats on day 29 in the recovery group at the highest dose (1200 mg/kg). This was also observed in the recovery group of control animals in a separate study that tested doses up to 360 mg/kg. These changes are likely related to the onset of sexual maturity in male rats of that age.
Plasma concentrations
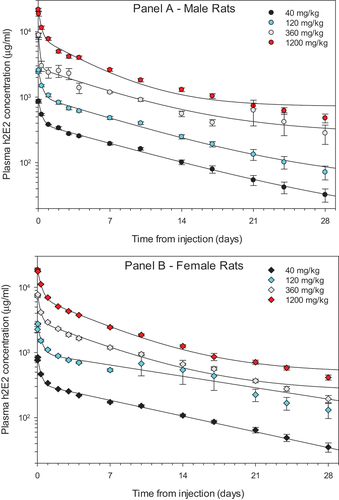
The plasma concentrations of h2E2 in both male and female rats across all four doses versus time are shown in . Following administration of h2E2 at 40, 120, 360, and 1200 mg/kg, there was a decrease in h2E2 concentrations in the plasma from 30 min up to 672 h (28 d) after end of infusion (EOI) across all dose levels in both male and female rats. There were no measurable h2E2 concentrations in the vehicle control group at all collection periods (data not shown). Peak concentrations of h2E2 were observed from 0.5 hr to 1 hr following EOI, with most of the peak plasma concentrations occurring at 1-h post EOI. The peak plasma concentrations of h2E2 were also proportional to the dose administered.
Figure 1. The pharmacokinetics of h2E2 mAb in male (Panel A) and female rats (Panel B).
Pharmacokinetics
Plasma concentrations of h2E2 in both male and female rats displayed a bimodal pharmacokinetic distribution with a mean elimination half-life (t1/2β) of 7.5 ± 1.2 d across all doses (, ). displays the breakdown of the average pharmacokinetic parameters between male and female rats across all h2E2 doses tested in this study. There was no significant difference in elimination half-life (t1/2β) between male and female rats across all doses of h2E2 (p = .454, 2-way ANOVA) or volume of distribution at steady state (Vdss, p = .617, 2-way ANOVA). The exposure of h2E2, also known as the area under the time–concentration curve (AUC), was not significantly influenced by gender (p = .999, 2-way ANOVA). Exposure to h2E2 (AUC) did increase as the dose of h2E2 increased and occurred in both male and female rats (, ). The average peak plasma concentration normalized by dose (Cmax/D) did not differ significantly between male and female rats (p = .105, 2-way ANOVA. As the dose of h2E2 increased, so did the Cmax or peak plasma concentration (). Both AUC and Cmax for plasma h2E2 concentrations increased linearly across this range of doses. The linear regression had a positive slope that was significantly different from zero for both parameters.
Figure 2. Area under the curve (AUC)and peak plasma concentration (Cmax) for h2E2.
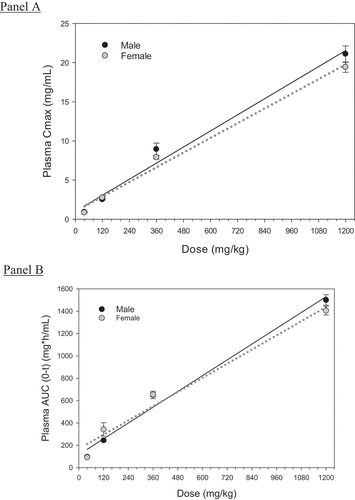
Table 1. Complete pharmacokinetic profile across all doses in male and female rats.
Ligand cross-reactivity
Based on the EC50, the binding affinity of cocaethylene to h2E2 was the highest, and EME was the lowest. As shown in , the rank order affinity of binding is Cocaethylene>Cocaine>BE>EME. This order of binding affinity is very similar to previously published data.Citation23 shows a representative 4-parameter logistic curve fit obtained with cocaine and its metabolites. The assay was repeated to test the binding affinity of the catecholamines. In these experiments (), cocaine was used as the control to compare the binding affinity of the catecholamines. For all catecholamines tested, except epinephrine, no inhibition of h2E2 binding was observed at any concentration tested (up to 60,000 µM). shows a comparison of EC50 values obtained for cocaine and the other amines tested. Epinephrine showed some binding at extremely high concentrations in two out of three experiments, but the standard error on the fit was very high. Methamphetamine was tested in only one experiment no inhibition of binding up to 60,000 µM was observed.
Figure 3. ELISA binding curves for cocaine, its metabolites and other amines.
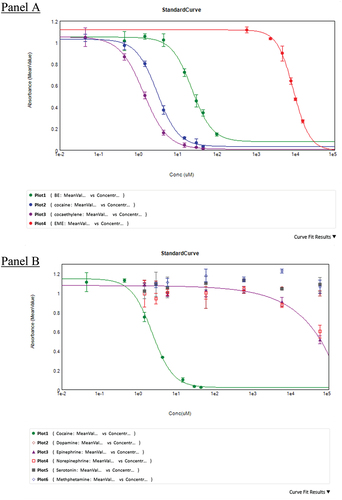
Table 2. Relative binding affinities of cocaine, its metabolites and other amines for h2E2.
Discussion
Studies reported here tested the formulation of h2E2 that will be closely similar to the proposed first-in-human clinical studies except for the concentration of sucrose and histidine will be lower in the clinical formulation. This study also confirms our earlier published data with antibodies from the same clone when administered in PBS in mice.Citation16 Elimination half-life and volume of distribution (Vdss) obtained in this study were almost identical to what was obtained in the mice study using a single dose of the antibody (120 mg/kg). Elimination half-life in this study was 7.0 ± 1.2 () d for male rats at a dose of 120 mg/kg, and it was 7.1 ± 1.6 d in the mice study. The volume of distribution here was 0.11 L/kg in this study and 0.13 L/kg in the mice study. The linear response observed for both AUC and Cmax concerning plasma h2E2 concentrations indicates that the pharmacokinetics of h2E2 are independent of the dose of h2E2 over the range of doses used here. Interestingly, the AUC responses are similar to results obtained in an earlier study, albeit with 10 mg/kg IgG plus 2 g/kg IVIG in rats (private communication), and yet different from what was observed in mice in other studies.Citation24 In our study, it may be concluded that there is no evidence that the mechanisms underlying the elimination of h2E2 (for example, FcRn recycling) in male and female rats are saturated even at the highest levels of h2E2 used. Whether this is the case in humans has yet to be determined.
We have also previously reported a terminal elimination half-life of 9.0 d and a Vdss of 0.3 L/kg in a study performed using an initial production run of h2E2 prepared in PBS.Citation15 The fact that two different laboratories, using two different assay systems, observed such similar pharmacokinetics indicates that measurements of h2E2 concentrations across studies are likely to be reproducible. The GLP protocol study was in rats using a goat anti-human antibody for h2E2 capture and quantification. In our previously published studies, ELISA was done using whole blood from mice.Citation16 In the mice study, an antigen to h2E2, BE-diab BSA was used for capture and quantification of h2E2.
h2E2 has a high affinity for cocaine with low or no affinity for cocaine metabolites (except cocaethylene), endogenous monoamines, and methamphetamine. The safety of h2E2 is predicted by the lack of significant binding to other possible endogenous targets such as neurotransmitters (, ). h2E2 should not therefore interfere with normal neurotransmission or endocrine function, and the lack of cross reactivity with other stimulants indicates that h2E2 will not interfere with the use of other medications that act on these systems.
Human tissue cross-reactivity and in vivo, rat toxicology studies performed under GLP conditions also do not indicate that h2E2 should cause toxicity in humans. A panel of 36 tissues from three human donors were tested with h2E2 and no cross reactivity was observed, further confirming that this antibody has no affinity for endogenous proteins. The lack of any cytoplasmic or membrane staining by h2E2 in the tissues examined is highly suggestive of the fact that h2E2 will not bind or activate any nonspecific cellular components. Furthermore, toxicology studies in rats showed an absence of any effects on the central nervous, respiratory, or cardiovascular systems following the administration of single intravenous (IV) doses of 40 to 1200 mg/kg to rats. Based on the minimal magnitude of the changes and the lack of correlating microscopic findings in the observations of mild testis degeneration, these changes were not considered adverse. Doses up to 1200 mg/kg are well tolerated in rats and can be considered the no-observed-adverse-effect level (NOAEL). Ligand cross-reactivity data show that h2E2 binds with a high degree of specificity to cocaine over its inactive metabolites like BE or EME. Significantly, the antibody showed almost no binding to catecholamines tested in this system. This is highly important information to ensure that the antibody efficacy is not altered by binding to either closely similar molecules or completely dissimilar off-target molecules. The lack of cross-reactivity to monoamines predicts that h2E2 will not affect monoamine-mediated physiological functions.
The terminal elimination half-life and C max of h2E2 in both male and female rats were not significantly different (p> .05, 2-way ANOVA). This finding indicates that there are no sex differences in the way this antibody is metabolized and cleared in rats. These results are reassuring as it is generally believed that drugs are metabolized slower in women in comparison to men. If metabolism is slowed, then there is a greater exposure of the drug which could directly result in an increased risk of side effects. The FDA reports that women experience more adverse events than men and that these adverse events also tend to be more severe.Citation25 Eight out of the ten drugs withdrawn from the market from January of 1997 to December of 2000 were withdrawn due to greater occurrence of adverse events in women compared to men.Citation25 The generally lower body weight and organ size, higher percentage of body fat, lower glomerular filtration rate, and different gastric motility in women compared with men can lead to differences in the metabolism and distribution of any drug.Citation26 The volume of distribution and clearance of monoclonal antibodies could vary with body size, which could affect dosing regimens that are not based on body weight. A 40% higher rate of clearance occurs in males treated with adalimumab or rituximabCitation27,Citation28 Dose calculations need to be adjusted to consider differences of bodyweight and clearance rates.
In the case of alemtuzumab, no differences were observed in PK and PD in men and women.Citation29,Citation30 Ocrelizumab and natalizumab also did not show any difference in clinical efficacy in men and women.Citation31–34 The efficacy and safety of erenumab, a monoclonal antibody antagonizing the calcitonin gene-related peptide (CGRP) receptor, showed comparable safety and efficacy in men and women.Citation35 Antidrug antibody (ADA) responses to infliximab appear to exhibit gender-specific differences.Citation36 One study shows a higher ADA response in women,Citation37 whereas another shows a higher response in males.Citation38
It is important to note that the lack of differences in PK (observed here with h2E2) may not be indicative of the lack of difference in other effects due to the antibody treatment. The use of monoclonal therapies does run the risk of anaphylaxis, cytokine storm, and ADA formation. ADA formation can affect exposure, clearance, safety, and efficacy.Citation39 In contrast, to complement-dependent cytotoxicity and antibody-dependent cell cytotoxicity, the two principal mechanisms of action for antibodies for anticancer or anti-lymphoproliferative disease, h2E2 has a simpler and possibly more of an antidotal mechanism of action. Our antibody would work by binding the exogenous low molecular weight cocaine, blocking its action and distribution to its sites of action, thus potentially being less problematic than the other monoclonal antibodies.Citation40
The results from this GLP study indicate that the formulation tested is not different from the earlier formulation of h2E2 in terms of its pharmacokinetic parameters. In clinical trials, h2E2 is predicted to have the same pharmacokinetic profiles in both male and female patients. The lack of any adverse effects in doses up to 1200 mg/kg in rats is a significant finding from this study. Therapeutic monoclonal antibodies are rarely, if ever, tested at such high doses. A review of the literature suggests that the highest doses of monoclonal antibodies tested specifically against infectious diseases in use rarely exceed 40 mg/kg.Citation41 With the tenfold higher level testing requirement of the FDA in preclinical animal studies, this would suggest most of these antibodies were not tested above 400 mg/kg levels. In the case of other humanized antibodies also this stands out. For example, bevacizumab, a humanized antibody against vascular endothelial factor, the highest dose tested was 50 mg/kg in cynomolgus monkeys.Citation42 In a more recent preclinical study, Omalizumab and a biosimilar were tested at 150 mg/kg and 300 mg/kg in cynomolgus monkeys.Citation43 An antibody directed against methamphetamine abuse was tested at 150 mg/kg in preclinical studies in rats. It is to be emphasized that intravenous immunoglobulin (IVIG) preparations are typically used in the range of 2 g/kg for autoimmune disorders, but these are different from a humanized monoclonal antibody produced by recombinant technology. IVIG preparations are polyclonal and could contain 95% IgG but also IgA and IgM, typically harvested from plasma.Citation44,Citation45 We do not foresee the use of 1200 mg/kg of h2E2 in humans, and the testing in rats was performed to satisfy an FDA requirement. Other drug interactions of h2E2 or its possible potential to saturate FcRn is yet to be investigated and could be done using animal systems or physiologically based PK (PBPK) modeling approach.Citation24 A possible limitation of this approach would be the need to not include in the clinical trial patients with renal dysfunction, diabetes, or taking nephrotoxic drugs as has been done previously for IVIG, if higher doses are to be tested.Citation40
The effectiveness of h2E2 will vary between early users and chronic cocaine abusers, primarily based on the amount of cocaine in circulation. An equimolar dose of h2E2 (assuming bivalent binding) that could bind nearly (89%) of 0.56 mg/kg b.w cocaine HCl is expected to be the highest dose tested in clinical trials. Treatment with h2E2 will be most effective in patients undergoing rehabilitation who relapsed using a dose of cocaine HCl (0.56 mg/kg b.w. ~33 mg for a 60 kg person)Citation46 With the limited availability of cocaine in rehabilitation, it is anticipated that most of the used cocaine could be completely bound by 120 mg/kg, the highest dose to be used in clinical studies. There will also be FDA limitations on the allowed amounts of cocaine to be used in Phase 1b h2E2-cocaine cross-reactivity studies.
Importantly, this monoclonal antibody h2E2 has been generated from the same HuMAb transgenic mouse platform that has produced 22 or more human monoclonal antibodies that have undergone clinical trials with little or no immunogenicity.Citation47 Yet another indirect confirmation that this antibody-based approach will be safe is also provided by the clinical trials of the cocaine vaccine.Citation11,Citation48,Citation49 Overall, h2E2 exhibits characteristics that predict that it could potentially be safe and effective for the prevention of relapse in patients with cocaine user disorder.
Materials & methods
Test materials
Catalent Pharma Solutions (Madison, WI) manufactured the antibody, h2E2. Two different lots of h2E2, one from the first scale-up production run and the other from an engineering run [50LSUB340285.6.001 and D5 -340,201-01] manufactured at Catalent, were used in this study. The engineering run is the formulation that is similar to the clinical formulation. The antigen, benzoylecgonine-1,4-diaminobutane-BSA, conjugate (referred to hereafter as BE-diab-BSA) used in the ligand-binding assay was synthesized in the laboratory at the Department of Pharmacology & Systems Physiology, College of Medicine, University of Cincinnati. The secondary antibody, the biotin conjugate-goat anti-human IgG (GAH), and alkaline phosphatase substrate, para-nitro phenyl phosphate (pNPP), were purchased from Sigma Aldrich (St. Louis, MO). Streptavidin linked-alkaline phosphatase (AP) was purchased from Roche (Indianapolis, IN). Cocaine (Coc), cocaethylene (CE), benzoylecgonine (BE) and ecgonine methyl ester (EME) were supplied by RTI via the National Institute of Drug Abuse (NIDA) drug supply program under Dr Norman’s DEA license. Dopamine (Cat#H8502) and Serotonin (Cat #H9523) hydrochloride were also purchased from Sigma (St. Louis, MO).
Goat anti-human IgG (cat: 2014–01) and peroxidase conjugate anti-human IgG (cat: 2014–05) were purchased from Southern Biotech (Birmingham, AL) for the GLP study. Clear flat bottom Immuno nonsterile MaxiSorp 96-well plates (cat: 439454) for ELISA and casein blocker in PBS (cat: 37528) were purchased from ThermoScientific (Waltham, MA). 3,3‘5,5’- tetramethylbenzidine (cat: 50-76-01) and peroxidase substrate Solution B (cat: 50-65-00) were purchased from KPL laboratories. 1N sulfuric acid was purchased from Fisher (cat: SA21–1). The vehicle and h2E2 antibody were shipped from Catalent (Madison, WI). ELISA (96 well) plates from Thermofisher (Cat# 14245142) were used for the in vitro ligand-binding competition assay.
In vivo pharmacokinetics study
Animals
Male and female Sprague Dawley rats (192–304 g) were purchased from Charles River Laboratories, Inc. (Raleigh, NC). Upon arrival, animals were housed in groups (two to three animals of the same sex/cage) until randomization. Animals for the 1200 mg/kg study were approximately 7 weeks old at the initiation of dosing (Day−1) and weighed an average of 183.5 ± 10.1 g for females and 225.5 ± 8.6 g for males. Following randomization, animals were divided based on sex and dosing into two to three animals per group/cage. Animals were assigned to groups by a stratified randomization scheme designed to achieve similar group mean body weights. Males and females were randomized separately. All rats had free access to food and water and were kept on a 12/12-h light/dark cycle. These studies were carried out following the Guide for the Care and Use of Laboratory Animals under a protocol approved by the Charles River Ashland laboratory (Ashland, OH), which is accredited by AAALAC International.Citation50
Dose formulation
The formulation used for the GLP rat study was h2E2 (~20 mg/mL) in a 10 mM histidine, 10% sucrose, and 0.01% polysorbate 80 buffer solution (pH 6.0). In all previously published animal studies involving h2E2, the antibody was in a phosphate-buffered solution (pH 7.2). The dose levels were 40, 120, and 360 mg/kg/d in the first study. The dose volume was 18 mL/kg for all groups and used h2E2 at a concentration of 20 mg/ml. The highest dose of h2E2 was 1200 mg/kg, by concentrating the h2E2 in the formulation to 103 mg/ml. The concentration was then adjusted to 66.7 mg/ml to inject equal volumes for the study.
Experimental design
Five cohorts of both male and female rats were used for the pharmacokinetic study. The first cohort of rats (vehicle control, n = 3) for each sex received an infusion of vehicle of equal volume as h2E2 treated cohorts. The second through the fourth cohorts (n = 7) received h2E2 at a dose of 40 mg/kg, 120 mg/kg, and 360 mg/kg. h2E2, in the vehicle (10 mM histidine, 10% sucrose, 0.01% polysorbate 80, pH 6.0), was administered as a single IV infusion (60 min ±3 min) to three groups of seven males and seven female rats. A control group, consisting of three animals/sex, received the vehicle on the same regimen. On Day 1 (day of dosing), blood samples were collected from all animals at the end of infusion (EOI; within 5 min), 30 min, and 8, 24, 48, 72, 96, 168, 240, 336, 408, 504, 576, and 672 h following the EOI. In the vehicle control group, blood samples were collected on the day of dosing at the EOI (within 5 min), 24, and 672 h following EOI only. In a separate study control, animals (n = 3) received a vehicle, and the treatment group (n = 9) received h2E2 at a dose of 1200 mg/kg. The concentration of h2E2 increased as the dose increased to keep the volume injected the same between each cohort. Each cohort of rats received either h2E2 or vehicle control infusion over 60 min via the tail vein. A calibrated syringe pump was used for the infusion. Sample collection for this study followed the exact time intervals as for the first study. Toxicological studies were carried out in a separate study with 10 animals per group in vehicle and h2E2 doses (40, 120, 360, and 1200 mg/kg). Five additional animals in the vehicle and h2E2 doses (360 & 1200 mg/kg) were followed for 29 d.
Blood samples (0.3 ml/time point) were collected via the retro-orbital sinus into an anticoagulant dipotassium ethylene diamine tetra-acetic acid (K2EDTA) and placed on ice starting from 0 to 672 hours. Samples were then centrifuged at 4οC at 2400–2700 rpm for 10 min. The recovered plasma was then transferred to a new vial and stored at −55 to −85 οC until quantification. All samples were shipped on dry ice to another site for quantification of h2E2 in the plasma samples.
Plasma h2E2 concentrations
A validated ELISA assay performed at Charles River, Canada, in their GLP laboratories was used to quantitate h2E2 in plasma samples. Calibration standards, ranging from 100 to 5000 ng/mL (with accessory standards at 50 and 10,000 ng/mL), were prepared by spiking blank rat plasma with appropriate amounts of h2E2 stock solutions. Aliquots of the calibration standards were stored in a freezer set to maintain −80°C. The measured concentrations of at least 75% of the calibration standards were to be within ± 20% of their theoretical concentrations (within ± 25% at the upper and lower limits of quantification). Additionally, the coefficient of variation of the instrument response between duplicate wells for each standard and sample had to be ≤ 20%. Samples were examined for hemolysis and excluded based on validated criteria. A multi-species adsorbed goat anti-human IgG was coated on the ELISA plate. h2E2 in the sample binds to the antibody and was estimated using peroxidase-conjugated goat anti-human IgG. Nonspecific binding was blocked using a casein blocker in PBS. After washing away unbound material, the substrate 3,3’5,5’- tetramethylbenzidine and peroxidase substrate was applied. The intensity of the color produced was directly proportional to the quantity of bound h2E2 antibody. Absorbance was read at 650 nm before stopping and at 450 nm post-stopping with 1N sulfuric acid. Data capture of the ELISA reader (Spectra MAX UV/VIS) was performed with Softmax Pro GxP version 5.4.6 or later. Plates were washed with Skan Plate Washer 400 (Skatron Instruments). Runs were accepted only if the back-calculated concentrations of all standards were within 20% of the theoretical except for the ULOQ (upper limit of quantitation) and LLOQ (lower limit of quantitation) which should be within 25% of the theoretical value. At least 67% (4/6) of the quality controls met the same acceptance criteria.
Pharmacokinetic modelling
A 4-parameter logistic regression model with a weighing factor of 1/Y was used for curve fitting to quantify the antibody PK in rat plasma samples. PK analyses were carried out using WinNonlin software (Phoenix, Certara, USA) using a non-compartmental approach consistent with the route of administration. All statistical analyses were performed using Watson Laboratory Information Management System version 7.4.2 SP1 or later in the GLP laboratory. The graphing of the PK data from male and female rats used Sigma Plot 14.0.
In vitro ligand binding
A modification of a previously published ELISA protocol was used to develop this competition ELISA using the drugs in this study (Coc, CE, BE, EME and catecholamines).Citation16 ELISA plate was incubated with BE-diab-BSA at 0.5 µg/ml in Tris-EGTA 1 mM, pH 7.2 at RT for 1 hr on a shaker. Following incubation, the plate was washed twice and then blocked using the same BSA-Tris buffer (0.5% BSA in 10 mM Tris, 140 mM NaCl, and 0.02% NaN3, pH 7.2) for 15 min. Subsequently, a 1:1 solution of h2E2 combined with cocaine or its metabolites or catecholamines was applied to the plate. All catecholamines were prepared fresh with 50 µM sodium thiosulfate to prevent oxidation and experiments were carried out in the dark to protect from light. After preliminary screening over a concentration range of 0–75 µM (data not shown) for all drugs, subsequent experiments extended or narrowed the range based on the results from the screen. The monoclonal antibody h2E2 from the h2E2-analyte solution was bound to the plate-adsorbed antigen and was reacted with the secondary goat-antihuman Fc-specific antibody. The colorimetric signal due to biotin-bound streptavidin-AP conjugate hydrolysis of pNPP was recorded as optical density (OD) at 405 nm. All the incubations except that with substrate were carried out at room temperature for 1 h. All washes following primary antibody incubation were performed with BSA-PBS buffer (0.5% BSA, 10 mM sodium phosphate, 145 mM NaCl, 1.5 mM MgCl2, 0.05% Triton X-100, and 0.02% NaN3, pH 7) manually. Mean OD values at every concentration were obtained after subtracting the OD obtained with the absence of h2E2. Plates were read and data analyzed on SpectraMax M3 and SoftMax Pro 6.4. A 4-parameter logistic curve was used to analyze the data, and the EC50 (effective concentration) reported in the analysis is considered as the IC50 (inhibitory concentration that causes 50% inhibition of antigen-antibody binding in the wells). Each drug was tested in triplicate (catecholamines were tested in duplicate) and each experiment was repeated three times.
Abbreviations
| ANOVA | = | analysis of variance |
| AUC | = | area under the time-concentration curve |
| EOI | = | end of infusion |
| BE | = | benzoylecgonine |
| BE-diab BSA | = | benzoylecgonine diamino bovine serum albumin |
| CNS | = | central nervous system |
| ELISA | = | enzyme-linked immunosorbent assay |
| EME | = | ecogonine methylecogonine |
| FDA | = | Food and Drug Administration |
| GLP | = | good laboratory practice |
| mAbs | = | monoclonal antibodies |
| IVIG | = | intravenous immunoglobulin |
| PK | = | pharmacokinetics |
| PBS | = | phosphate buffered saline |
| pNPP | = | para nitrophenyl phosphate |
Acknowledgments
We thank Charles River Laboratories, Ashland, OH USA for conducting the pharmacokinetic studies under a GLP protocol. We thank Mackenzie Turner and Hanna Wetzel for useful discussions. We acknowledge Dr Marepalli Rao for providing statistical insights. Supported by NIDA grant U01DA050330.
Disclosure statement
Dr Norman is named as a co-inventor on a portfolio of patents for the matter and use of the h2E2 humanized anti-cocaine monoclonal antibody. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Funding
References
- Richards JR, Le JK. Cocaine toxicity. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jun 8. PMID: 28613695
- Hantson P. Mechanisms of toxic cardiomyopathy. Clin Toxicol (Philadelphia, Pa). 2019;57:1–11. PMID: 30260248. doi:10.1080/15563650.2018.1497172.
- Mikhail A, Tanoli O, Légaré G, Dubé PA, Habel Y, Lesage A, Low NCP, Lamarre S, Singh S, Rahme E. Over-the-counter drugs and other substances used in attempted suicide presented to emergency departments in Montreal, Canada. Crisis. 2019;40:166–75. PMID: 30215303. doi:10.1027/0227-5910/a000545.
- Narula N, Siddiqui F, Katyal N, Krishnan N, Chalhoub M. Cracking the crack dance: a case report on cocaine-induced choreoathetosis. Cureus. 2017;9:e1981. PMID: 29503775. doi:10.7759/cureus.1981.
- EMCDD. Treatment for cocaine dependence — reviewing current evidence. http://www.emcdda.europa.eu/topics/pods/treatment-for-cocaine-dependence_en:2014Date.
- NIDA. Cocaine. Vol. 2018. https://www.drugabuse.gov/publications/research-reports/cocaine/what-treatments-are-effective-cocaine-abusers2016.
- Vocci FJ Ph.D., Acri J Ph.D, Elkashef A M.D. Medication development for addictive disorders: the state of the science. Am J Psychiatry. 2005;162(8):1432–40. PMID: 16055764. doi:10.1176/appi.ajp.162.8.1432.
- Kosten T, Domingo C, Orson F, Kinsey B. Vaccines against stimulants: cocaine and MA. Br J Clin Pharmacol. 2014;77(2):368–74. doi:10.1111/bcp.12115.
- Kosten TR, Domingo CB, Shorter D, Orson F, Green C, Somoza E, Sekerka R, Levin FR, Mariani JJ, Stitzer M, et al. Vaccine for cocaine dependence: a randomized double-blind placebo-controlled efficacy trial. Drug Alcohol Depen. 2014;140:42–7. PMID: 24793366. doi:10.1016/j.drugalcdep.2014.04.003.
- Havlicek DF, Rosenberg JB, De BP, Hicks MJ, Sondhi D, Kaminsky SM, Crystal RG. Cocaine vaccine dAd5gne protects against moderate daily and high-dose “binge” cocaine use. PloS One. 2020;15(11):e0239780. doi:10.1371/journal.pone.0239780.
- Martell BA, Orson FM, Poling J, Mitchell E, Rossen RD, Gardner T, Kosten TR. Cocaine vaccine for the treatment of cocaine dependence in methadone-maintained patients: a randomized, double-blind, placebo-controlled efficacy trial. Arch Gen Psychiatry. 2009;66:1116–23. PMID: 19805702. doi:10.1001/archgenpsychiatry.2009.128.
- Lin M, Marin A, Ellis B, Eubanks LM, Andrianov AK, KD J. Polyphosphazene: a new adjuvant platform for cocaine vaccine development. Mol Pharm. 2022;19(9):3358–66. doi:10.1021/acs.molpharmaceut.2c00489.
- Koch SE, Marckel JA, Rubinstein J, Norman AB. A humanized anti-cocaine mAb antagonizes the cardiovascular effects of cocaine in rats. Pharmacol Res Perspect. 2023;11(1):e01045. doi:10.1002/prp2.1045.
- Scendoni R, Bury E, Ribeiro ILA, Cameriere R, Cingolani M. Vaccines as a preventive tool for substance use disorder: a systematic review including a meta-analysis on nicotine vaccines’ immunogenicity. Hum Vaccin Immunother. 2022;18:2140552. doi:10.1080/21645515.2022.2140552.
- Norman AB, Gooden FC, Tabet MR, Ball WJ. A recombinant humanized anti-cocaine monoclonal antibody inhibits the distribution of cocaine to the brain in rats. Drug Metab Dispos. 2014;42:1125–31. PMID: 24733787. doi:10.1124/dmd.114.057034.
- Wetzel HN, Webster RP, Saeed FO, Kirley TL, Ball WJ, Norman AB. Characterization of a recombinant humanized anti-cocaine monoclonal antibody produced from multiple clones for the selection of a master cell bank candidate. Biochem Biophys Res Commun. 2017;487(3):690–4. PMID: 28442345. doi:10.1016/j.bbrc.2017.04.117.
- Kirley TL, Norman AB. Characterization of a recombinant humanized anti-cocaine monoclonal antibody and its fab fragment. Hum Vaccin Immunother. 2015;11:458–67. PMID: 25692880. doi:10.4161/21645515.2014.990856.
- Norman AB, Norman MK, Buesing WR, Tabet MR, Tsibulsky VL, Ball WJ. The effect of a chimeric human/murine anti-cocaine monoclonal antibody on cocaine self-administration in rats. J Pharmacol Exp Ther. 2009;328:873–81. PMID: 19088302. doi:10.1124/jpet.108.146407.
- Wetzel HN, Tsibulsky VL, Norman AB. The effects of a repeated dose of a recombinant humanized anti-cocaine monoclonal antibody on cocaine self-administration in rats. Drug Alcohol Depen. 2016;168:287–92. PMID: 27736682. doi:10.1016/j.drugalcdep.2016.09.024.
- Turner ME, Wetzel HN, Zinani DB, Crutchfield CA, Norman AB. Effects of a recombinant humanized anti-cocaine monoclonal antibody on the metabolism and distribution of cocaine in vitro and in mice. Pharmacol Res Perspect. 2022;10(5):e01009. PMID: 36121122. doi:10.1002/prp2.1009.
- Bazin-Redureau MI, Renard CB, Scherrmann JM. Pharmacokinetics of heterologous and homologous immunoglobulin G, F(ab‘)2 and fab after intravenous administration in the rat. J Pharm Pharmacol. 1997;49(3):277–81. PMID: 9231345. doi:10.1111/j.2042-7158.1997.tb06795.x.
- Joos B, Trkola A, Kuster H, Aceto L, Fischer M, Stiegler G, Armbruster C, Vcelar B, Katinger H, Günthard HF. Long-term multiple-dose pharmacokinetics of human monoclonal antibodies (MAbs) against human immunodeficiency virus type 1 envelope gp120 (MAb 2G12) and gp41 (MAbs 4E10 and 2F5). Antimicrob Agents Chemother. 2006;50(5):1773–9. PMID: 16641449. doi:10.1128/aac.50.5.1773-1779.2006.
- Paula S, Tabet MR, Keenan SM, Welsh WJ, Ball WJ Jr. Three-dimensional structure–activity relationship modeling of cocaine binding to two monoclonal antibodies by comparative molecular field analysis. J Mol Biol. 2003;325(3):515–30. PMID: 12498800. https://www.ncbi.nlm.nih.gov/pubmed/12498800.
- Shah DK, Betts AM. Towards a platform PBPK model to characterize the plasma and tissue disposition of monoclonal antibodies in preclinical species and human. J Pharmacokinet Pharmacodyn. 2012;39:67–86. PMID: 22143261. doi:10.1007/s10928-011-9232-2.
- Soldin OP, Mattison DR. Sex differences in pharmacokinetics and pharmacodynamics. Clin Pharmacokinet. 2009;48:143–57. PMID: 19385708. doi:10.2165/00003088-200948030-00001.
- Zucker I, Prendergast BJ. Sex differences in pharmacokinetics predict adverse drug reactions in women. Biol Sex Differ. 2020;11:32. doi:10.1186/s13293-020-00308-5.
- Ng CM, Bruno R, Combs D, Davies B. Population pharmacokinetics of rituximab (anti-CD20 monoclonal antibody) in rheumatoid arthritis patients during a phase II clinical trial. J Clin Pharmacol. 2005;45:792–801. PMID: 15951469. doi:10.1177/0091270005277075.
- Ternant D, Ducourau E, Fuzibet P, Vignault C, Watier H, Lequerré T, Le Loët X, Vittecoq O, Goupille P, Mulleman D, et al. Pharmacokinetics and concentration–effect relationship of adalimumab in rheumatoid arthritis. Br J Clin Pharmacol. 2015;79(2):286–97. PMID: 25223394. doi:10.1111/bcp.12509.
- Daniels GH, Vladic A, Brinar V, Zavalishin I, Valente W, Oyuela P, Palmer J, Margolin DH. Alemtuzumab-related thyroid dysfunction in a phase 2 trial of patients with relapsing-remitting multiple sclerosis. J Clin Endocr Metab. 2014;99:80–9. doi:10.1210/jc.2013-2201.
- Li Z, Richards S, Surks HK, Jacobs A, Panzara MA. Clinical pharmacology of alemtuzumab, an anti-CD52 immunomodulator, in multiple sclerosis. Clin Exp Immunol. 2018;194:295–314. doi:10.1111/cei.13208.
- Lublin FD, Cutter G, Giovannoni G, Pace A, Campbell NR, Belachew S. Natalizumab reduces relapse clinical severity and improves relapse recovery in MS. Mult Scler Relat Disord. 2014;3:705–11. doi:10.1016/j.msard.2014.08.005.
- Radue E-W, Stuart WH, Calabresi PA, Confavreux C, Galetta SL, Rudick RA, Lublin FD, Weinstock-Guttman B, Wynn DR, Fisher E, et al. Natalizumab plus interferon beta-1a reduces lesion formation in relapsing multiple sclerosis. J Neurol Sci. 2010;292(1–2):28–35. doi:10.1016/j.jns.2010.02.012.
- Turner B, Cree BAC, Kappos L, Montalban X, Papeix C, Wolinsky JS, Buffels R, Fiore D, Garren H, Han J, et al. Ocrelizumab efficacy in subgroups of patients with relapsing multiple sclerosis. J Neurol. 2019;266:1182–93. doi:10.1007/s00415-019-09248-6.
- Wolinsky JS, Montalban X, Hauser SL, Giovannoni G, Vermersch P, Bernasconi C, Deol-Bhullar G, Garren H, Chin P, Belachew S, et al. Evaluation of no evidence of progression or active disease (NEPAD) in patients with primary progressive multiple sclerosis in the ORATORIO trial. Ann Neurol. 2018;84(4):527–36. doi:10.1002/ana.25313.
- Ornello R, Baraldi C, Guerzoni S, Lambru G, Fuccaro M, Raffaelli B, Gendolla A, Barbanti P, Aurilia C, Cevoli S, et al. Gender differences in 3-month outcomes of erenumab treatment-study on efficacy and safety of treatment with erenumab in men. Front Neurol. 2021;12:774341. PMID: 34975732. doi:10.3389/fneur.2021.774341.
- Maranini B, Bortoluzzi A, Silvagni E, Govoni M. Focus on sex and gender: what we need to know in the management of rheumatoid arthritis. J Pers Med. 2022;12(3):499. doi:10.3390/jpm12030499.
- Hambardzumyan K, Hermanrud C, Marits P, Vivar N, Ernestam S, Wallman JK, van Vollenhoven RF, Fogdell-Hahn A, Saevarsdottir S. Association of female sex and positive rheumatoid factor with low serum infliximab and anti-drug antibodies, related to treatment failure in early rheumatoid arthritis: results from the SWEFOT trial population. Scand J Rheumatol. 2019;48(5):362–6. doi:10.1080/03009742.2019.1602670.
- Shehab M, Alasfour H, Abdullah I, Alhendi G, Alhadab A, Alfadhli A, Ziyab AH, Battat R. Relationship between patient sex and serum tumor necrosis factor antagonist drug and anti-drug antibody concentrations in inflammatory bowel disease; a nationwide cohort study. Front Med. 2021;8:801532. PMID: 35004778. doi:10.3389/fmed.2021.801532.
- Hansel TT, Kropshofer H, Singer T, Mitchell JA, George AJT. The safety and side effects of monoclonal antibodies. Nat Rev Drug Discov. 2010;9:325–38. doi:10.1038/nrd3003.
- Norman AB, Ball WJ Jr. Predicting the clinical efficacy and potential adverse effects of a humanized anticocaine monoclonal antibody. Immunotherapy. 2012;4:335–43. PMID: 22401638. doi:10.2217/imt.12.19.
- Ovacik M, Lin K. Tutorial on monoclonal antibody pharmacokinetics and its considerations in early development. Clin Transl Sci. 2018;11:540–52. PMID: 29877608. doi:10.1111/cts.12567.
- Gerber H-P, Ferrara N. Pharmacology and pharmacodynamics of bevacizumab as monotherapy or in combination with cytotoxic therapy in preclinical studies. Cancer Res. 2005;65:671–80. doi:10.1158/0008-5472.671.65.3.
- Wang Y, Zheng C, Zhuang C, Fu Q, Qin J, Zhang B, Bian Y, Qi N, Zhu J. Preclinical pharmacology and toxicology evaluation of an anti-CD52 monoclonal antibody produced by perfusion fermentation process. J Ind Microbiol Biotechnol. 2021;48(9–10):48. PMID: 34669957. doi: 10.1093/jimb/kuab078.
- Durandy A, Kaveri SV, Kuijpers TW, Basta M, Miescher S, Ravetch JV, Rieben R. Intravenous immunoglobulins–understanding properties and mechanisms. Clin Exp Immunol. 2009;158(Supplement_1):2–13. PMID: 19883419. doi:10.1111/j.1365-2249.2009.04022.x.
- Velikova T, Sekulovski M, Bogdanova S, Vasilev G, Peshevska-Sekulovska M, Miteva D, Georgiev T. Intravenous immunoglobulins as immunomodulators in autoimmune diseases and reproductive medicine. Antibodies. 2023;12:20. doi:10.3390/antib12010020.
- Treadwell SD, Robinson TG. Cocaine use and stroke. Postgrad Med J. 2007;83:389–94. doi:10.1136/pgmj.2006.055970.
- Fishwild DM, O’Donnell SL, Bengoechea T, Hudson DV, Harding F, Bernhard SL, Jones D, Kay RM, Higgins KM, Schramm SR, et al. High-avidity human IgG kappa monoclonal antibodies from a novel strain of minilocus transgenic mice. Nat Biotechnol. 1996;14(7):845–51. PMID: 9631008. doi:10.1038/nbt0796-845.
- Haney M, Gunderson EW, Jiang H, Collins ED, Foltin RW. Cocaine-specific antibodies blunt the subjective effects of smoked cocaine in humans. Biol Psychiatry. 2010;67:59–65. PMID: 19846066. doi:10.1016/j.biopsych.2009.08.031.
- Martell BA, Mitchell E, Poling J, Gonsai K, Kosten TR. Vaccine pharmacotherapy for the treatment of cocaine dependence. Biol Psychiatry. 2005;58:158–64. PMID: 16038686. doi:10.1016/j.biopsych.2005.04.032.
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press; 2011. doi:10.17226/12910.
