ABSTRACT
Antibody obtained by the coronavirus disease-19 (COVID-19) mRNA vaccine declines over time, and additional vaccinations are offered. It is not clear how repeated vaccination affects humoral immunity in uninfected individuals. We analyzed immunoglobulin G for spike protein (S-IgG) titers in COVID-19 uninfected and infected individuals vaccinated up to six times. The geometric mean S-IgG titers were 575.9 AU/mL and 369.0 AU/mL in those who received 6 and 5 doses less than 180 days after the last vaccination in uninfected subjects. In the 180–360 days after the last vaccination, the geometric mean S-IgG titers were 237.9 AU/mL and 128.6 AU/mL in the uninfected subjects who underwent five-dose and four-dose groups, respectively. Multivariate analysis showed that S-IgG titer increased 1.261-fold with each additional dose of mRNA vaccine. The S-IgG titers were 2.039-fold higher in the COVID-infected subjects compared to uninfected subjects. The positivity rate of nucleocapsid antibodies, suggesting a history of COVID-19, decreased 82% and 30% of COVID-infected cases after 180 and 360 days of infection, respectively. This result suggested that repeated vaccination with the COVID-19 mRNA vaccine may increase antibody titer in uninfected subjects.
Introduction
Severe acute respiratory virus-2 (SARS-CoV-2) infection (coronavirus disease-19, COVID-19) has spread worldwide and is observed to cause multiple infections. Initially, vaccines against COVID-19 were highly effective,Citation1 however, antibody titers, as a protective effect against SARS-CoV-2 infection, observed declined over time after vaccination.Citation2 It is reported that hybrid immunity combining vaccination and natural infection produces higher antibody titers and reduces the risk of reinfection compared to vaccination or natural infection alone.Citation3,Citation4 Although there have been reports comparing the effectiveness of the fourth dose of vaccination in preventing infection as the third dose, there have been no reports evaluating up to the sixth dose in a population with a majority of uninfected individuals.Citation5 However, the significance of repeated vaccination in uninfected individuals was not clear.
In Japan, the COVID-19 vaccine is supplied by the government. As for adults, the supply of mRNA vaccine began on February 17, 2021, and 76.9% of the population completed the initial two doses. Initial two doses were followed by a third booster vaccination on December 1, 2021; additional vaccinations for the elderly, immunocompromised, and medical personnel on July 22, 2022; supply of Omicron strain-compatible bivalent vaccine began on September 20, 2022; and additional vaccinations for the elderly, immunocompromised, and medical personnel on May 8, 2023.Citation6 As a result, there will be up to six mRNA vaccination opportunities for health-care workers by August 2023.
The prevalence of COVID-19 rate using blood donations was estimated to be low at 42.3% as of February 2023 in Japan.Citation7 We evaluated the antibody titers for spike protein of SARS-CoV-2 in the health-care workers to analyze the significance of the repeated vaccination, especially the fifth and sixth mRNA vaccinations, especially in the COVID-naïve subjects. There was a large infection epidemic in Japan from August to September 2023, and collecting samples of antibody titers over time from uninfected individuals was difficult after the study period.
Nucleocapsid antibodies are also widely used in epidemiological analyses to estimate the rate of previous infection since they are elevated in patients with previous infection.Citation8 Although it was previously thought that Nucleocapsid antibodies remained positive for years, we also report that they became negative in the first year after infection.
Subjects and methods
Study participants were recruited from employees aged 20 years or older working at Yokohama City University Hospital (672 beds, approximately 1800 employees). Study participants were enrolled upon written informed consent, and blood samples were collected during the annual health check between June 26 and July 26, 2023. The serum of the blood samples was stored at −80°C. Stored sera were brought to room temperature on July 31, 2023, and assayed by Lumipulse G1200 plus (Fujirebio Inc. Tokyo) and chemiluminescent enzyme immunoassay (CLEIA) for SARS-CoV-2 S-IgG (S-IgG) and N-IgG (nucleocapsid IgG). The titer of 20 AU/mL in the S-IgG and 1.0 AU/mL of N-IgG was considered positive.
Study subjects were asked about the number of vaccinations with the mRNA vaccine, the date of last vaccination, the number of COVID-19 infections, and the date of last date of SARS-CoV-2 infection. In Japan, BNT162b2 (Pfizer, U.S.A.) and mRNA-1273, Spikevax (Moderna, U.S.A.) are available as mRNA vaccines, and either vaccination was counted as one. The history of COVID-19 was defined as a laboratory-confirmed COVID-19 diagnosis or positive N-IgG was also considered as a history of COVID-19. All patients with laboratory-confirmed positive for SARS-CoV-2 were registered in Japan until May 2023. The date of the last vaccination or the onset of COVID-19 symptoms (for those with laboratory-confirmed positive) was defined as the date of last exposure.
Continuous data are reported as means and 95% confidence intervals (CIs) or medians and interquartile ranges (IQRs). Categorical data were presented as numbers and percentages. Data were analyzed using two-tailed Mann–Whitney U-tests to compare continuous variables between two groups. To calculate the S-IgG titers, values below the detection threshold for S-IgG (value of <20 AU/mL) were assigned half the threshold value of 10 AU/mL. Multivariate regression analyses were performed to investigate predictors of log-transformed S-IgG titer, and adjustments were made to potential confounders, including age, mRNA vaccine doses, received Omicron bivalent vaccine, COVID-19 infection history, days after the last vaccine or infection. Because nucleocapsid antibody were assumed to be 100% positive at the time of infection and then become negative over time,Citation9 the positivity rate of N-IgG was calculated by the number of subjects for each day since infection Statistical analyses were performed by using Prism 10 (GraphPad Software, San Diego, CA, USA). A p value < .05 was considered statistically significant. This study was approved by the institutional ethics board (F230700042).
Results
Two hundred and thirty-five patients were analyzed in this study. The median age was 45 [37–52], 98 males and 157 females. The doses of mRNA vaccination were 6 (n = 96), 5 (n = 74), 4 (n = 40), 3 (n = 17), 2 (n = 2), 1 (n = 3) and none (n = 3). Two-hundred-fourteen individuals had a history of vaccination with the Omicron bivalent vaccine. A history of COVID-19 infection was found in 84 (36%); 67 had a history of laboratory-diagnosed infection (of which 18 were N-IgG positive), and 17 subjects were N-IgG positive without symptomatic COVID-19 history. No one had more than two COVID-19 infections.
Differences in antibody titers in the study subjects by number of vaccination and COVID-19 history
The S-IgG titers and the days since the last exposure (vaccination or natural infection), by the number of vaccinations, for 151 subjects with no history of COVID-19 and 84 subjects with a history of infection were shown (). Since the chances of additional vaccination were provided approximately every 6 months in Japan, it can be seen that many subjects are vaccinated every 180 days. The geometric mean titer of S-IgG was 701.1 AU/mL, 513.1 AU/mL, 188.1 AU/mL, 128.8 AU/mL, 96.19 AU/mL, 110.7 AU/mL, and 33.03 AU/mL in the subjects underwent 6, 5, 4, 3, 2, 1 and none mRNA doses vaccination, respectively. The geometric mean S-IgG titers were 342.7 AU/mL and 586.6 AU/mL in the uninfected and infected subjects.
Figure 1. S-IgG titer and the days since the last vaccination or infection date for each mRNA vaccination dose in uninfected (COVID-naïve) (a) and infected (COVID-infected) individuals (b). Cases in which more than 600 days had elapsed since the last vaccination or infection (two and one case, respectively) are not shown in the graph.
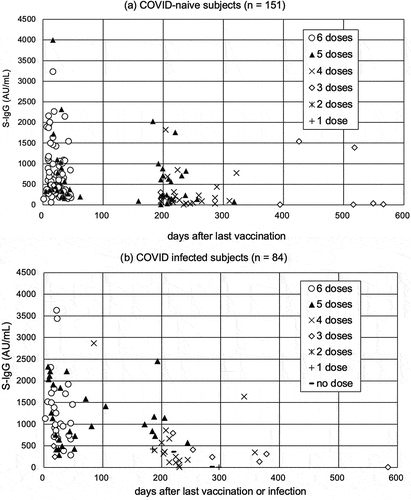
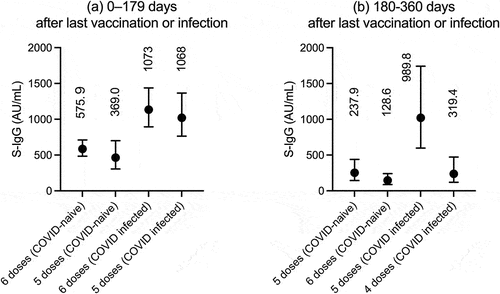
Focusing the subjects with 4–6 vaccinations, the S-IgG titer up to 180 days after the last vaccination or infection was 575.9 (IQR 326.2–1077) AU/mL for those with six vaccinations (N = 70, median days after the last vaccination, 20 days) and 369.0 (IQR 218.9–934.7) AU/mL for those with five vaccinations (N = 22, 31 days) in the uninfected subjects (). As same, 1073 (IQR 749.8–1703) AU/mL for those with six vaccinators (N = 26, 22 days) and 1068 (IQR 603.7–1949) AU/mL for those with five vaccinations (N = 22, 26 days) in the infected subjects. S-IgG titer at 180–360 days after the last vaccination or infection was 237.9 (IQR 94.63–713.3) AU/mL for those with five vaccinations (N = 28, 208 days) in the uninfected subjects and 128.6 (IQR 66.78–294.0) AU/mL (N = 24, 225 days) for those with four vaccinations in the uninfected subjects, and 989.8 (IQR 685.4–1491) AU/mL for those with five vaccinations (N = 6, 191 days) and 237.9 (94.63–713.3) AU/mL for those with four vaccinations (N = 28, 208 days) in the infected subjects.
Figure 2. S-IgG titers in the mRNA vaccinations in uninfected (COVID-naïve) and infected (COVID-infected) subjects (a) up to 180 days after vaccination and (b) at 180–360 days. Numbers represent median values. 6 no vaccinators had been vaccinated for 180 days during the survey. In addition, most 4-dose vaccinees were more than 180 days free from the last vaccination.
Analysis of the effect of infection history and number of mRNA vaccination on S-IgG titer
As shown in , S-IgG titer decreases exponentially from the last exposure (last vaccination or infection). Since it has been reported that antibody titer decreases with age,Citation10 multivariate analysis was performed on the log-transformed S-IgG titer using age, number of mRNA vaccinations, history of vaccination with Omicron-bivalent vaccines, history of infection, and days since last exposure as variables (). Log transformation back to real numbers showed that the S-IgG titer increased 1.261-fold with each increase in mRNA vaccinations. COVID-infected subjects also had a 2.039-fold higher S-IgG titer compared to uninfected individuals.
Table 1. Multivariate analysis adjusted for each variable against log-transformed S-IgG titer. Log-reversed regression coefficients showed that the S-IgG for COVID-infected subjects was 2.039-fold higher than that for COVID-naïve subjects. S-IgG titers were 1.261-fold higher for with each additional vaccination.
Duration of nucleocapsid antibody positivity
Of the 84 cases determined to have a history of COVID-19, the date of infection was clearly reported in 67 cases. When divided by the number of days since infection, all subjects (12/12) collected within 124 days of infection were positive. The positive rate for N-IgG in the subjects examined within 185 and 361 days after infection was 82% (14/17) and 30% (17/57), respectively.
Discussions
Additional mRNA vaccination in COVID-19 uninfected individuals was shown to result in higher spike protein antibodies (S-IgG). The present study shows that S-IgG titers were elevated in repeat vaccinators after the fifth and sixth vaccinations, suggesting that humoral immunity was maintained and possibly further enhanced. COVID-infected individuals were also found to have twice as much S-IgG compared to uninfected individuals who had been vaccinated in the same number of doses as we previously reported in standard population.Citation11 This is consistent with previous reports that vaccination reduces the risk of reinfection in infected individuals and is supported by the antibody titers.Citation12 The strategy of preventing infection by humoral immunity in COVID-19 is facing challenges due to the emergence of mutant strains.
Further investigation is warranted to determine if the enhanced antibody titer contributes to preventing infection. Our result may contribute to providing basic data for coming mRNA vaccines against other pathogens in the future.Citation13
There has been controversy regarding the effectiveness of multiple mRNA inoculations. It was suggested that a fourth vaccination in health-care workers may not adequately prevent infection during an outbreak of the Omicron strain.Citation14 The study mentioned that the level of humoral immunity did not differ after the third and fourth vaccinations. On the contrary, studies investigating vaccine efficacy after the fourth vaccination in elderly subjects observed higher vaccine efficacy after the fourth vaccination than after the third vaccination.Citation15,Citation16 There are few reports on vaccination after the fifth vaccination. In Japan, it was reported that the fifth vaccination in dialysis patients resulted in 85% of the subjects obtaining 1000 AU/mL of IgG to spike protein, and a high antibody titer level was maintained.Citation17 It was also reported that the fifth immunization resulted in higher antibody titers in lung transplant patients than after the fourth vaccination.Citation18 The present study also showed that subjects with a history of infection had twice the antibody titers of non-infected subjects, consistent with previous reports.Citation4 The present study suggested that humoral immunity was enhanced even in COVID-naïve subjects. Re-infected individuals now account for 40% of COVID-infected individuals,Citation19 and health hazards due to post-acute sequelae of SARS-CoV-2 may be elevated in re-infected individuals.Citation20 Our study suggests that additional mRNA vaccinations may be provided in an aspect of controlling social health.
Nucleocapsid antibodies (N-IgG) had been considered an indicator of a history of COVID-19 infection was found to become negative over time. According to previous reports, nucleocapsid antibodies were positive up to 100 days after infection. They were estimated to be detectable up to 500 days,Citation21 which has been used as an indicator to analyze the prevalence of COVID-19 in a population using blood donations. It has been reported to become negative over time in study.Citation8 In our study, nucleocapsid antibody positivity was 82% and 30% after a half and 1 year from COVID-19 infection, respectively. It will be necessary to be careful in estimating the rate of existing infections in the future based on the nucleocapsid antibody positivity.
There are several limitations to this study. The study participants were healthy hospital workers. However, it is possible that they were not interviewed sufficiently in the questionnaire for all medicines and medical histories. It was reported that 40.8% of the infected patients were assumed to be asymptomatic and possibly included in the COVID-naive group in this study.Citation22 In addition, the date of vaccination or infection was not confirmed by documents, and recall bias cannot be ruled out. S-IgG titers were measured by the CLEIA method using Lumipulse in this study. A few studies were comparing the antibody titers measured by Lumipulse and other automated methods,Citation23 and also, it is not clear whether the antibodies have direct neutralizing activity against mutant strains, including the Omicron strain. It was reported that the neutralizing antibody titers and IgG antibody titers correlate.Citation24 We also did not measure cellular immunity, which was also a limitation of this study. This study analyzed cross-sectional antibody titers by a single point. As mentioned earlier, the subsequent major epidemic made it difficult to evaluate antibody titers over time in the COVID-naïve subjects.
In conclusion, this study suggests that repeated mRNA vaccination may further increase antibody titers in COVID-19 uninfected persons and may assist in the consideration of future vaccine programs.
Author contributions
HK contributed to the study design, data collection, statistical analysis, and interpretation of data, as well as the drafting and editing of the manuscript. TK and KH contributed data collection and performed the laboratory tests. AR and KM contributed to the laboratory tests and supervision of the analysis. YK and AG contributed to the study design and supervision of the analysis. All authors made critical revisions to the manuscript for important intellectual content and approved the final manuscript. All authors meet the ICMJE authorship criteria.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603–5. doi:10.1056/NEJMoa2034577.
- Kato H, Miyakawa K, Ohtake N, Yamaoka Y, Yajima S, Yamazaki E, Shimada T, Goto A, Nakajima H, Ryo A, et al. Vaccine-induced humoral response against SARS-CoV-2 dramatically declined but cellular immunity possibly remained at 6 months post BNT162b2 vaccination. Vaccine. 2022;40(19):2652–5. doi:10.1016/j.vaccine.2022.03.057.
- Wang Q, Iketani S, Li Z, Liu L, Guo Y, Huang Y, Bowen AD, Liu M, Wang M, Yu J, et al. Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants. Cell. 2023;186(2):279–86.e8. doi:10.1016/j.cell.2022.12.018.
- Tan ST, Kwan AT, Rodríguez-Barraquer I, Singer BJ, Park HJ, Lewnard JA, Sears D, Lo NC. Infectiousness of SARS-CoV-2 breakthrough infections and reinfections during the Omicron wave. Nat Med. 2023;29:358–65. doi:10.1038/s41591-022-02138-x.
- Magen O, Waxman JG, Makov-Assif M, Vered R, Dicker D, Hernán MA, Lipsitch M, Reis BY, Balicer RD, Dagan N, et al. Fourth dose of BNT162b2 mRNA COVID-19 vaccine in a nationwide setting. N Engl J Med. 2022;386(17):1603–14. doi:10.1056/NEJMoa2201688.
- Prime minister’s Office of Japan. COVID-19 vaccines. [accessed 2023 Sept 7]. https://www.kantei.go.jp/jp/headline/kansensho/vaccine_supply.html.
- Ministry of Health Labour and Welfare. Survey of COVID-19 antibody prevalence using blood donation samples. [accessed 2023 Sep 7]. https://www.mhlw.go.jp/content/10906000/001070846.pdf.
- Krutikov M, Palmer T, Tut G, Fuller C, Azmi B, Giddings R, Shrotri M, Kaur N, Sylla P, Lancaster T, et al. Prevalence and duration of detectable SARS-CoV-2 nucleocapsid antibodies in staff and residents of long-term care facilities over the first year of the pandemic (VIVALDI study): prospective cohort study in England. Lancet Heal Longev. 2022;3:e13–e21. doi:10.1016/S2666-7568(21)00282-8.
- Burbelo PD, Riedo FX, Morishima C, Rawlings S, Smith D, Das S, Strich JR, Chertow DS, Davey RT, Cohen JI, et al. Sensitivity in detection of antibodies to nucleocapsid and spike proteins of severe acute respiratory syndrome coronavirus 2 in patients with coronavirus disease 2019. J Infect Dis. 2020;222(2):206–13. doi:10.1093/infdis/jiaa273.
- Kato H, Miyakawa K, Ohtake N, Go H, Yamaoka Y, Yajima S, Shimada T, Goto A, Nakajima H, Ryo A, et al. Antibody titers against the alpha, beta, Gamma, and Delta variants of SARS-CoV-2 induced by BNT162b2 vaccination measured using automated chemiluminescent enzyme immunoassay. J Infect Chemother. 2022;28(2):273–8. doi:10.1016/j.jiac.2021.11.021.
- Goto A, Miyakawa K, Nakayama I, Yagome S, Xu J, Kaneko M, Ohtake N, Kato H, Ryo A. Prediction models for neutralization activity against emerging SARS-CoV-2 variants: a cross-sectional study. Front Microbiol. 2023;14:1126527. doi:10.3389/fmicb.2023.1126527.
- Bobrovitz N, Ware H, Ma X, Li Z, Hosseini R, Cao C, Selemon A, Whelan M, Premji Z, Issa H, et al. Protective effectiveness of previous SARS-CoV-2 infection and hybrid immunity against the Omicron variant and severe disease: a systematic review and meta-regression. Lancet Infect Dis. 2023;23(5):556–67. doi:10.1016/S1473-3099(22)00801-5.
- National Institutes of Health. Decades in the making: mRNA COVID-19 vaccines. [accessed 2023 Sep 7]. https://covid19.nih.gov/nih-strategic-response-covid-19/decades-making-mrna-covid-19-vaccines.
- Regev-Yochay G, Gonen T, Gilboa M, Mandelboim M, Indenbaum V, Amit S, Meltzer L, Asraf K, Cohen C, Fluss R, et al. Efficacy of a fourth dose of COVID-19 mRNA vaccine against Omicron. N Engl J Med. 2022;386(14):1377–80. doi:10.1056/NEJMc2202542.
- Grewal R, Kitchen SA, Nguyen L, Buchan SA, Wilson SE, Costa AP, Kwong JC. Effectiveness of a fourth dose of COVID-19 mRNA vaccine against the Omicron variant among long term care residents in Ontario, Canada: test negative design study. BMJ. 2022;378:e071502. doi:10.1136/bmj-2022-071502.
- Nordström P, Ballin M, Nordström A. Effectiveness of a fourth dose of mRNA COVID-19 vaccine against all-cause mortality in long-term care facility residents and in the oldest old: a nationwide, retrospective cohort study in Sweden. Lancet Reg Heal Eur. 2022;21:100466. doi:10.1016/j.lanepe.2022.100466.
- Tani Y, Takita M, Wakui M, Saito H, Nishiuchi T, Zhao T, Yamamoto C, Kawamura T, Sugiyama A, Nakayama A, et al. Five doses of the mRNA vaccination potentially suppress ancestral-strain stimulated SARS-CoV2-specific cellular immunity: a cohort study from the Fukushima vaccination community survey, Japan. Front Immunol. 2023;14:1240425. doi:10.3389/fimmu.2023.1240425.
- van Gemert J, Steenberg F, van Leer-Buter C, Kerstjens H, Steenhuis W, Akkerman O, Verschuuren E, Gan T. Increasing antibody responses to five doses of SARS-CoV-2 mRNA vaccine in lung transplant patients. J Clin Med. 2023;12:12. doi:10.3390/jcm12124125.
- Ma KC, Dorabawila V, León TM, Henry H, Johnson AG, Rosenberg E, Mansfield JA, Midgley CM, Plumb ID, Aiken J, et al. Trends in laboratory-confirmed SARS-CoV-2 reinfections and associated hospitalizations and deaths among adults aged ≥18 years — 18 U.S. Jurisdictions, September 2021–December 2022. Morb Mortal Wkly Rep. 2023;72:683–9. doi:10.15585/mmwr.mm7225a3.
- Bowe B, Xie Y, Al-Aly Z. Acute and postacute sequelae associated with SARS-CoV-2 reinfection. Nat Med. 2022;28(11):2398–405. doi:10.1038/s41591-022-02051-3.
- Swartz MD, DeSantis SM, Yaseen A, Brito FA, Valerio-Shewmaker MA, Messiah SE, Leon-Novelo LG, Kohl HW, Pinzon-Gomez CL, Hao T, et al. Antibody duration after infection from SARS-CoV-2 in the Texas coronavirus antibody response survey. J Infect Dis. 2023;227(2):193–201. doi:10.1093/infdis/jiac167.
- Ma Q, Liu J, Liu Q, Kang L, Liu R, Jing W, Wu Y, Liu M. Global percentage of asymptomatic SARS-CoV-2 infections among the tested population and individuals with confirmed COVID-19 diagnosis: a systematic review and meta-analysis. JAMA Netw Open. 2021;4:e2137257. doi:10.1001/jamanetworkopen.2021.37257.
- Hibino M, Watanabe S, Kamada R, Tobe S, Maeda K, Horiuchi S, Kondo T. Antibody responses to the BNT162b2 mRNA vaccine in healthcare workers in a general Hospital in Japan: a comparison of two assays for anti-spike protein immunoglobulin G. Intern Med. 2022;61:811–9. doi:10.2169/internalmedicine.8704-21.
- Higashimoto Y, Kozawa K, Miura H, Kawamura Y, Ihira M, Hiramatsu H, Suzuki R, Haga K, Takai-Todaka R, Sawada A, et al. Correlation between anti-S IgG and neutralizing antibody titers against three live SARS-CoV-2 variants in BNT162b2 vaccine recipients. Hum Vaccin Immunother. 2022;18(6):2105611. doi:10.1080/21645515.2022.2105611.
