ABSTRACT
The evidence on the waning protection of COVID-19 vaccines has been reviewed by the World Health Organization and has led to consideration of the need for booster doses. This study aimed to evaluate vaccine effectiveness against COVID-19, and the COVID-19 infections among healthcare workers who received various types (inactive or m-RNA) and doses (2 to 4 doses) of the COVID-19 vaccine. The study was conducted with a total of 3,009 healthcare workers between August 1 and November 30, 2021 at a university hospital. Six different vaccination statuses were evaluated in the study. The effectiveness for COVID-19 infection, after adjusting for age, sex, and position, was highest in those who received two doses of CoronaVac and two doses of BNT162b2 (89.3%, 95% CI 72.2–95.9%) and was lowest in those who received two doses of CoronaVac (29%, 95% CI − 8–53%). The adjusted effectiveness of two doses of CoronaVac for COVID-19 infection was not significant (21.0%, 95% CI − 20.7–48.3%) but increased significantly with a booster dose of CoronaVac or BNT162b2. One or two doses of the BNT162b2 booster demonstrated higher effectiveness in comparison to a single dose of the CoronaVac booster. These results indicate the need for a booster dose, and heterologous boosting with BNT162b2 may be a better option for higher effectiveness for those who received two doses of CoronaVac. Future studies should evaluate the need for further booster doses and their long-term effects.
Introduction
Previous studies have demonstrated reduced vaccine effectiveness against COVID-19 due to waning vaccine effectiveness over time or the immune-escape properties of the variants when compared to the original strain.Citation1–5 The evidence on the waning protection of COVID-19 vaccines and waning immunity has been reviewed by the World Health Organization (WHO) and has led to consideration of the need for booster doses.Citation6 Israel became the first country in the world to provide COVID-19 booster doses, and afterward other countries have also started to implement booster doses to prevent the rise in COVID-19 cases.Citation7,Citation8
In Turkiye, the CoronoVac vaccine was granted Emergency Use Authorization on January 13, 2021. Initially, healthcare workers (HCWs) were eligible for vaccination, and subsequently, vaccination was extended to other high-risk groups such as the elderly and residents of nursing homes.Citation9,Citation10 In April 2021, the BNT162b2 vaccine was incorporated into the vaccination program and, the choice of vaccine to be administered remained at the individuals’ discretion.Citation11 Turkiye has initiated the administration of the third dose of the vaccine to healthcare workers and individuals aged 50 and above who have already received two doses of the vaccine. The vaccine type of the third dose was also left to the person’s sole discretion and there was no restriction on the selection of the vaccine.Citation12 On the other hand, as certain countries required two doses of m-RNA vaccine to accept passengers, Turkiye administered a second dose of BNT162b2 vaccine upon request to people who received two doses of CoronaVac and one dose of BNT162b2.Citation13 This circumstance has led to various vaccination statuses in the population.
This study aimed to assess the vaccine effectiveness against COVID-19 during the predominance of the Delta variant,Citation14 and the COVID-19 infections among healthcare workers in a university hospital who received various types (inactive or m-RNA) and doses (two to four doses) of COVID-19 vaccines.
Methods
Study design and participants
This retrospective cohort study was conducted between August 1 and November 30, 2021, at a university hospital in Istanbul (Turkiye) with a total of 4067 healthcare workers. The data regarding vaccination status and polymerase chain reaction (PCR) test results were retrieved from the hospital’s medical records with permission from both the Republic of Turkiye’s Ministry of Health and the faculty administration. The demographic data used in the study were obtained from records of faculty administration. Previous studies have shown that education level and occupational exposure to patients infected with COVID-19 are associated with vaccine acceptance.Citation4 Therefore, in the present study, position groups were categorized based on the similarity of healthcare workers in terms of these two components. Of the 4067 healthcare workers, 1057 were excluded from the study for various reasons: being previously infected with COVID-19, taking part in vaccine trials or having only one dose of vaccine.
Six different vaccination statuses were evaluated in the study:
Unvaccinated (UV)
Vaccinated with two doses of CoronaVac (2C)
Vaccinated with two doses of BNT162b2 (2B)
Vaccinated with three doses of CoronaVac (3C)
Vaccinated with two doses of CoronaVac and one dose of BNT162b2 (2C1B)
Vaccinated with two doses of CoronaVac and two doses of BNT162b2 (2C2B)
The study period was divided into two halves: from 1 August to 30 September and from 1 October to 30 November. For an adequate follow-up period, only vaccines administered until September 30th, 2021 were considered when determining vaccination status. HCWs who were vaccinated after September 30 or who had a positive PCR test result were subjected to censorship.
According to vaccination status on September 30, 2021:
Follow-up started on August 1, 2021 for the unvaccinated group.
For other groups, follow-up started either 14 days after receiving the last dose or on 1 August 2021, whichever was later.
The study permission was granted by the Ethics Committee of Istanbul University – Cerrahpasa (Approval number: 327664 Approval date: 02.03.2022). The study was conducted in accordance with the Declaration of Helsinki and was reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement.
Statistical analysis
Statistical analyses were performed by using SPSS v21.0 (SPSS Inc., Chicago, IL, USA). Continuous variables were given as mean ± standard deviation or median (interquartile range), and categorical variables were reported as frequencies and percentages. The Chi-square test was used in the analysis of categorical variables and the Mann-Whitney U test was used in the analysis of continuous variables. The incidence rate (IR) of a group was calculated by dividing the number of COVID-19 positive cases by the cumulative follow-up time of the same group. The unadjusted incidence rate ratio (uIRR) was used to estimate the unadjusted effectiveness (1−uIRR) of the vaccine. Details about uIRR were given in the previous study.Citation4 The Cox regression was used to determine the adjusted (by age, sex, and position) effectiveness of vaccines. The results were given as hazard ratios (HR) and 95% confidence intervals (95% CI). A p-value < .05 was considered statistically significant.
Results
The study included 3,009 healthcare workers of whom 6.7% were unvaccinated. The majority of healthcare workers (51%) received the 2C1B vaccine schedule, followed by 2C2B (12%), 2B (11.7%), 2C (10%), and 3C (8.5%), respectively. The demographics and history of COVID-19 infection among healthcare workers based on their vaccination status are presented in Supplementary Table S1.
During the study period, 5.7% (n = 171) of healthcare workers had a positive PCR result for COVID-19. There was no difference in sex between COVID positive and negative HCWs (p = .658). The median ages of COVID positive and negative HCWs were 34 (26–44) years and 37 (29–47) years, respectively. It was found that COVID positive HCWs were slightly younger than the others (p = .001). The percentage of COVID-19 positivity was highest among nurses (7.5%) and cleaning staff (7.4%) and the lowest among academic staff (2.7%). The highest COVID-19 positivity was found in the unvaccinated group (21.7%) followed by the 2C group (14.2%), while the lowest was found in the 2C2B group (1.4%) (p < .001). The demographic characteristics and vaccination status of COVID-19 positive and negative HCWs were given in .
Table 1. Demographics and vaccination status of COVID negative and positive health care workers.
The incidence rate of SARS-CoV-2 infection was 246.3 per 100.000 person-days in the unvaccinated HCWs, whereas it varied between 18.1 and 174.4 in vaccinated HCWs depending on the type of vaccine. Even though the unadjusted effectiveness for COVID-19 infection appeared to be lowest in the 2C vaccines (29%, 95% CI − 8–53%), this effectiveness was not statistically significant. In contrast, the unadjusted effectiveness for COVID-19 infection was significant for other vaccination groups, of which 3C vaccines demonstrated the lowest unadjusted effectiveness (62%, 95% CI 38–77%) while 2C2B vaccines demonstrated the the highest unadjusted effectiveness (93%, 95% CI 81–97%). presents the incidence rates, unadjusted incidence rate ratios, and unadjusted effectiveness of the vaccines.
Table 2. The incidence rates, unadjusted incidence rate ratios and unadjusted effectiveness of vaccines.
The age-, sex-, and position-adjusted effectiveness for COVID-19 infection was not significant for 2C vaccines (21%, 95% CI − 20.7–48.3%) whereas it was significant for other vaccination groups. Among those, the adjusted effectiveness was the highest in 2C2B vaccines (89.3%, 95% CI 72.2–95.9%) and was the lowest in 3C vaccines (56.4%, 95% CI 26.8–74.0%). The adjusted effectiveness by vaccination status was given in . During the median 120 days of follow-up the cumulative incidence of SARS-CoV-2 infection was 2.8% for HCWs vaccinated with 2C2B, 3.2% for 2C1B, 4.6% for 2B, 11.1% for 3C, and 19.1% for 2C. The cumulative incidence was 23.5% for unvaccinated HCWs ().
Figure 1. The cumulative incidence for COVID-19 infection by vaccination status during the median 120 days of follow-up.
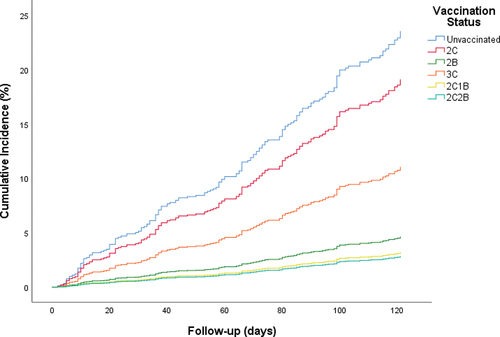
Table 3. Results of cox regression analysis×.
Discussion
This retrospective cohort study was conducted with healthcare workers during the Delta variant predominance. It was determined that two doses of BNT162b2 were effective against COVID-19 infection, while two doses of CoronaVac vaccine required a booster dose to be effective. Furthermore, when CoronaVac and BNT162b2 were compared as booster doses, the effectiveness was higher with BNT162b2.
Clinical evidence suggests that the COVID-19 vaccine protects against the severe symptoms of the disease and is an important tool for reducing the spread of the virus and the infection rate.Citation15 Our study revealed that the highest COVID-19 positivity rate was found in the unvaccinated group (21.7%), while the infection rates for the groups that received two doses of CoronaVac and two doses of BNT162b2 were 14.2% and 4.3%, respectively. In a study among healthcare workers in Israel, BNT162b2 vaccine was associated with a significantly lower incidence of SARS-CoV-2 infection after the second dose compared to no vaccination.Citation16 Both efficacy and effectiveness studies conducted in Turkiye have shown that receiving two doses of CoronaVac vaccine provides protection against symptomatic COVID-19 infection.Citation4,Citation17 On the other hand, in a study conducted in Singapore, it was observed that the group administered two doses of inactivated virus vaccine had lower protection against COVID-19 infection as compared to those administered m-RNA vaccine.Citation18 In the present study, the adjusted effectiveness of two doses of BNT162b2 was higher than two doses of CoronaVac. However, the adjusted effectiveness of two doses of CoronaVac for COVID-19 infection was not statistically significant in our study. This may be due to the predominance of the Delta variant during the study period. This finding is relatively consistent with the results of a study conducted in Azerbaijan within the framework of the WHO’s Unity platform, which reported an effectiveness of 19% (95% CI − 81% to 64%).Citation19
The increasing clinical evidence that indicates waning effectiveness of the vaccine against COVID-19 has led to consideration of the need for booster doses and booster doses have started to be implemented. In our study, HCWs who had completed primary vaccination with two doses of CoronaVac and received a third heterologous dose of BNT162b2 (2C1B) or homologous CoronaVac (3C), or fourth heterologous dose of BNT162b2 (2C2B) were evaluated for their booster effectiveness against COVID-19. It was found that the booster dose of CoronaVac (3C vaccines) had significant effectiveness, while the effectiveness of 2C vaccines for COVID-19 infection was not significant. Moreover, the effectiveness of one booster dose of BNT162b2 (2C1B, 88.0%) or two booster doses of BNT162b2 (2C2B, 89.3%) was higher in comparison to single booster dose of CoronaVac (3C, 56.4%). However, no significant difference was observed between the effectiveness of one booster dose of BNT162b2 compared to two booster doses of BNT162b2. The findings of this study provide further evidence for the need for booster doses, as has been reported in the existing literature.Citation6,Citation7 A study conducted in Chile also recommended administering a booster vaccine dose, whether homologous or heterologous, after completion of the primary vaccination with CoronaVac, as it resulted in a higher effectiveness against COVID-19. Similar to our study, it also demonstrated a superior effectiveness of the BNT162b2 booster over the CoronaVac booster (96.5% vs 78.8%).Citation20 In a test-negative study, the effectiveness of homologous and heterologous BNT162b2 and CoronaVac booster vaccination against SARS-CoV-2 infection was evaluated in predominant-Delta and predominant-Omicron periods. In both periods, homologous CoronaVac boosting demonstrated a lower effectiveness than heterologous boosting for CoronaVac. The study suggested that heterologous boosting using BNT162b2 should be preferred for inactivated primary vaccination recipients.Citation21 Furthermore, several immunological studies from various countries have indicated that the BNT162b2 booster stimulates a significantly higher and longer-lasting immune response compared to the CoronaVac booster.Citation22–25 In a randomized, observer-masked, controlled trial which was published in January 2023, showed that heterologous boosting with BNT162b2 induced a higher immune response than homologous boosting with CoronaVac.Citation26
Although there have been several studies in Turkiye investigating the impact of booster doses on the immune response,Citation25,Citation27–29 only a limited number have examined their effectiveness against COVID-19 or their effect on infection rates.Citation30–32 One such study, conducted at an Adult Vaccine Center in Turkiye, evaluated the relative effectiveness of a third dose of CoronaVac or BNT162b2 following two doses of CoronaVac. The study revealed that the effectiveness of the third dose of CoronaVac against symptomatic COVID-19 was 58.24%, whereas the effectiveness of the third dose of BNT162b2 was 87.27%. Despite the similarity of our findings (56.4% vs 88.0%), the effectiveness of the study has not been adjusted for significant confounding factors such as age and sex, nor has it been evaluated separately for predominant variants (alpha and delta) observed during the study period.Citation32 The fact that Delta was the only dominant variant during our study period and that the effectiveness was adjusted for age, sex, and position constitutes the strengths of our study.
The present study has several limitations. First, it is a single-center retrospective cohort study conducted exclusively on healthcare workers, which may limit the generalizability of our findings to the general population. Second, since demographic data were obtained from the hospital database, confounding factors such as comorbidity and compliance with prevention recommendations could not be questioned. Third, it is possible that asymptomatic cases without PCR tests were included in our cohort, as the hospital lacked a screening protocol for SARS-CoV-2 infection. However, it is unlikely that this situation introduced any bias since it was present across all vaccine groups in the study. The final limitations concern the time since the last dose and SARS-CoV-2 variants. To evaluate effectiveness in a period when a single variant was dominant, the study period was selected as 1 August − 30 November. The follow-up period started 14 days after receiving the last dose or on August 1, 2021, whichever was later. Since antibody levels were not evaluated in the study, it is not known if there was any decrease in antibody levels from the last dose to the beginning of the follow-up period. On the other hand, our results indicate the effectiveness of vaccines against the Delta variant, but the effectiveness of vaccines may differ against existing variants such as Omicron or ErisCitation33 or in current pandemic conditions.
Conclusion
In this study, conducted during the predominance of the Delta variant, the effectiveness of two doses of CoronaVac against COVID-19 infection was not significant but increased significantly with a booster dose with CoronaVac or BNT162b2. The effectiveness was higher in HCWs who received one or two doses of BNT162b2 as a booster dose. These findings suggest that a booster dose is needed, and that heterologous boosting with BNT162b2 may be a better choice for higher effectiveness for those fully vaccinated with CoronaVac. However, these results must be confirmed by research designs with a higher level of evidence. On the other hand, there was no significant difference in effectiveness between one and two booster doses of BNT162b2. It is recommended that future studies evaluate the need for further booster doses and their long-term effects.
Author contributions
HCÇ, İİB, RK, NS and GC designed the study. EŞ, BB, YK collated the data. HCÇ, US and GC performed the statistical analysis. HCÇ, SNA, AU and US interpreted the results. HCÇ, SNA, and AU wrote the manuscript. EŞ and BB created the tables and figures. İİB, RK, and YK edited the manuscript. HCÇ, SNA, AU and US revised the manuscript. GC and NS supervised the whole study. All authors read and approved the final manuscript.
Ethics approval statement
The study permission was given by the Ethics Committee of Istanbul University – Cerrahpasa (Approval number: 327664 Approval date: 02.03.2022). The study was conducted in accordance with the Declaration of Helsinki and it was reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement.
Supplemental Material
Download PDF (300.2 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data and materials that have been used in this current study can be obtained by making a reasonable request to the corresponding author https://dataverse.harvard.edu/dataset.xhtml?persistentId=doi:10.7910/DVN/GM57Q4.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2275445.
Additional information
Funding
References
- Nanishi E, Levy O, Ozonoff A. Waning effectiveness of SARS-CoV-2 mRNA vaccines in older adults: a rapid review. Hum Vaccin Immunother. 2022;18(5):2045857. doi:10.1080/21645515.2022.2045857.
- Kodera S, Rashed EA, Hirata A. Estimation of real-world vaccination effectiveness of mRNA COVID-19 vaccines against delta and omicron variants in Japan. Vaccines (Basel). 2022;10(3):430. PMID: 35335062; PMCID: PMC8953128. doi:10.3390/vaccines10030430.
- Tartof SY, Slezak JM, Fischer H, Hong V, Ackerson BK, Ranasinghe ON, Frankland TB, Ogun OA, Zamparo JM, Gray S, et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398(10309):1407–6. doi:10.1016/S0140-6736(21)02183-8.
- Can G, Acar HC, Aydin SN, Balkan II, Karaali R, Budak B, Saltoglu N. Waning effectiveness of CoronaVac in real life: a retrospective cohort study in health care workers. Vaccine. 2022;40(18):2574–9. doi:10.1016/j.vaccine.2022.03.032.
- Fiolet T, Kherabi Y, MacDonald CJ, Ghosn J, Peiffer-Smadja N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: a narrative review. Clin Microbiol Infect. 2022;28(2):202–21. doi:10.1016/j.cmi.2021.10.005.
- WHO. Interim statement on COVID-19 vaccine booster doses. [accessed 2022 Apr 20]. https://www.who.int/news/item/10-08-2021-interim-statement-on-covid-19-vaccinebooster-doses.
- Juno JA, Wheatley AK. Boosting immunity to COVID-19 vaccines. Nat Med. 2021;27(11):1874–5. doi:10.1038/s41591-021-01560-x.
- Andrews N, Stowe J, Kirsebom F, Toffa S, Sachdeva R, Gower C, Ramsay M, Lopez Bernal J. Effectiveness of COVID-19 booster vaccines against COVID-19-related symptoms, hospitalization and death in England. Nat Med. 2022;28(4):831–7. doi:10.1038/s41591-022-01699-1.
- Turkish medicines and medical devices agency. [accessed 2022 Apr 20]. https://www.titck.gov.tr/haber/kamuoyunun-dikkatine-13012021185623.
- Turkey Ministry of Health. List of COVID-19 vaccination groups. [accessed 2022 Apr 20]. https://covid19asi.saglik.gov.tr/EN-80295/list-of-covid-19-vaccination-groups.html.
- Turkey Ministry of Health. [accessed 2022 Apr 20]. https://www.saglik.gov.tr/EN,81812/minister-koca-receives-questions-from-parliamentary-reporters.html.
- Turkey Ministry of Health. [accessed 2022 Apr 20]. https://www.saglik.gov.tr/TR,84343/koronavirus-bilim-kurulu-toplantisina-iliskin-aciklama-30062021.html.
- Reuters. [accessed 2022 Apr 20]. https://www.reuters.com/world/middle-east/turkey-offering-extra-pfizer-shots-those-wanting-travel-2021-08-16/.
- DeGrace MM, Ghedin E, Frieman MB, Krammer F, Grifoni A, Alisoltani A, Alter G, Amara RR, Baric RS, Barouch DH, et al. Defining the risk of SARS-CoV-2 variants on immune protection. Nature. 2022;605(7911):640–52. doi:10.1038/s41586-022-04690-5.
- Vitiello A, Ferrara F, Troiano V, La Porta R. COVID-19 vaccines and decreased transmission of SARS-CoV-2. Inflammopharmacology. 2021;29(5):1357–60. doi:10.1007/s10787-021-00847-2.
- Angel Y, Spitzer A, Henig O, Saiag E, Sprecher E, Padova H, Ben-Ami R. Association between vaccination with BNT162b2 and incidence of symptomatic and asymptomatic SARS-CoV-2 infections among health care workers. JAMA. 2021;325(24):2457–65. doi:10.1001/jama.2021.7152.
- Tanriover MD, Doğanay HL, Akova M, Güner HR, Azap A, Akhan S, Köse Ş, Erdinç FŞ, Akalın EH, Tabak ÖF, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021;398(10296):213–22. doi:10.1016/S0140-6736(21)01429-X.
- Premikha M, Chiew CJ, Wei WE, Leo YS, Ong B, Lye DC, Lee VJ, Tan KB. Comparative effectiveness of mRNA and inactivated whole-virus vaccines against coronavirus disease 2019 infection and severe disease in Singapore. Clin Infect Dis. 2022;75(8):1442–5. doi:10.1093/cid/ciac288.
- Katz MA, Rojas Castro MY, Seyidov N, Herdman MT, Mehdiyev S, McKnight CJ, Guseinova A, Cojocaru R, Doran J, Mühlemann B, et al. The effectiveness of primary series CoronaVac vaccine in preventing COVID-19 illness: a prospective cohort study among healthcare workers in Azerbaijan, May–November 2021. Influenza Other Respir Viruses. 2023;17(10):e13147. doi:10.1111/irv.13147.
- Jara A, Undurraga EA, Zubizarreta JR, González C, Pizarro A, Acevedo J, Leo K, Paredes F, Bralic T, Vergara V, et al. Effectiveness of homologous and heterologous booster doses for an inactivated SARS-CoV-2 vaccine: a large-scale prospective cohort study. Lancet Glob Health. 2022;10(6):e798–e806. doi:10.1016/S2214-109X(22)00112-7.
- Suah JL, Tng BH, Tok PSK, Husin M, Thevananthan T, Peariasamy KM, Sivasampu S. Real-world effectiveness of homologous and heterologous BNT162b2, CoronaVac, and AZD1222 booster vaccination against delta and omicron SARS-CoV-2 infection. Emerg Microbes Infect. 2022;11(1):1343–5. doi:10.1080/22221751.2022.2072773.
- Barin B, Kasap U, Selçuk F, Volkan E, Uluçkan Ö. Comparison of SARS-CoV-2 anti-spike receptor binding domain IgG antibody responses after CoronaVac, BNT162b2, ChAdOx1 COVID-19 vaccines, and a single booster dose: a prospective, longitudinal population-based study. Lancet Microbe. 2022;3(4):e274–e83. doi:10.1016/S2666-5247(21)00305-0.
- Costa Clemens SA, Weckx L, Clemens R, Almeida Mendes AV, Ramos Souza A, Silveira MBV, da Guarda SNF, de Nobrega MM, de Moraes Pinto MI, Gonzalez IGS, et al. Heterologous versus homologous COVID-19 booster vaccination in previous recipients of two doses of CoronaVac COVID-19 vaccine in Brazil (RHH-001): a phase 4, non-inferiority, single blind, randomised study. Lancet. 2022;399(10324):521–9. doi:10.1016/S0140-6736(22)00094-0.
- Vargas L, Valdivieso N, Tempio F, Simon V, Sauma D, Valenzuela L, Beltrán C, Castillo-Delgado L, Contreras-Benavides X, Acevedo ML, et al. Serological study of CoronaVac vaccine and booster doses in Chile: immunogenicity and persistence of anti-SARS-CoV-2 spike antibodies. BMC Med. 2022;20(1):216. doi:10.1186/s12916-022-02406-0.
- Yavuz E, Günal Ö, Başbulut E, Şen A. SARS-CoV-2 specific antibody responses in healthcare workers after a third booster dose of CoronaVac or BNT162b2 vaccine. J Med Virol. 2022;94(8):3768–75. doi:10.1002/jmv.27794.
- Fadlyana E, Setiabudi D, Kartasasmita CB, Putri ND, Rezeki Hadinegoro S, Mulholland K. BCOV21 study group. Immunogenicity and safety in healthy adults of full dose versus half doses of COVID-19 vaccine (ChAdox1-S or BNT162b2) or full-dose CoronaVac administered as a booster dose after priming with CoronaVac: a randomised, observer-masked, controlled trial in Indonesia. Lancet Infect Dis. 2023;23(5):S1473-3099(22)00800–3. doi:10.1016/S1473-3099(22)00800-3.
- Saltoğlu N, Dinç HÖ, Balkan İİ, Can G, Özbey D, Beytur AN, Keskin E, Budak B, Aydoğan O, Mete B, et al. Heterologous booster COVID-19 vaccination elicited potent immune responses in HCWs. Diagn Microbiol Infect Dis. 2022;104(2):115758. doi:10.1016/j.diagmicrobio.2022.115758.
- Çağlayan D, Süner AF, Şiyve N, Güzel I, Irmak Ç, Işik E, Appak Ö, Çelik M, Öztürk G, Alp Çavuş S, et al. An analysis of antibody response following the second dose of CoronaVac and humoral response after booster dose with BNT162b2 or CoronaVac among healthcare workers in Turkey. J Med Virol. 2022;94(5):2212–21. doi:10.1002/jmv.27620.
- Gareayaghi N, Demirci M, Ozbey D, Dasdemir F, Dinc HO, Balkan II, Saribas S, Saltoglu N, Kocazeybek B. Comparison of SARS-CoV-2 antibody levels after a third heterologous and homologous BNT162b2 booster dose. Vaccines (Basel). 2022;10(10):1672. doi:10.3390/vaccines10101672.
- Uzun O, Akpolat T, Varol A, Turan S, Bektas SG, Cetinkaya PD, Dursun M, Bakan N, Ketencioglu BB, Bayrak M, et al. COVID-19: vaccination vs. hospitalization. Infection. 2022;50(3):747–52. doi:10.1007/s15010-021-01751-1.
- Aykurt O. İki Doz Coronavac Aşısı Sonrası Heterolog veya Homolog Rapel Dozun Etkinliği, Çankırı Örneği. Avrasya Sağlık Bilimleri Dergisi. 2022;6(1):16–20. doi:10.53493/avrasyasbd.1157196.
- Sonmezer MC, Dizman GT, Erul E, Sahin TK, Saricaoglu T, Alp A, Tanriover MD, Uzun O, Unal S, Akova M, et al. Relative vaccine effectiveness of the third dose of CoronaVac or BNT162b2 following a two-dose CoronaVac regimen: a prospective observational cohort study from an adult vaccine center in Turkey. Vaccines (Basel). 2022;10(7):1140. doi:10.3390/vaccines10071140.
- Parums DV. Editorial: a rapid global increase in COVID-19 is due to the emergence of the EG.5 (Eris) subvariant of omicron SARS-CoV-2. Med Sci Monit. 2023;29:e942244. doi:10.12659/MSM.942244.
