ABSTRACT
A serum-free, highly purified rabies vaccine produced in Vero cells is under development. The initial formulation, PVRV-NG, was evaluated in five Phase II studies and subsequently reformulated (PVRV-NG2). This multicenter, observer-blinded Phase II study investigated the safety and immune response of three different doses (antigen content) of PVRV-NG2 versus a licensed human diploid cell rabies vaccine (HDCV; Imovax rabies®). Healthy adults (N = 320) were randomized to receive PVRV-NG2 (low, medium, or high dose), PVRV-NG, or HDCV (2:2:2:1:1 ratio), according to a five-dose Essen simulated post-exposure regimen (Days [D] 0, 3, 7, 14, and 28). All participants received human rabies immunoglobulin intramuscularly on D0. Immunogenicity was assessed at D0, 14, 28, 42, and 6 months after the final injection using the rapid fluorescent focus inhibition test. Seroconversion rates were calculated as the percentage of participants achieving rabies virus neutralizing antibody titers ≥0.5 IU/mL. All analyses were descriptive. At each timepoint, geometric mean titers (GMTs) increased with antigen content (measured using an enzyme-linked immunosorbent assay). High-dose PVRV-NG2 GMTs were the highest at all timepoints, medium-dose PVRV-NG2 GMTs were similar to those with HDCV, and low-dose PVRV-NG2 GMTs were similar to PVRV-NG. The safety profile of PVRV-NG2 was comparable to PVRV-NG; however, fewer injection site reactions were reported with PVRV-NG2 or PVRV-NG (range 36.7–47.5%) than with HDCV (61.5%). This study demonstrated a dose–effect of antigen content at all timepoints. As post-exposure prophylaxis, the safety and immunogenicity profiles of the high-dose PVRV-NG2 group compared favorably with HDCV. Clinicaltrials.gov number: NCT03145766.
Introduction
The World Health Organization (WHO) estimates that there are over 59,000 deaths worldwide each year due to rabies following lyssavirus infection.Citation1–3 Nevertheless, human rabies is preventable by vaccination when used as part of pre- or post-exposure prophylaxis for participants with a high risk of exposure.Citation4 Despite over 29 million people receiving post-exposure prophylaxis (PEP) every year, many exposed individuals do not have access to effective vaccines.Citation4 With approximately 95% of deaths due to rabies occurring in Africa and Asia,Citation4 rabies prevention is highly dependent on raising disease awareness and improving access to vaccines and rabies immunoglobulin (RIG) for high-risk populations.Citation3,Citation5,Citation6
The development of rabies vaccines for human use has progressed over time, with new technological approaches to vaccine production driving their continued improvement.Citation7 A serum- and antibiotic-free, highly purified, freeze-dried Vero cell rabies vaccine candidate (PVRV-NG) was developed using the same Pitman-Moore viral strain as the already licensed HDCV (Imovax® rabies, Sanofi) and PVRV (Verorab®, Sanofi) Vero cell rabies vaccines. No components of animal or human origin are introduced during the manufacturing process for PVRV-NG, which eliminates the risk of contamination by adventitious agents.Citation8,Citation9 PVRV-NG has reduced residual DNA content (<100 pg/dose) compared with the licensed Vero cell rabies vaccine, is antibiotic-free and displays a higher purity profile.Citation10 Two formulations of PVRV-NG have been developed (PVRV-NG and PVRV-NG2). The PVRV-NG vaccine has been investigated in pre- and post-exposure settings within five clinical trials in Europe (VRV01: NCT00948272),Citation11 China (VRV08: NCT01339312),Citation10 the US (VRV02: NCT01784874Citation12 and VRV04: NCT01877395), and the Philippines (VRV06: NCT01930357).Citation13 Notably, in four of these studies (VRV01, VRV02, VRV06, and VRV08), PVRV-NG demonstrated non-inferiority to the licensed PVRV or HDCV. Furthermore, in the VRV01 and VRV08 studies, PVRV-NG displayed similar immunogenic and safety profiles to PVRV;Citation10,Citation11 however, since non-inferiority to HDCV was not demonstrated in VRV04,Citation12 PVRV-NG2 was developed with increased antigen content compared with PVRV-NG.
This Phase II study was designed to investigate the antigen dose response of the PVRV-NG2 formulation to enable selection of a desired dose to be further evaluated in Phase III studies. PVRV-NG and a licensed HDCV were included as control vaccines.
Methods
This observer-blind, controlled, randomized Phase II study was conducted in five US centers (www.clinicaltrials.gov identifier: NCT03145766; VRV11).
Participants
Participants were eligible for inclusion if they were aged 18–64 years, had a body mass index of 18.5–30 kg/m2, and were able to attend all scheduled visits and comply with all study procedures. Key exclusion criteria included any vaccination in the 4 weeks preceding the first study vaccination or planned prior to Day (D) 42; any previous rabies vaccination; receipt of immunoglobulins, blood or blood-derived products within the past 3 months; at high risk of rabies infection during the study; known or suspected congenital or acquired immunodeficiency; immunosuppressive therapy within the preceding 6 months; long-term systemic corticosteroid therapy; known systemic hypersensitivity to any of the vaccine or human rabies immunoglobulins (HRIG) components; history of a life-threatening reaction to a vaccine containing any of the same substances as the study vaccines.
Ethics
This study was conducted in accordance with the Declaration of Helsinki and International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) guidelines for Good Clinical Practice. All participants provided informed consent prior to study inclusion.
Interventions
PVRV-NG, PVRV-NG2, and HDCV (Sanofi, Marcy-l’Étoile, France) are derived from the same Wistar Rabies Virus Pitman Moore/WI 38 1503-3 M strain and all tested batches of each vaccine fulfill the national Institutes of Health (NIH) potency of ≥2.5 IU per dose.
The three PVRV-NG2 formulations were prepared with different levels of antigen content: ‘low’ (antigen content 3.8 IU/dose; batch number: S4485F01), ‘medium’ (antigen content 5.4 IU/dose; batch number: S4484F01), or ‘high’ (antigen content 7.6 IU/dose; batch number: S4483F01). For PVRV-NG (batch number: S4486S01) and HDCV (batch number: K14141M), the actual antigen content was 3.3 IU/dose and 6.3 IU/dose, respectively. The antigen content of each vaccine was measured by enzyme-linked immunosorbent assay (ELISA), as previously described.Citation14,Citation15
All vaccines were provided as freeze-dried powders and were reconstituted immediately before use with 0.5 mL saline diluent for PVRV-NG and PVRV-NG2 and 1.0 mL sterile water for HDCV. Vaccines were administered intramuscularly (IM) into the deltoid muscle on alternate sides in accordance with prescribing information.Citation16 Participants also received concomitant HRIG (Imogam® Rabies – HT #1361; Sanofi, Marcy-l’Étoile, France) on D0, which was supplied as a ready-to-use 2.0 mL solution at a minimum concentration of 150 IU/mL, for injection. HRIG was administered IM at a site distant from vaccine injection (anterolateral thigh) as recommended.Citation17 A maximum of 5.0 mL was to be injected, with the total volume divided and administered at separate sites (at least 3 cm apart). The vaccine schedule complied with United States Advisory Committee on Immunization Practice (US ACIP)Citation18 and WHO recommendationsCitation17 at the time of study conduct.
Study design
Participants were allocated in a 2:2:2:1:1 ratio into one of the five vaccine groups: low-dose PVRV-NG2, medium-dose PVRV-NG2, high-dose PVRV-NG2, PVRV-NG, or HDCV. Randomization was performed using the permuted block method with stratification on centers and was managed by an interactive response technology system, which provided a vaccine dose identification number for each enrolled participant. The study was conducted in an observer-blind manner. Vaccines were prepared and administered by unblinded qualified staff members who were not authorized to collect safety data. Investigators were blinded to the PVRV-NG and PVRV-NG2 groups, but not the HDCV group.
Participants received a vaccine injection on D0, D3, D7, D14, and D28, as per the PEP 5-dose Essen regimen,Citation19 and were followed for 6 months after the final injection.
For immunogenicity assessments, blood samples were collected prior to vaccination (D0) and on D14, D28, D42, and 6 months after the final injection.
Participants recorded safety data using diary cards. Data on solicited and unsolicited injection site and systemic reactions were collected (including immediate reactions occurring within 30 minutes of a vaccination). Safety data were collected after each vaccination, and up to 28 days after the complete vaccination series. Adverse reactions (ARs) were defined as adverse events (AEs) which were considered at least possibly related to the vaccine and coded using the Medical Dictionary for Regulatory Activities (MedDRA).Citation20 Serious AEs (SAEs) were recorded for all participants up to 6 months after the final injection.
Objectives and endpoints
The objectives of this study were to describe the immune responses and safety of increasing doses of PVRV-NG2, in comparison with PVRV-NG and HDCV. Endpoints included rabies virus neutralizing antibody (RVNA) titers, which were used to calculate percentage of subjects who achieved post-vaccination RVNA titers ≥0.5 IU/mL as defined by the WHO;Citation17 geometric mean titers (GMTs), and ratios of GMTs between vaccine groups; and complete or incomplete virus neutralization at the starting dilution in the rapid fluorescent focus inhibition test (RFFIT) assay, as defined by the US ACIP.Citation18 Safety endpoints included unsolicited systemic AEs within 30 minutes after each vaccine administration and solicited and unsolicited injection site and systemic adverse reactions or AEs.
Statistical analysis
This study was designed to be descriptive. Point estimates and 95% confidence intervals (CIs) were calculated for all endpoints. The full analysis set (FAS) was defined as the subset of randomized participants who received at least one dose of the study vaccine. A modified FAS (mFAS) was defined post-hoc (considering only those with a baseline RVNA titer <0.5 IU/mL) to limit the impact of corresponding post-vaccination high values on the immune response assessment. The safety analysis sets (SafAS) were defined for each vaccine injection as subsets of participants who received the corresponding dose. The per-protocol analysis set (PPAS) was a subset of the FAS and was defined based on the D14 timepoint. Exclusions from this set included participants with protocol or inclusion/exclusion criteria violations, a baseline RVNA titer higher than the lower limit of quantitation (LLOQ), an incorrect number of doses or type of vaccine for the first three doses, and incorrectly prepared/administered vaccines. Immunogenicity analysis was performed on the FAS and PPAS.
For the calculation of GMTs and ratios of GMTs, the RVNA titers were log-transformed, means and difference of means were computed, respectively, and normal approximation was used to calculate the 95% CI. GMT ratios were calculated at D14, D28, and D42 for the following between-group comparisons: each PVRV-NG2 group (low, medium, and high dose) and PVRV-NG versus HDCV vaccine; each PVRV-NG2 group (low, medium, and high dose) versus PVRV-NG; PVRV-NG2 medium and high doses versus low dose; and PVRV-NG2 high versus medium dose.
In a post-hoc exploratory analysis, a regression model was used to determine the effect of the PVRV-NG2 antigen content on the immunogenicity response (log-transformed RVNA titer) at different timepoints in the mFAS. A significance level of 0.05 was applied.
Additionally, a post-hoc analysis of complete/incomplete neutralization was conducted among a subset of participants with a determined neutralization status.
Sample size
A limited number of 80 participants per PVRV-NG2 group and 40 participants in the PVRVNG and HDCV groups were defined. If more than 95–100% of patients achieved RVNA titer ≥0.5 IU/mL at D14 in each PVRV-NG2 group, the 95% CIs around the point estimate would be between 87.7–98.6 and 95.5–100. Additionally, the 95% CIs would be between 4.4–18.8 and 20.3–41.3 for observed safety incidence rates between 10% and 30%.
Laboratory methods
RVNA was measured using the RFFIT method, with laboratory personnel blinded to the sample treatments. The RFFIT method was performed according to the protocols of Smith, et al.Citation21 and Timiryasova, et al.Citation22 Two-fold dilutions of test serum samples and controls were incubated with a fixed pre-determined amount of Challenge Virus Standard-11 strain of rabies virus prior to addition of diethylaminoethyl-treated BHK-21 cells. After incubation, un-neutralized rabies virus was detected using a fluorescein isothiocyanate conjugated anti-rabies nucleocapsid monoclonal antibody (Merck, catalog #5500). The RVNA titer at the median effective dose (ED50) was mathematically interpolated.Citation23 The LLOQ of the assay was 0.2 IU/mL. Each serum sample was tested in two independent assay runs, with each value reported. The geometric mean was then calculated for immunogenicity analysis according to rules governing the handling of extreme values: if a value was <LLOQ, then the computed value LLOQ/2 was used; if a value was ≥LLOQ, then the value itself was used. Titers were determined to the endpoint value, and samples with very high antibody levels were pre-diluted and retested. The rabies RFFIT method was validated in accordance with the ICH guidelinesCitation24 and demonstrated to be precise, accurate, linear, specific, and robust to quantitate specific RVNA levels in human serum samples. The conversion from titer to IU/mL was calculated using a WHO-1 standard rabies immunoglobulin reference.
Results
Participants
Between April 17 and May 19, 2017, 320 participants (FAS) were included in the study and randomized (). Six-month follow-up was completed by January 8, 2018. In total, 279 (87.2%) participants completed the study. Twenty-one (6.6%) participants did not complete the active phase of the study (prior to D56): six in the low-dose PVRV-NG2 group, three in the medium-dose PVRV-NG2 group, two in the high-dose PVRV-NG2 group, seven in the PVRV-NG group, and three in the HDCV group ().
Figure 1. Participant flow from visits 1–8 for all groups.
Baseline characteristics were similar across study groups (). About half of the participants were male (50.6%) and the majority were Caucasian/white (67.8%); the mean age (standard deviation [SD]) of participants was 41.4 (13.5) years. Six participants had a baseline RVNA titer ≥0.5 IU/mL, and one had invalid results at the first visit and high post-vaccination titers (142.7 and 108.2 IU/mL as duplicates at V04) and was presumed to have existing titers at baseline. These seven participants were therefore excluded from the mFAS population, resulting in a total of 313 participants in the mFAS. The PPAS population comprised 287 participants and the SafAS populations comprised 320 participants.
Table 1. Baseline characteristics for the full analysis set (N = 320).
Immunogenicity
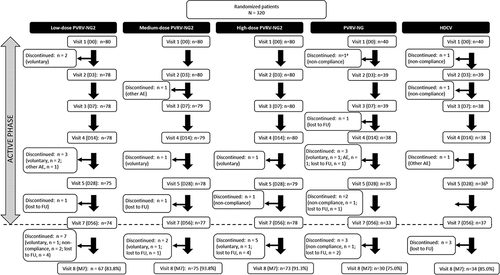
Across the PVRV-NG2 groups, GMTs ranged from 1.28 (95% CI 0.944, 1.73) in the low-dose group to 2.52 (95% CI 1.93, 3.28) in the high-dose group at D14 and increased through D42 (). A strong dose–response relationship between the antigen content (as measured by ELISA) and GMTs was observed for all batches, at all timepoints. Notably, GMTs in the high-dose PVRV-NG2 group were consistently higher than other vaccine groups, including HDCV, at all timepoints (); this observation persisted for 6 months after the final injection. The highest GMTs were obtained with high-dose PVRV-NG2, followed by HDCV (), with GMT ratios between these two groups in the range of 1.30–1.54 at each timepoint (). At all timepoints, GMTs were higher in the high-dose PVRV-NG2 group than in the PVRV-NG group (i.e., 95% CI lower bounds of the PVRV-NG2/PVRV-NG GMT ratios were higher than 1) and were numerically higher compared with the HDCV group, albeit with 95% CIs of the GMT ratios including 1. GMTs were comparable between the medium-dose PVRV-NG2 and HDCV groups (range 0.999–1.19), and between the low-dose PVRV-NG2 and PVRV-NG groups (range 0.958–1.18).
Figure 2. Post-vaccination geometric mean rabies virus neutralizing antibody (RVNA), determined by RFFIT (modified full analysis set).
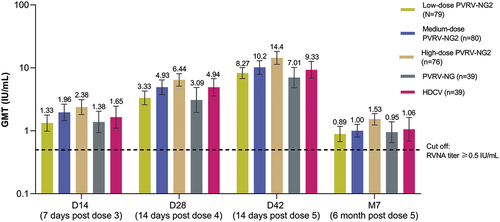
Table 2. Immunogenicity summary of RVNA titers and GMTs on D14, 28, and 42 (per protocol analysis set).
Table 3. Ratio of geometric mean titer (GMT) between groups on D14, 28, and 42 (modified full analysis set).
The proportion of participants with RVNA titers ≥0.5 IU/mL in the PVRV-NG2 groups ranged from 75.0% in the low-dose group to 90.1% in the high-dose group at D14, indicating a positive dose–response relationship, although the 95% CIs of these point estimates overlapped (). The proportion of participants with RVNA titers ≥0.5 IU/mL increased through D42, with the dose–response effect evident at all timepoints; this was confirmed with the regression model (p-values: 0.0062, 0.0004, 0.0008, and 0.0017 on D14, D28, D42, and 6 months after the final injection, respectively). In both the high-dose PVRV-NG2 and HDCV groups, RVNA titers ≥0.5 IU/mL were achieved by 100% of participants on both D28 and D42, and titers remained ≥0.5 IU/mL 6 months after the final injection in most participants (); the dose–response effect was less pronounced at later timepoints since almost all participants across all groups had RVNA titers ≥0.5 IU/mL.
Figure 3. Post-vaccination: rabies virus neutralizing antibody (RVNA) titers determined by RFFIT≥0.5 IU/mL (per-protocol analysis set).
Table 4. Immunogenicity summary of RVNA titers 6 months after the final injection in participants with a baseline titer <0.5 IU/mL (modified full analysis set).
Overall, 10–12% of participants did not achieve RVNA titers ≥0.5 IU/mL 14 days post-vaccination. Three participants also failed to exceed a RVNA titer of 0.5 IU/mL by D42 (one participant in each of the medium-dose PVRV-NG2 and PVRV-NG groups, and one participant in the high-dose PVRV-NG2 group who was receiving immunosuppressive treatment at the time of study participation [excluded from the PPAS; protocol deviation]) ().
Evaluation of the immune response with complete neutralization at the 1:5 serum dilution (corresponding to 0.1–0.3 IU/mL at the lowest concentration)Citation25 showed adequate response to vaccination for PVRV-NG2 versus HDCV (). In the high-dose PVRV-NG2 and HDCV groups, among the subset of participants with a determined neutralization status, complete neutralization (100%) was reached at D14, D28, and D42. Six months after the final injection, complete neutralization (100%) was reported in all participants with high-dose PVRV-NG2.
Table 5. Number of patients with determined virus neutralization results and achieving complete neutralization at 1:5 dilution in RFFIT (per-protocol analysis set).
Safety
The overall rate of solicited reactions was similar across all PVRV-NG and PVRV-NG2 groups (range 47.5–55.0%) and was lower than in the HDCV group (71.8%) (). This trend was observed for both solicited injection site and systemic reactions (). The most common injection site reaction was pain, and the most commonly reported systemic reactions were headache, malaise, and myalgia.
Table 6. Safety profile of low-, medium-, and high-doses of PVRV-NG2, PVRV-NG, or HDCV for solicited and unsolicited reactions (safety analysis set).
In the PVRV-NG2 groups, the proportion of participants reporting at least one solicited injection site reaction slightly increased in proportion with antigen content (). Solicited reactions were most frequently reported after the first injection in all vaccine groups (except for low-dose PVRV-NG2 and injection site reactions), and most resolved spontaneously within 1–3 days. Rates of unsolicited AEs were also lower in all PVRV-NG and PVRV-NG2 groups (range 13.8–25.6%) than with HDCV (43.9%).
Similarly, rates of unsolicited ARs were lower across all the PVRV-NG and PVRV-NG2 groups (range 2.5–6.3%) compared with the HDCV group (19.5%). Unsolicited ARs included abdominal pain, injection site bruising, pruritus, paresthesia and hemorrhage, presyncope, rash, diarrhea, vomiting, dizziness, fatigue, and nausea; most were Grade 1 or 2. Five participants discontinued due to AEs: one participant in each of the low- (abdominal pain) and medium- (peripheral edema, muscle spasms, peripheral and local swelling, limb discomfort, chest pain, and fibrin D-dimer increase) dose PVRV-NG2 groups, one in the PVRV-NG (pleurisy) group, and two in the HDCV group (muscle pain, bacterial enteritis). Only one AE (abdominal pain in one participant receiving low-dose PVRV-NG2) was considered related to the vaccine. One SAE was reported in a participant with a history of diabetes who experienced facial paralysis 23 days after receiving the fifth dose of high-dose PVRV-NG2. This event, which resolved with sequelae, was considered unrelated to the vaccine.
Discussion
This Phase II study showed that the PVRV-NG2 rabies vaccine candidate is immunogenic and well tolerated in a simulated PEP setting, where HRIG was administered IM concomitantly with the first vaccine injection. The increased antigen dose of PVRV-NG2 increased antibody response, and this dose–response relationship was detected at all timepoints up to 6 months after the final injection. To our knowledge, these observations of a dose–response relationship for a rabies vaccine are novel; prior meta-analysis by Sudarshan et al. explored historical data on the relationship between antigenicity and immunogenicity of ten cell culture human rabies vaccines administered at one center between 1993 and 2004 but not in a randomized controlled study.Citation26 In our study, high-dose PVRV-NG2 compared favorably with the HDCV vaccine in terms of immune response, with higher GMTs and GMTRs noted at each timepoint, a comparable proportion of participants displaying RVNA titers ≥0.5 IU/mL and fewer reports of ARs.
In this study, 10–12% of participants did not achieve RVNA titers ≥0.5 IU/mL 14 days post-vaccination, with similar proportions observed in both the high-dose PVRV-NG2 and the licensed vaccine HDCV groups. However, all participants achieved complete neutralization at the 1:5 serum dilution as early as 14 days post-vaccination, as per the US ACIP criterion of adequate response to vaccination. These results are consistent with other studies that have evaluated licensed vaccines using the same regimen in healthy participants or in those with suspected rabies exposure,Citation27–34 and can be explained by different factors, including HRIG interference, variations in threshold definition and differences between guidelines and RFFIT test variability.
In this study, the RVNA results observed at D14 were likely due to co-administration of HRIG with the first vaccine injection on D0, which may have led to a delayed immune response in some participants. This effect has been observed in several studies, which noted that co-administration of licensed rabies vaccines with HRIG resulted in rates of 91–99% of patients achieving an RVNA titer ≥0.5 IU/mL at D14, without impact on the effectiveness of the vaccines.Citation29–Citation30-34–Citation37 Currently, WHO guidelines recommend that RIGs be injected only around the wound in exposed patients, with IM injection of the remaining rabies RIG no longer recommended.Citation17 As such, the co-administration of HRIG in this simulated PEP regimen was the most stringent evaluation of the rabies vaccines since a full dose was injected into the participants’ adjacent thigh – with the practice of delivering excess HRIG by IM at a site distant from vaccine administration adopted globally.Citation38,Citation39
Protection against rabies correlates with the presence of rabies-specific virus-neutralizing antibodies.Citation40 Historically, an RNVA titer of 0.5 IU/mL by D14 is considered proof that a PEP regimen produces an adequate immune response,Citation40 with this threshold level accepted by the WHO as a study endpoint for clinical trials of novel rabies vaccines.Citation17,Citation35,Citation41 Although RVNA levels are considered the main indicator of immunity, they do not represent the totality of immune response to the vaccine as low titers are not necessarily indicative of low immunity. Studies have also highlighted the involvement of innate and adaptive cellular immunity; however, methods to measure these effectors are less established.Citation25,Citation42
The US ACIP 2010 guidelines recommended that the majority of individuals display the ability to completely neutralize rabies challenge virus at least at a 1:5 serum dilution using the RFFIT assay after completion of a 4-dose regimen.Citation43 The 1:5 serum dilution by RFFIT corresponds to an RVNA titer range of 0.1–0.3 IU/mL, which varies both within and between laboratories.Citation25 Notably, although no infections among vaccinated individuals have occurred using the US ACIP guidelines, a RVNA titer of 0.5 IU/mL was implemented as a conservative threshold for boosters following pre-exposure prophylaxis;Citation44 however, the 0.5 IU/mL threshold for PEP is challenged by some experts, with several considering any antibody level after vaccination protective, as long as response occurs rapidly after exposure.Citation45
With reports of poor antibody response to licensed rabies vaccines not unprecedented, a recent meta-analysis evaluated the factors associated with lower GMTs and fewer participants achieving RVNA titers ≥0.5 IU/mL post vaccination.Citation46 Despite accounting for variability between laboratories, the co-administration of RIGs and vaccine potency, the authors found a statistically significant trend of lower GMTs over time with licensed vaccines.Citation46 However, true PEP failures remain rare, suggesting that the observed trend of lower GMTs has limited impact on clinical effectiveness.Citation34,Citation36,Citation37 Although this observation included the HDCV rabies vaccine, substantial experience with this vaccine supports its safety and effectiveness despite such findings.
The variability of RFFIT should also be considered when evaluating rabies studies. For example, it is important to note that a serological titer of 0.5 IU/mL by RFFIT may vary between 0.4 and 0.6 IU/mL day to day purely due to the nature of the test.Citation25 To account for this, the RFFIT performed in this study was validated,Citation22,Citation47 with two individual titer determinations performed by two different analysts to decrease variability and subjectivity and to confirm sample quality. The kinetics of the immune response is also relevant when evaluating the efficacy of rabies vaccines.Citation40 In this study, all participants achieved RVNA titers ≥0.5 IU/mL at D28 and D42 in the high-dose PVRV-NG2 and HDCV groups. Six months after the final injection, the dose effect was still observed; high-dose PVRV-NG2 resulted in a higher proportion of participants reaching RVNA titers ≥0.5 IU/mL compared with lower doses, which favored the selection of this dose for future trials.
The high baseline RVNAs (≥0.5 IU/mL) observed in six participants may be due to receipt of a previous rabies vaccine (confirmed in one participant; protocol deviation). High RVNA titers of unknown origin were also present at baseline in 6–9% of healthy participants in a study of a purified chick embryo cell rabies vaccine.Citation48 A post-hoc adjustment was therefore performed to limit the impact of the unusually high values on the immune response assessment, leading to an overestimation of the GMTs in a study with limited sample size, such as this one. Overall, excluding participants with a baseline RVNA titer ≥0.5 IU/mL, a similar trend was observed, with GMTs among the PVRV-NG2 groups increasing with antigen content at all timepoints.
The frequency and severity of AEs reported in the PVRV-NG2 groups were broadly similar to those reported for the PVRV-NG group. The frequency of reactions (injection site pain) slightly increased with PVRV-NG2 dose; however, there was no impact on the benefit-risk ratios, which were within similar ranges across the three vaccine groups. The low-dose PVRV-NG2 and PVRV-NG groups displayed similar reactogenicity profiles, indicating that the PVRV-NG2 formulation did not impact the safety profile. The proportion of participants reporting solicited injection site reactions was lower for all PVRV-NG and PVRV-NG2 groups compared with the HDCV vaccine group.
This study had limitations that should be considered. Firstly, this study was designed to be descriptive with no formal statistical analyses planned; it was designed to investigate the antigen response of the PVRV-NG2 formulation to select which dose could be further evaluated in Phase III studies. As such, the formal dose–effect demonstration was only assessed through post hoc and exploratory analyses. Secondly, as all participants received HRIG, there was no vaccine-only group and the interference on immune response after vaccination could not be assessed. However, this study aimed to evaluate the current standard of care for PEP, which recommends the use of HRIG in all not previously vaccinated individuals, as per the US ACIP recommendations.Citation43 Finally, no further immunological assessment of participants who did not achieve RVNA ≥ 0.5 IU/mL at D14 or after the full PEP course at D42 was undertaken. As such, although this study excluded participants with any immunosuppression [confirmed in one participant; protocol deviation], the immunological mechanisms of slow- or non-response to vaccination remain unknown.Citation28,Citation45
In conclusion, this study showed that PVRV-NG2 displays a favorable immunogenicity and safety profile, similar to that of the licensed HDCV control vaccine with the high-dose formulation. Therefore, the high-dose formulation was chosen to conduct Phase III studies.
Acknowledgments
The authors would like to thank the volunteers who enrolled in this study. They would also like to thank the following Sanofi employees for their valuable contributions: Manon Croix, Jose Rivera, and Pascale Chavand for study coordination; Joanna Korejwo for the safety evaluation during the study; Gee Marsh for critical review; Kathryn Bonaparte, PhD and Anirban Sanyal, PhD for coordinating the manuscript preparation. Medical writing support was provided by Katherine St. John (Zoetic Science, an Ashfield company, Glasgow, UK) and Nichola Cruickshanks (inScience Communications, Springer Healthcare Ltd, UK), and was funded by Sanofi.
All authors attest that they meet the ICMJE criteria for authorship.
Disclosure statement
JS and BE declare they have no conflicts of interest. SP, FGM, ACPP, AM, CP, and AMM are employees of Sanofi.
Data availability statement
Qualified researchers may request access to patient-level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications. Patient-level data will be anonymized, and study documents will be redacted to protect the privacy of study participants. Further details on Sanofi’s data-sharing criteria, eligible studies, and process for requesting access can be found at: https://www.vivli.org/.
Additional information
Funding
References
- Hampson K, Coudeville L, Lembo T, Sambo M, Kieffer A, Attlan M, Barrat J, Blanton JD, Briggs DJ, Cleaveland S, et al. Correction: estimating the global burden of endemic canine rabies. PLoS Negl Trop Dis. 2015;9(5):e0003786. doi:10.1371/journal.pntd.0003786.
- World Health Organization. Rabies epidemiology and burden of disease; 2022 [accessed 2023 Sept 1]. https://www.who.int/teams/control-of-neglected-tropical-diseases/rabies/epidemiology-and-burden.
- Hampson K, Coudeville L, Lembo T, Sambo M, Kieffer A, Attlan M, Barrat J, Blanton JD, Briggs DJ, Cleaveland S, et al. Estimating the global burden of endemic canine rabies. PLoS Negl Trop Dis. 2015;9(4):e0003709. doi:10.1371/journal.pntd.0003709.
- World Health Organization. Rabies fact sheet; 2021 [accessed 2023 Sept 1]. https://www.who.int/news-room/fact-sheets/detail/rabies.
- Hampson K, Dobson A, Kaare M, Dushoff J, Magoto M, Sindoya E, Cleaveland S, Kieny MP. Rabies exposures, post-exposure prophylaxis and deaths in a region of endemic canine rabies. PLoS Negl Trop Dis. 2008;2(11):e339. doi:10.1371/journal.pntd.0000339.
- Anderson A, Shwiff SA. The Cost of canine rabies on four continents. Transbound Emerg Dis. 2015;62(4):446–11. doi:10.1111/tbed.12168.
- Patil SU, Shreffler WG. Novel vaccines: technology and development. J Allergy Clin Immunol. 2019;143(3):844–51. doi:10.1016/j.jaci.2018.05.021.
- Montagnon B, Fanget B. Chapter 26: purified Vero cell vaccine for humans. In: Meslin F-X-K Koprowski H, World Health Organization, editors. Laboratory techniques in rabies. 4th ed. Geneva (Switzerland): WHO; 1996. p. 285.
- World Health Organization. WHO consultation on public health and animal transmissible spongiform encephalopathies: epidemiology, risk and Research requirements, Geneva, Switzerland, 1-3 December 1999. Geneva: World Health Organization; 2000.
- Li R, Huang L, Li J, Mo Z, He B, Wang Y, Wu X, Minutello M, Guinet-Morlot F, Pichon S. A next-generation, serum-free, highly purified Vero cell rabies vaccine is safe and as immunogenic as the reference vaccine verorab(R) when administered according to a post-exposure regimen in healthy children and adults in China. Vaccine. 2013;31:5940–7. doi:10.1016/j.vaccine.2013.10.043.
- Pichon S, Guinet-Morlot F, Minutello M, Donazzolo Y, Rouzier R, Chassard D, Fitoussi S, Hou V. A serum-free, purified Vero cell rabies vaccine is safe and as immunogenic as the reference vaccine verorab™ for pre-exposure use in healthy adults: results from a randomized controlled phase-II trial. Vaccine. 2013;31(18):2295–301. doi:10.1016/j.vaccine.2013.02.058.
- Pichon S, Moureau A, Petit C, Chu L, Essink B, Muse D, Saleh J, Guinet-Morlot F, Minutello AM. Safety and immunogenicity of a serum-free purified Vero rabies vaccine in healthy adults: a randomised phase II pre-exposure prophylaxis study. Vaccine. 2022;40(33):4780–7. doi:10.1016/j.vaccine.2022.06.040.
- Quiambao B, Montalban C, Minutello AM, Guinet-Morlot F, Moureau A, Petit C, Pichon S. Serum-free purified Vero rabies vaccine is safe and immunogenic in children: results of a randomized phaseII pre-exposure prophylaxis regimen study. Vaccine. 2022;40(35):5170–8. doi:10.1016/j.vaccine.2022.06.061.
- Morgeaux S, Poirier B, Ragan CI, Wilkinson D, Arabin U, Guinet-Morlot F, Levis R, Meyer H, Riou P, Shaid S, et al. Replacement of in vivo human rabies vaccine potency testing by in vitro glycoprotein quantification using ELISA - Results of an international collaborative study. Vaccine. 2017;35:966–71. doi:10.1016/j.vaccine.2016.12.039.
- Chabaud-Riou M, Moreno N, Guinchard F, Nicolai MC, Niogret-Siohan E, Sève N, Manin C, Guinet-Morlot F, Riou P. G-protein based ELISA as a potency test for rabies vaccines. Biologicals. 2017;46:124–9. doi:10.1016/j.biologicals.2017.02.002.
- Mulder R, Singh AB, Hamilton A, Das P, Outhred T, Morris G, Bassett D, Baune BT, Berk M, Boyce P, et al. The limitations of using randomised controlled trials as a basis for developing treatment guidelines. Evid Based Ment Health. 2018;21(1):4–6. doi:10.1136/eb-2017-102701.
- World Health Organization. Rabies vaccines: WHO position paper, April 2018. Wkly Epidemiol Rec. 2018;16:201–20. doi:10.1016/j.vaccine.2018.06.061.
- Manning SE, Rupprecht CE, Fishbein D, Hanlon CA, Lumlertdacha B, Guerra M, Meltzer MI, Dhankhar P, Vaidya SA, Jenkins SR, et al. Human rabies prevention–United States, 2008: recommendations of the advisory committee on immunization practices. MMWR Recomm Rep. 2008;57:1–28.
- World Health Organization. WHO expert consultation on rabies: third report. Geneva: World Health Organization; 2018.
- Beaujouan É, Toulemon L. European countries with delayed childbearing are not those with lower fertility. Genus. 2021;77:2. doi:10.1186/s41118-020-00108-0.
- Smith JS, Yager PA, Baer GM. A rapid reproducible test for determining rabies neutralizing antibody. Bull World Health Organ. 1973;48:535–41.
- Timiryasova TM, Luo P, Zheng L, Singer A, Zedar R, Garg S, Petit C, Moore S, Hu BT, Brown M. Rapid fluorescent focus inhibition test optimization and validation: improved detection of neutralizing antibodies to rabies virus. J Immunol Methods. 2019;474:112626. doi:10.1016/j.jim.2019.06.017.
- Reed LJ, Muench H. A simple method of estimating fifty per cent endpoints12. Am J Epidemiol. 1938;27(3):493–7. doi:10.1093/oxfordjournals.aje.a118408.
- Mehari MA, Maeruf H, Robles CC, Woldemariam S, Adhena T, Mulugeta M, Haftu A, Hagose H, Kumsa H. Advanced maternal age pregnancy and its adverse obstetrical and perinatal outcomes in ayder comprehensive specialized hospital, Northern Ethiopia, 2017: a comparative cross-sectional study. BMC Pregnancy Childbirth. 2020;20(1):60. doi:10.1186/s12884-020-2740-6.
- Moore SM. Challenges of rabies serology: defining context of interpretation. Viruses. 2021;13(8):1516. doi:10.3390/v13081516.
- Sudarshan MK, Mahendra BJ, Madhusudana SN, Narayana DHA, Sanjay TV, Gangaboraiah, Anandagiri MS. Assessing the relationship between antigenicity and immunogenicity of human rabies vaccines: results of a metaanalysis. Hum Vaccin. 2005;1:187–90. doi:10.4161/hv.1.5.2110.
- Miranda E, Lacuesta TL, Lacuesta V, Suquila J, Manalo M, Dimaano E . Safety and immunogenicity of purified Vero cell rabies vaccine versus purified chick embryo cell rabies vaccine using pre-exposure and post exposure regimen among healthy volunteers in San Lazaro hospital. Philip J Inte Med. 2014;52:1–7.
- Uwanyiligira M, Landry P, Genton B, de Valliere S. Rabies postexposure prophylaxis in routine practice in view of the new centers for disease control and prevention and World Health Organization recommendations. Clin Infect Dis. 2012;55(2):201–5. doi:10.1093/cid/cis384.
- Gogtay NJ, Munshi R, Ashwath Narayana DH, Mahendra BJ, Kshirsagar V, Gunale B, Moore S, Cheslock P, Thaker S, Deshpande S, et al. Comparison of a novel human rabies monoclonal antibody to human rabies immunoglobulin for postexposure prophylaxis: a Phase 2/3, randomized, single-blind, noninferiority, controlled study. Clin Infect Dis. 2018;66(3):387–95. doi:10.1093/cid/cix791.
- Sampath G, Banzhoff A, Deshpande A, Malerczyk C, Arora AK, Vakil H, Preiss S. Comparison of the immunogenicity and safety of the purified chick embryo cell rabies vaccine manufactured in India and Germany: a randomized, single blind, multicentre, phase IV clinical study. Hum Vaccin Immunother. 2017;13:1531–8. doi:10.1080/21645515.2017.1307483.
- Preiss S, Chanthavanich P, Chen LH, Marano C, Buchy P, van Hoorn R, Vonk Noordegraaf M, Mukherjee P. Post-exposure prophylaxis (PEP) for rabies with purified chick embryo cell vaccine: a systematic literature review and meta-analysis. Expert Rev Vaccines. 2018;17(6):525–45. doi:10.1080/14760584.2018.1473765.
- Wang SY, Sun JF, Liu P, Luo L, Li JX, Zhu FC, Shen XX, Meng FY. Immunogenicity and safety of human diploid cell vaccine (HDCV) vs. purified Vero cell vaccine (PVRV) vs. purified chick embryo cell vaccine (PCECV) used in post-exposure prophylaxis: a systematic review and meta-analysis. Hum Vaccin Immunother. 2022;18(1):2027714. doi:10.1080/21645515.2022.2027714.
- Matson MA, Schenker E, Stein M, Zamfirova V, Nguyen HB, Bergman GE. Safety and efficacy results of simulated post-exposure prophylaxis with human immune globulin (HRIG; KEDRAB) co-administered with active vaccine in healthy subjects: a comparative phase 2/3 trial. Hum Vaccin Immunother. 2020;16(2):452–9. doi:10.1080/21645515.2019.1656967.
- Hobart-Porter N, Stein M, Toh N, Amega N, Nguyen HB, Linakis J. Safety and efficacy of rabies immunoglobulin in pediatric patients with suspected exposure. Hum Vaccin Immunother. 2021;17(7):2090–6. doi:10.1080/21645515.2020.1854000.
- Bernard MC, Boudet F, Pineda-Pena AC, Guinet-Morlot F. Inhibitory effect of concomitantly administered rabies immunoglobulins on the immunogenicity of commercial and candidate human rabies vaccines in hamsters. Sci Rep. 2022;12(1):6570. doi:10.1038/s41598-022-10281-1.
- Lang J, Simanjuntak GH, Soerjosembodo S, Koesharyono C. Suppressant effect of human or equine rabies immunoglobulins on the immunogenicity of post-exposure rabies vaccination under the 2-1-1 regimen: a field trial in Indonesia. MAS054 clinical investigator group. Bull World Health Organ. 1998;76:491–5.
- Salva EP, Dimaano EM, Villarama JBR, Suquila JT. An evaluation of the safety and potency of equine rabies immunoglobulin through measurement of suppression on vaccine-induced antibody production among healthy volunteers. Philip J Inte Med. 2014;52:1–7.
- Soentjens P, Declercq S. Prophylaxie post-exposition contre la rage, Institute of tropical medicine Antwerp, September 2020. 2020.
- UK Health Security Agency. Administration of rabies vaccine and iImmunoglobulin. 2022.
- Briggs DJ, Moore SM. The Route of administration of rabies vaccines: comparing the data. Viruses. 2021;13(7):1252. doi:10.3390/v13071252.
- Rodriguez MC, Fontana D, Garay E, Prieto C. Detection and quantification of anti-rabies glycoprotein antibodies: current state and perspectives. Appl Microbiol Biotechnol. 2021;105(18):6547–57. doi:10.1007/s00253-021-11515-4.
- Wilde H. Failures of post-exposure rabies prophylaxis. Vaccine. 2007;25(44):7605–9. doi:10.1016/j.vaccine.2007.08.054.
- Rupprecht CE, Briggs D, Brown CM, Franka R, Katz SL, Kerr HD, Lett SM, Levis R, Meltzer MI, Schaffner W, et al. Use of a reduced (4-dose) vaccine schedule for postexposure prophylaxis to prevent human rabies: recommendations of the advisory committee on immunization practices. MMWR Recomm Rep. 2010;59:1–9.
- Rao AK, Briggs D, Moore SM, Whitehill F, Campos-Outcalt D, Morgan RL, Wallace RM, Romero JR, Bahta L, Frey SE, et al. Use of a modified preexposure prophylaxis vaccination schedule to prevent human rabies: recommendations of the advisory committee on immunization practices - United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:619–27. doi:10.15585/mmwr.mm7118a2.
- Audran R, Depraetere G, Genton B, Spertini F, Uwanyiligira M, Mayor C, Thierry A, Zanoni R, Valliere S. Poor immune response to rabies post-exposure prophylaxis: an attempt to understand the underlying mechanisms. JSM Trop Med Res. 2016;1:1009.
- Morelli F, Augard C, Bourhy H, Bravo C, Coudeville L, Moore S, Quiambao B, Recuenco S. Immunogenicity of rabies vaccines in postexposure prophylaxis (PEP) or simulated PEP regimens: a systematic literature review and meta-analysis. Presented at: the International Conference on Rabies in the Americas Meeting; 2022 Oct 23–28; Querétaro, Mexico.
- Timiryasova TM, Hodge SA, Zheng LY, Singer A, Vincent D, Rahman M, Petit C, Brown M. Preparation and qualification of internal rabies reference standards for use in the rabies rapid fluorescent focus inhibition test. Sci Rep-Uk. 2020;10(1):10. doi:10.1038/s41598-020-66754-8.
- Mahendra BJ, Narayana DA, Agarkhedkar S, Ravish HS, Harish BR, Agarkhedkar S, Madhusudana SN, Belludi A, Ahmed K, Jonnalagedda R, et al. Comparative study on the immunogenicity and safety of a purified chick embryo cell rabies vaccine (PCECV) administered according to two different simulated post exposure intramuscular regimens (Zagreb versus Essen). Hum Vaccin Immunother. 2015;11:428–34. doi:10.4161/21645515.2014.995059.
