ABSTRACT
The Regional Immunization Calendar in Calabria, a region of Italy, was updated in 2022, introducing optional co-administration of three injectable vaccines, with one oral vaccine, at 3 and 5 months old, and three injectable vaccines at 13–14 months old. In this project, the opinions and expectations of healthcare professionals (HCPs) in Calabria were investigated, with respect to the updated recommended practices. An 11-question survey was developed, which addressed concepts and topics related to immunization calendar implementation. Focus group discussions were also organized to provide further insight on the survey findings. A total of 132 HCPs completed the survey (86 public health providers [PHs] and 46 family pediatricians [FPs]). Overall, ≥50% of respondents agreed that vaccine co-administration would be advantageous for public health by reducing the number of vaccination sessions required. Most PHs and FPs agreed that dissemination of available safety data on vaccine co-administration is a necessary action to facilitate effective implementation of the strategy into clinical practice. The importance of safety data related to vaccine co-administration was supported further by discussions held in PH and FP focus groups. Overall, these findings demonstrate support from HCPs in Calabria for vaccine co-administration, and highlight key activities needed for successful uptake.
Introduction
Immunization calendars are tools implemented by many countries worldwide in order to guide healthcare providers in vaccination activities within a national immunization program (NIP). Up to 3 million deaths among children are prevented annually as a result of vaccinating against diphtheria, tetanus, pertussis, and measles,Citation1 highlighting the importance of effective NIPs. NIPs typically evolve over time, either through the introduction of new vaccines or in an effort to optimize existing vaccination strategies. To this end, several Italian scientific societies (Italian Society of Hygiene, Preventive Medicine and Public Health [SItI], the Italian Society of Pediatrics [SIP], the Italian Federation of Pediatricians [FIMP], and the Italian Federation of General Practitioners [FIMMG]) support the co-administration of routine vaccines within a NIP, in light of positive experiences observed in other countries.Citation2–4
In August 2023, the Italian NIP was updated (edition: 2023–2025), supporting the option to co-administer mandatory and recommended pediatric vaccines.Citation5 Among pediatric populations, mandatory vaccines include diphtheria, tetanus, acellular pertussis, poliomyelitis, hepatitis B, Haemophilus influenzae type b (DTaP-IPV-HB-Hib) and measles, mumps, rubella, and varicella (MMRV) vaccines, whilst recommended vaccines include pneumococcal conjugate vaccine (PCV), meningococcal B (MenB), meningococcal C (MenC), and rotavirus (RV) vaccines.Citation5 In 2021, the mean vaccine coverage reported for mandatory vaccines across Italy was estimated at 92.1–94.0%, whereas coverage rates reported for most recommended vaccines were notably lower, such as RV (70.4%), MenC (73.4%), and MenB (79.7%) vaccines.Citation6 Interestingly, PCV coverage rate (91.3%) was similar to that of the mandatory vaccines, likely due to being co-administered with DTaP-IPV-HB-Hib.Citation6
Calabria is one of 20 regions in Italy and reports similar vaccination coverage patterns to national rates.Citation6 In April 2022, the Calabria Immunization Calendar (CIC) was updated, according to recommendations outlined by the Regional Technical Coordination for Vaccinations, which introduced the option to co-administer three injectable vaccines at 3 and 5 months old (PCV, DTaP-IPV-HB-Hib, and MenB), with the oral RV vaccine, in addition to three injectable vaccines at 13–14 months old (MMRV, meningococcal ACWY, and MenB) (Supplementary Table S1). These updates were consistent with recommendations from the Ministry of Health, Board of the Calendar for Life, and the 2023–2025 National Vaccine Prevention Plan (NVPP).Citation2,Citation5,Citation7 Families otherwise have the option to choose the original co-administration regimen also outlined in the 2023–2025 NVPP, which allows the co-administration of DTaP-IPV-HB-Hib/PCV and RV, followed by MenB vaccination after a further 15–30 days.Citation5
Adherence to the updated co-administration strategy outlined in the CIC could provide many advantages, such as fewer vaccination appointments during the first year of life, completion of the primary vaccination cycle in two sessions (compared with the standard of four), and thereby earlier vaccination, which may provide greater protection against some diseases.Citation8,Citation9 Similar vaccination strategies have also been used previously in the United Kingdom and Portugal.Citation10,Citation11 In the United Kingdom, vaccine co-administration led to no reductions in vaccine coverage or efficacy, and no negative impact on the overall tolerability of the vaccines.Citation11
The option to co-administer three injectable vaccines with one oral vaccine, as recommended in the updated CIC, may present an easy-to-implement strategy for optimizing and improving vaccine coverage in the region. However, this strategy will require efficient implementation into current healthcare practices and would need to be favored over the previous vaccination approach by healthcare professionals (HCPs). The aim of this project was to investigate the opinions and expectations of HCPs in Calabria, with respect to the updated, optional practices presented in the CIC.
Methods
Project design
An 11-question online survey (Supplementary Material) was conducted between September 2022 and November 2022, with the aim of collecting the opinions of PHs and FPs on the feasibility of the new pediatric CIC updated in April 2022 (Supplementary Table S1). The survey was hosted and distributed online using the Google Forms electronic platform and was strictly anonymized, avoiding the need for any personal identifiers from participants.
Following the survey, remote focus group meetings were organized with PH and FP respondents to further explore key outcomes of the survey. Online focus group meetings were carried out as recommended by Kite and Phongsavan (2017),Citation12 in which a homogeneous group of PHs or FPs, selected on the basis of their own interest and availability to participate, discussed and compared their personal attitudes toward the survey topics. During the meetings, questions were asked interactively (unstructured), and group participants could freely communicate with other attendees. Two focus group meetings were held, which comprised six FP and seven PH participants, respectively. Each session lasted between 1.5–2 hours and was chaired by a facilitator who moderated and encouraged the free flow of ideas.
Statistical analysis
The necessary sample size to obtain sufficient data was calculated based on the responses to Question 4 in the survey (Supplementary Material), which explored activities that may be necessary to support the co-administration of three injectable vaccines (DTaP-IPV-HB-Hib, PCV, and MenB) and one oral vaccine (RV) at 3 and 5 months old. A difference of 18% in the frequencies of the group responses to “assess the degree of acceptance by parents” was assumed. In accordance with an alpha (α) value = 0.05 and beta (β) value = 0.19 (study power = 81%), a minimum sample size of 131 respondents was required.
A descriptive analysis of the responses was conducted, reporting absolute number and percentage frequency of responses. For questions where multiple answers could be selected, the frequency of responses was calculated for each available answer. The statistical significance, when comparing data recorded from PH and FP participants for each response, was assessed by Pearson’s chi-square tests, or Fisher’s exact tests if the expected frequencies were less than five.
The statistical package STATA/BE 17.0 was used for the data analysis and the significance level was set at p < .05.
Results
A total of 132 completed questionnaires were collected (comprised of responses from 86 PHs and 46 FPs). Respondents were well represented across five local health units (LHUs; Catanzaro, Cosenza, Crotone, Reggio Calabria, and Vibo Valentia). However, when comparing the geographical representation of PH and FP respondents, PHs accounted for almost 10-fold more respondents from the LHU of Cosenza compared with FPs (39.35% vs 4.35%; p < .001), whereas more FP respondents were from the LHU of Crotone (19.57% vs 3.49%; p = .004).
Overall, most PHs and FPs were in favor of the co-administration of vaccines, recognizing the benefits that are generated for vaccinated individuals, caregivers, and public health organizations (; multiple answers could be selected). Over 50% of respondents agreed that vaccine co-administration would be advantageous for public health, because it would reduce the overall number of vaccination sessions per individual (PHs: 54.65%, FPs: 50.00%). Additionally, ≥50% of respondents agreed that a benefit of vaccine co-administration is that it would reduce the number of lost working hours for users and caregivers (PHs: 58.14%, FPs: 50.00%). Less than 7% of respondents from either the PH or FP groups reported that vaccine co-administration would be challenging as a result of HCPs being unaware of its value/utility. Similarly, fewer than 5% in either group reported that co-administration would be too demanding for HCPs due to increased doubt among citizens, vaccine hesitation, or refusal to be vaccinated.
Figure 1. Respondents’ perception of the co-administration of vaccines. Question three in the full survey shared with the respondents (Supplementary Material). Survey participants were able to select multiple answers.
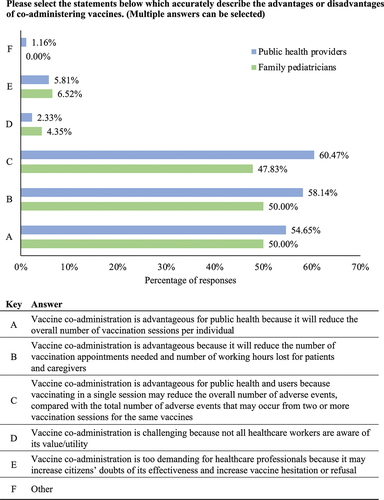
In light of the updated CIC, PHs and FPs were asked to indicate any activities that may help to support the implementation of DTaP-IPV-HB-Hib, PCV, and MenB vaccine co-administration (with the oral RV vaccine) in children aged 3 and 5 months old. Most FP (91.30%) and PH (70.93%) respondents voted that disseminating data on the overall safety/tolerability profile of the co-administered vaccines would be necessary (); although, the proportion of PH respondents who voted for this action was significantly less than the proportion reported among FP respondents (p = .008). Consistent with an emphasis on safety, >50% of FPs and > 40% of PHs also agreed that further information on vaccine co-administration within vaccine data sheets would be needed. A significantly greater proportion of PH respondents compared with FP respondents voted that assessing degree of acceptance by parents for the co-administration of DTaP-IPV-HB-Hib, PCV, and MenB is needed to help support its implementation in practice (51.16% vs 32.61%; p = .041). Comparatively, the frequency reported for all other options was similar between FPs and PHs ().
Figure 2. Activities deemed necessary by healthcare professionals to support the implementation of the updated Calabria immunization Calendar. Question four in the full survey shared with the respondents (Supplementary Material). Survey participants were able to select multiple answers. DTaP-IPV-HB-Hib: diphtheria, tetanus, acellular pertussis, poliomyelitis, hepatitis B, haemophilus influenzae type b.
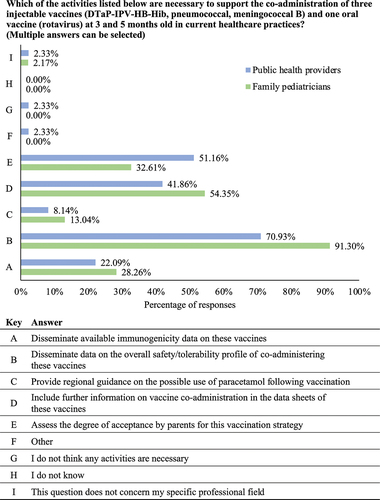
PHs and FPs were asked to suggest any activities that may help to support the implementation of MMRV, meningococcal ACWY, and MenB vaccine co-administration at 13–14 months old. Consistent with results in , the most common response voted for by PH and FP respondents was the dissemination of data on the overall safety/tolerability profile of the vaccines (69.77% and 73.91%, respectively; ). A significantly greater proportion of PH respondents compared with FP respondents had also voted that assessing the degree of acceptance by parents would be necessary (51.16% vs 17.39%; p < .001). The second most common response from the FP respondents was to include further information on co-administration within vaccine data sheets, with frequencies comparable to those reported in the PH group (39.13% vs 39.53%; p = .964; ).
Figure 3. Activities deemed necessary by healthcare professionals to support the implementation of MMRV, meningococcal ACWY, and meningococcal B vaccine co-administration. Question five in the full survey shared with the respondents (Supplementary Material). MenB: meningococcal B; MMRV: measles, mumps, rubella, and varicella.
Survey participants were also asked to indicate their preferred modalities to receive training on the updated CIC. Multidisciplinary Continued Medical Education (CME) training courses, organized by the district/LHU of affiliation, was the preferred option among the PH group, which was significantly greater than the frequency reported in the FP group (60.47% vs 39.96%; p = .010). The preferred option indicated by the FP group was general accredited residential training; there was no significance in the proportion of votes for this option when compared with the PH group (45.65% vs 39.53%; p = .497).
Following the survey analyses, two focus groups were organized according to professional roles (FPs and PHs). Both focus groups agreed that the initial survey covered all issues and major topics related to the updated CIC. Participants in both groups emphasized the importance of the opinions of HCPs on vaccine co-administration (), which was to reassure FPs and consequently reassure parents on the safety of vaccine co-administration. In the meeting, participants had discussed three large European studies,Citation13–15 considered important in providing key data on the safety of vaccine co-administration. FP and PH groups also noted that the most common adverse events following immunization (AEFIs) are expected to be fever and pain/swelling at the site of injection. Moreover, PHs noted that the main advantages of co-administering vaccines from the perspective of parents were lower rates of AEFIs, and reduced discomfort experienced by their newborn, due to fewer vaccination visits.
Both FP and PH groups further discussed parents’ degree of acceptance of vaccine co-administration. From the experience of FPs in the LHU of Reggio Calabria, approximately 80% of parents, an increase from 35%, had chosen for their child to receive the full co-administration regimen, compared with separate vaccination. These findings were also reported at an Italian CME webinar hosted in April 2023.Citation16 The PH group suggested that consulting mothers on available vaccination practices for their child, at the time of maternal tetanus-diphtheria-acellular pertussis (Tdap)/influenza vaccination, could improve vaccine adherence. According to PHs, when vaccine co-administration was recommended to parents by HCPs, refusals were rare.
In the context of vaccine co-administration, the topic of regional indications of paracetamol was also raised by FPs, as paracetamol is recommended for prophylactic use in the UK following MenB vaccination,Citation17 whereas it was not foreseen in the 2017–2019 NVPP.Citation18 The overall consensus from the focus group was that paracetamol should be prescribed only for discomfort, rather than fever; except in cases of high or severe fever. It was also expressed that regional indications should include further guidance on when to use paracetamol (e.g., always, never, or only in children at risk).
Lastly, the PH focus group reported that vaccine co-administration at the first visit for newborns is preferable. It was suggested that this allows for an increase in the interim period between the first and second vaccination session, and therefore may help to avoid the perdurance of swelling at injection sites caused by the first round of injections. PHs emphasized that all HCPs across the LHUs should adopt the same positive attitude toward vaccine co-administration, assisting in demonstrating the advantages to parents. This was a message that was not in place among the LHUs at the time of the meeting.
Discussion
In summary, the findings of this project indicate that the majority of PHs and FPs in Calabria, Italy, support the co-administration of three injectable vaccines in children aged 3 and 5 months old (with one oral vaccine), and 13–14 months old. The survey suggested that HCPs in the region consider vaccine co-administration, as proposed in the updated CIC, to have a range of benefits to caregivers, vaccinated individuals, and public health organizations.
A key advantage of vaccine co-administration is the need for fewer vaccination appointments in the first year of life. In accordance with the updated CIC, newborns could complete the primary vaccination cycle in only two sessions, compared with a standard of four. Furthermore, from an epidemiological perspective, optimizing the co-administration of vaccines would likely lead to earlier vaccination of newborns, which may provide greater protection against diseases. For example, the first peak in invasive MenB infections occurs in the first year of life.Citation19 A recent retrospective cohort screening study, carried out in Italy, reported that earlier vaccination with the four-component MenB vaccine (4CMenB) may lead to greater protection against meningococcal infection.Citation9 Pellegrino et al. Citation20 also reported that co-administration of DTaP-IPV-HB-Hib, PCV, and MenC vaccines has led to increased vaccine coverage in the LHU of Cuneo-1, from 47% to 65%, in only one year of implementation. According to evidence from experience in the United Kingdom, co-administration of such vaccines does not affect vaccine efficacy. Indeed, between 2015–2020, the United Kingdom NIP recommended co-administration of the MenB vaccine with the first dose of DTaP-IPV-HB-Hib, PCV, and RV vaccines at 2 months old.Citation11,Citation21 No notable impact on MenB or RV effectiveness was reported.Citation11,Citation21 However, the United Kingdom later switched to the 1 + 1 schedule of PCV (one dose at the second month and one dose during the first year of life), meaning that the co-administration of MenB with three other injectable vaccines now only occurs at 2 years of age.Citation22
The results of this survey and focus group meetings have highlighted the importance of safety data in supporting the implementation of vaccine co-administration in the context of the CIC. The survey outcomes also indicated that further information on vaccine co-administration should be included in the respective data sheets of vaccines, which would include the summary of product characteristics (SmPC). The SmPC is useful for HCPs to understand how to safely use a product.Citation7 However, information on the co-administration of vaccines is sometimes missing, even in cases where vaccines are commonly co-administered in practice, such as for the MenB and RV vaccines.Citation7 Since this survey was conducted, the Italian NIP (edition: 2023–2025) was updated, which now provides guidance to HCPs indicating that pediatric vaccines can be fully co-administered.Citation5
For future work, the generation of two main sets of data are proposed in order to support the uptake of vaccine co-administration. Firstly, a retrospective analysis of the available evidence on the safety/tolerability of vaccine co-administration is needed, which should include data available from four large studies of European randomized-controlled clinical trials, and real-life experience reported in the UK.Citation13–15,Citation23 The risks of AEFIs when co-administering pediatric vaccines should be compared with the cumulative risk reported when administering the vaccines individually.Citation13 Secondly, it would be valuable to provide data on the prospective monitoring of AEFIs identified from the passive AEFI surveillance system in Calabria, ensuring that this follows the appropriate European Medicines Agency guidelines for signal detection.Citation24–26
During the PH focus group, it was suggested that the consulting of pregnant women on the vaccination plan scheduled for their newborn child could improve vaccine adherence. A cross-sectional survey carried out in Italy in 2017 reported that pregnant women were most interested in information on the safety profile of Tdap vaccination during pregnancy, and how it may affect them, the fetus, or their newborn child.Citation27 Consistent with this, a second survey among pregnant women in Italy in 2018 reported that the main barriers to influenza and Tdap vaccination were a lack of recommendations from HCPs (81%) and safety concerns (18%).Citation28 The main facilitators for vaccination were a willingness to protect their child (82%) and themselves (66%), and having received advice from their healthcare provider (62%).Citation28 It can therefore be expected that if pregnant women agree to receive influenza and/or Tdap vaccinations to protect themselves or their offspring, they likely trust the value of vaccination and the opinions of HCPs. Hence, recommendations from HCPs may have a valuable impact when advising parents on the immunization pattern for their newborn child.
Following the completion of the survey, two (North and South Calabria) third party CME events were set up and released as online CME courses for one year. These courses included topics guided by the results of this survey, with a focus on the safety/tolerability of vaccines when co-administered. Interviews with parents, outlining their experience with vaccine co-administration, as well as video tutorials on the appropriate method to administer three injectable vaccines with one oral vaccine in a single session were included among the CME content. The experience of the LHU of Reggio Calabria with the updated CIC strategy was also presented.Citation16 In May 2022, 47/75 (62.67%) pediatric vaccination visits followed the NVPP (two injectable vaccines with one oral vaccine) schedule and 28/75 (37.33%) followed the CIC recommendations. In March 2023, 13/94 (13.83%) vaccination visits followed the NVPP and 81/94 (86.17%) followed the CIC, indicating an increasing uptake of the updated CIC.Citation16 Further to this, medical education via CME courses was also particularly valuable as a means to disseminate key safety data and reassuring HCPs of the availability, efficacy, and advantages of vaccine co-administration; key aspects highlighted in this survey.
To further assess the implementation and impact of vaccine co-administration in Italy, the next steps would include monitoring HCP adherence to CIC recommendations, assessing the impact of the updates on regional pediatric vaccination coverage, and maintaining passive collection of reported AEFIs. It is also important to note that efficient implementation of the updated CIC guidelines will likely require operational changes that may warrant consideration. Further to the scientific value and impact on public health, such operational changes require HCPs to be open to change. To achieve this, regular education and training are often necessary to support HCPs in implementing new vaccination regimes.Citation4,Citation29
Limitations
The results presented here are consistent with current recommendations and practices globally; however, there were limitations to this project. All findings were reported in a regional context in Italy. Therefore, these data only concern the geographical location of the respondents to the survey. The unbalanced participation of FPs and PHs in the LHU of Cosenza and Crotone was not considered a bias in the general outcome of this regional perspective. The composition of focus groups was also not reported; however, this was to protect the identity of the participants, given the low number of HCPs involved.
The findings reported here could be suggestive of insights for a wider group of HCPs; however, this was limited by the small sample size. The survey sample size did not reach a 90% confidence level, despite representing 22% of the whole target population. The number of focus groups conducted in this study also did not meet theoretical saturation of data for focus group discussions.Citation30 Another caveat of this project was that Calabria is among the smallest regions in Italy, representing 13,966/404,892 (3.45%) of all newborns in 2020;Citation31 therefore, Calabria’s social and professional demographics may not fully represent those of larger regions. Regarding the survey methodology, there were no technical restrictions in place that would prevent the occurrence of repeat entries and votes, and since the survey was anonymized, it was not possible to confirm if respondents had completed the survey more than once.
Lasty, as the primary objective of the project was to explore the insights and expectations of HCPs on the implementation of the updated CIC, questions were limited to this context. Therefore, an opportunity to collect information on observed vaccine-related side effects in the region may have been lost. Although, some general comments on AEFIs were raised during the focus group sessions.
Conclusions
In conclusion, this survey aimed to assess the insights and expectations of HCPs on the updated pediatric Regional Immunization Calendar in Calabria, Italy. The insights gained from FPs and PHs in the region will help guide future activities to support the full implementation of the CIC. Together with the recently updated guidance to the Italian NIP, the regional experience reported here may further support the adoption of the co-administration of pediatric vaccines in an increasing number of regions across Italy. Given that PHs and FPs are essential in mediating the vaccination of pediatric populations in Italy, their efforts, supported by the strategic value of vaccine co-administration, will help to provide further protection of infants from life-threatening transmittable infectious diseases.
Authors’ contributions
Substantial contributions to study conception and design: SG, GS, FF, FM; substantial contributions to analysis and interpretation of the data: SG, GS, FF, FM; drafting the article or revising it critically for important intellectual content: SG, GS, AG, FF, FM; final approval of the version of the article to be published: SG, GS, AG, FF, FM.
Data availablity statement
The authors confirm that the data supporting the findings of this project are available within the article.
Supplemental Material
Download MS Word (44.6 KB)Acknowledgments
The authors acknowledge Costello Medical for editorial assistance and publication coordination, on behalf of GSK, and acknowledge Samuel Shields, Costello Medical, UK for medical writing and editorial assistance based on authors’ input and direction.
Disclosure statement
SG, AG, and FF: no conflicts of interest to declare; GS and FM: employees and stock owners of the GSK group of companies.
Supplementary data
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2275475.
Additional information
Funding
References
- Duclos P, Okwo-Bele J-M, Gacic-Dobo M, Cherian T. Global immunization: status, progress, challenges and future. BMC Int Health Hum Rights. 2009;9(S1):S2. doi:10.1186/1472-698X-9-S1-S2.
- Vaccination Calendar for Life. 2019 [accessed 2023 June]. https://www.vaccinarsinveneto.org/assets/uploads/files/250/Calendario_Vaccinale_per_la_Vita_2019.pdf.
- Bonanni P, Angelillo IF, Villani A, Biasci P, Scotti S, Russo R, Maio T, Rosati GV, Barretta M, Bozzola E, et al. Maintain and increase vaccination coverage in children, adolescents, adults and elderly people: let’s avoid adding epidemics to the pandemic: appeal from the board of the vaccination Calendar for life in Italy: maintain and increase coverage also by re-organizing vaccination services and reassuring the population. Vaccine. 2021;39:1187–8. doi:10.1016/j.vaccine.2020.10.024.
- Poscia A, Lo GM, Nigri L, Perone V, Russo R, Bresesti I, Agosti M. Italian immunization calendar implementation: time to optimize number of vaccination appointments? Hum Vaccin Immunother. 2023;19:2156745. doi:10.1080/21645515.2022.2156745.
- Piano Nazionale Prevenzione Vaccinale 2023–2025. [accessed 2023 Sept]. https://www.trovanorme.salute.gov.it/norme/dettaglioAtto.spring?id=95963&page=newsett.
- Ministry of Health. Vaccinazioni dell’età pediatrica. Anno 2021 (coorte 2019). [accessed 2023 June]. https://www.salute.gov.it/imgs/C_17_tavole_20_10_0_file.pdf.
- Bonanni P, Boccalini S, Bechini A, Varone O, Matteo G, Sandri F, Gabutti, G. Co-administration of vaccines: a focus on tetravalent measles-mumps-rubella-varicella (MMRV) and meningococcal C conjugate vaccines. Hum Vaccin Immunother. 2020;16(6):1313–21. doi:10.1080/21645515.2019.1688032.
- Costantino C, Conforto A, Bonaccorso N, Cimino L, Sciortino M, Palermo M, Maiolo K, Tina LG, Betta PM, Caracciolo M, et al. Safety of rotavirus vaccination in preterm infants admitted in neonatal intensive care units in Sicily, Italy: a multicenter observational study. Vaccines (Basel). 2023;11(4):11. doi:10.3390/vaccines11040718.
- Lodi L, Barbati F, Amicizia D, Baldo V, Barbui AM, Bondi A, Costantino C, Da Dalt L, Ferrara L, Fortunato F, et al. Four-component recombinant protein–based vaccine effectiveness against serogroup B meningococcal disease in Italy. JAMA Netw Open. 2023;6(8):e2329678. doi:10.1001/jamanetworkopen.2023.29678.
- E-CDC Scheduler. Portugal: recommended vaccinations. [accessed Sept 2023]. https://vaccine-schedule.ecdc.europa.eu/.
- Martinelli D, Fortunato F, Marchetti F, Prato R. Rotavirus vaccine administration patterns in Italy: potential impact on vaccine coverage, compliance and adherence. Hum Vaccin Immunother. 2021;17(5):1546–51. doi:10.1080/21645515.2020.1816109.
- Kite J, Phongsavan P. Insights for conducting real-time focus groups online using a web conferencing service [version 1; peer review: 2 approved with reservations]. F1000Research. 2017;6:122. doi:10.12688/f1000research.10427.2.
- Zafack JG, Bureau A, Skowronski DM, De SG. Adverse events following immunisation with four-component meningococcal serogroup B vaccine (4CMenB): interaction with co-administration of routine infant vaccines and risk of recurrence in European randomised controlled trials. BMJ Open. 2019;9(5):e026953. doi:10.1136/bmjopen-2018-026953.
- Bryan P, Seabroke S, Wong J, Donegan K, Webb E, Goldsmith C, Vipond C, Feavers I. Safety of multicomponent meningococcal group B vaccine (4CMenB) in routine infant immunisation in the UK: a prospective surveillance study. Lancet Child Adolesc Health. 2018;2(6):395–403. doi:10.1016/S2352-4642(18)30103-2.
- Bauwens J, de LS, Weldesselassie YG, Sherlock J, Künzli N, Bonhoeffer J. Safety of routine childhood vaccine coadministration versus separate vaccination. BMJ Glob Health. 2022;7(9):e008215. doi:10.1136/bmjgh-2021-008215.
- Giuffrida SC L’esperienza della Regione Calabria sul calendario vaccinale. CME Webinar - 15 April 2023 - The Healthcare Assistant and Vaccinations In a Context Of Continuous Change. 2023 [accessed 2023 June]. https://www.vaccinarsintoscana.org/eventi/2023/15.04.2023-webinar-ecm-l%E2%80%99assistente-sanitario-e-le-vaccinazioni-in-un-contesto-di-cambiamento-continuo.
- Dubus M, Ladhani S, Vasu V. Prophylactic paracetamol after meningococcal B vaccination reduces postvaccination fever and septic screens in hospitalized preterm infants. Pediatr Infect Dis J. 2020;39(1):78–80. doi:10.1097/INF.0000000000002507.
- Ministero della Salute. Piano Nazionale Prevenzione Vaccinale 2017–2019. 2019 [accessed 2023 June]. https://www.salute.gov.it/imgs/C_17_pubblicazioni_2571_allegato.pdf.
- Azzari C, Canessa C, Lippi F, Moriondo M, Indolfi G, Nieddu F, Martini M, de Martino M, Castiglia P, Baldo V, et al. Distribution of invasive meningococcal B disease in Italian pediatric population: implications for vaccination timing. Vaccine. 2014;32(10):1187–91. doi:10.1016/j.vaccine.2013.09.055.
- Pellegrino A, Busellu G, Cucchi A, Cavallaro A, Gabutti G. Vaccine co-administration in paediatric age: the experience of the local health unit of cuneo-1 (Ambito di cuneo), Italy. Acta Biomed. 2010;81:204–9.
- UK Health Security Agency. Routine childhood immunisations. [accessed 2023 June]. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/465880/9406_PHE_2015_Routine_Childhood_Immunisation_Schedule_12.pdf.
- UK Health Security Agency. The complete routine immunisation schedule from February 2022. [accessed 2023 June]. https://www.gov.uk/government/publications/the-complete-routine-immunisation-schedule/the-complete-routine-immunisation-schedule-from-february-2022.
- EU Clinical Trials Register. 2019-002585-12. [accessed 2023 Sept]. https://www.clinicaltrialsregister.eu/ctr-search/trial/2019-002585-12/IT.
- European Medicines Agency. Guideline on good pharmacovigilance practices (GVP): product- or population-specific considerations IV: paediatric population. [accessed 2023 June]. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-good-pharmacovigilance-practices-gvp-product-population-specific-considerations-iv_en-0.pdf.
- European Medicines Agency. Guideline on good pharmacovigilance practices (GVP) module IX addendum I - methodological aspects of signal detection from spontaneous reports of suspected adverse reaction. [accessed 2023 June]. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-good-pharmacovigilance-practices-gvp-module-ix-addendum-i-methodological-aspects-signal_en.pdf.
- European Medicines Agency. Guideline on good pharmacovigilance practices (GVP) Module IX- Signal management (Rev 1). [accessed 2023 June]. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-good-pharmacovigilance-practices-gvp-module-ix-signal-management-rev-1_en.pdf.
- Marchetti F, Vilca LM, Cetin I. Insights and expectations for Tdap vaccination of pregnant women in Italy. J Matern-Fetal Neo Med. 2021;34(13):2132–9. doi:10.1080/14767058.2019.1659240.
- Vilca LM, Cesari E, Tura AM, Di SA, Vidiri A, Cavaliere AF, Cetin I. Barriers and facilitators regarding influenza and pertussis maternal vaccination uptake: a multi-center survey of pregnant women in Italy. Eur J Obstet Gynecol Reprod Biol. 2020;247:10–15. doi:10.1016/j.ejogrb.2020.02.007.
- Maltezou HC, Wicker S, Borg M, Heininger U, Puro V, Theodoridou M, Poland GA. Vaccination policies for health-care workers in acute health-care facilities in Europe. Vaccine. 2011;29(51):9557–62. doi:10.1016/j.vaccine.2011.09.076.
- Hennink MM, Kaiser BN, Weber MB. What influences saturation? Estimating sample sizes in focus group research. Qual Health Res. 2019;29(10):1483–96. doi:10.1177/1049732318821692.
- Il tuo accesso diretto alla statistica italiana. Resident population: Calabria. [accessed 2023 June]. http://dati.istat.it/index.aspx?queryid=18962.
