?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Varicella is an acute, highly contagious disease in susceptible individuals and is preventable through vaccination. This study aimed to determine the impact of varicella vaccination on hospitalizations and complications at a pediatric reference hospital in Panama before and after the vaccine introduction. This descriptive ecological study analyzed clinical records of patients diagnosed with varicella through a retrospective and interrupted time series analysis. An autoregressive integrated moving average model was built to compare the incidence rates observed after vaccination with those expected rates derived from the model. A statistical model was fitted to the observed interrupted time series data by regression and used to predict future trends. The mean difference in varicella hospital discharges before and after the introduction of the varicella vaccine was 47%. The rate of hospitalizations for varicella decreased to 52.3%. A declining trend in varicella hospitalizations was observed from 2015 after vaccine introduction in 2014. Complications in vaccinated patients were secondary skin and soft tissue infection, possibly due to bacterial superinfection. The impact of varicella vaccination on reducing varicella hospital discharges reported at a pediatric reference hospital in Panama was confirmed.
Introduction
Varicella is a common exanthem disease with worldwide distribution. It is highly contagious in susceptible individuals and has a secondary attack rate between 60% and 90%.Citation1
Varicella is spread by airborne, droplet, and contact transmission.Citation2 Some of the complications that may occur include encephalitis, pneumonitis, and secondary bacterial infections that could lead to hospitalization and, in some cases, deaths.Citation3 In 2014, the World Health Organization (WHO) estimated that approximately 4.2 million severe complications leading to hospitalization and 4200 related deaths occur globally each year.Citation4
Varicella is preventable through vaccination. The United States of America (USA) was the first country to include the varicella vaccine in its vaccination schedule in 1995. Before its introduction, it was estimated that close to 4 million cases 10,000 hospitalizations and 100 deaths occurred yearly due to varicella, with a higher incidence in children below 15 years of age.Citation3,Citation5,Citation6 After its introduction, outpatient visits reduced by 66%, reaching 98% in children under 5 years of age.Citation7 After implementing a second dose of the vaccine, the incidence, hospitalizations, and deaths due to varicella decreased by more than 90% compared to the pre-vaccine era, with a higher impact on the pediatric population.Citation8,Citation9
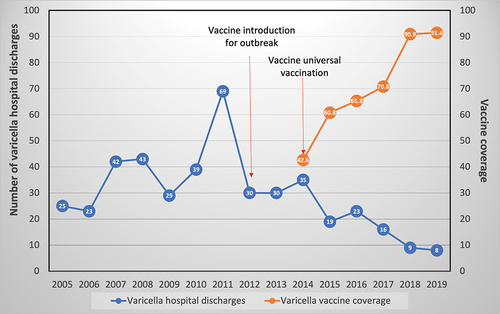
According to data obtained from the Expanded Program on Immunization (EPI), the varicella vaccine was included in Panama for outbreak control in 2012 and has been universally administered since 2014.Citation10 The first dose is administered at 15 months, with a booster at 4 years of age. However, its impact on the country, particularly the pediatric population, had not yet been documented.
As reported in the 2018 statistical bulletin of Panama’s EPICitation11 coverage of the first dose of varicella vaccine reached 90.9% in that year. However, there are no records of the impact of two doses of the varicella vaccine in the country.
The objective of this study was to evaluate the effectiveness of varicella vaccination in reducing the number of hospitalizations in Hospital del Niño Dr José Renán Esquivel (HNDJRE) and deaths and to evaluate the impact on hospitalization costs after its implementation in the national vaccination schedule.
This information will be useful to justify the government’s investment in programs to prevent the disease, as its benefit to health is universal.
Materials and methods
Study design
This was a descriptive, observational impact (ecological) study using an interrupted time series analysis of varicella hospital discharges in a reference pediatric hospital in Panama following universal varicella vaccination introduced in the country. An interrupted time series analysis was used to evaluate the effect of immunization before and after this intervention.
Data sources
Data of the available clinical records of varicella hospital discharges at the HNDJRE in Panama City between January 2005 and December 2019 were collected. The variables studied included age, sex, number of hospitalization days, current complications, treatment administered, and condition at discharge.
Descriptive analysis
Data of varicella hospital discharges observed between 2005 and 2014 (pre-vaccination) versus the expected number of discharges from 2015 to 2019 (post-vaccination) were plotted. The data were analyzed descriptively, using percentages, median, mean, and frequencies which are presented in tables and figures. Associated complications were also described.
Hospitalization rates were estimated based on the number of varicella admissions among the total number of admissions by age groups and expressed per 10,000 hospitalizations.
Comparisons between the pre- and postvaccination periods were reported.
Vaccine coverage for varicella information was obtained through the Expanded Program of Immunization from the Ministry of Health in Panama for years 2014 to 2019. Partial vaccine coverage included those children who received only one dose and complete vaccine coverage included children who received two doses. Denominator data for vaccine coverage were derived from the population of children aged less than 1 year from the administrative census in the country.
Interrupted time series (ITS) analysis
We analyzed annual hospital data using an ITS with a sequence of varicella outcomes observed during a continuous time period in Panama reported on an annual basis. Following varicella vaccination recommended universally in the country, we divided the dataset into three different periods as follows: the pre-vaccination period (2005–2011), a transition period (2012–2014), and the post-vaccination period (2015–2019). During the progression period, vaccine coverage progressively increased in the country.
As recommended by Linden,Citation12 a time series model was evaluated using pre-vaccination data to forecast predicted number of hospitalizations over time. Seasonality and noise in the time series were explored.
where Y was the varicella hospitalizations reported at each time point (t). Tt was the time since the initiation of the analysis. Xt indicated the introduction of varicella vaccination and XtTt was an interaction term. For single group analysis, β0 represented the number of varicella hospital discharges at the hospital in 2005, β1 was the slope of varicella hospital discharges during the pre-vaccination period, β2 was the change in the level after vaccine introduction and β3 represented the differences between the pre-vaccination and the post-vaccination period. Significance tests were applied to identify statistical differences during these two periods. The projected trend of the pre-vaccination period was used as counterfactual to determine differences between the predicted values as compared to the observed hospital discharges in the post-vaccination period.
We used two ordinary least-squares (OLS) regression-based approaches. The first was used to produce Newey-west standard errors to handle autocorrelation and possible heteroskedasticity and the second one in which the errors were assumed to follow an AR(1) process, as data points corresponded to annual data. The second one permits to transform the original observations based on the pooled autocorrelation.
We first fitted the OLS model and then tested for autocorrelation in the error distribution, allowing us to validate the adequacy of the time-series model. For this purpose, autocorrelation (ACF: comparing values of varicella hospital discharges comparing with previous years) and partial autocorrelations functions (PACF: comparing only with the previous data point) were employed. These analyses were stratified by age groups: children under 14 years of age, 1–4 years of age, and 5–9 years of age.
Autoregressive integrated moving average ARIMA model
An autoregressive integrated moving average ARIMA model was built, a variation of the interrupted time series analysis to evaluate the vaccination program’s impact by comparing the incidence rates observed after vaccination against the expected incidence rates derived from the model. A statistical model was adjusted to the temporary time series data observed by regression, and the adjusted model was used to forecast the future expected trends. The observed varicella incidence data were compared to the expected incidence rates forecast by the autoregressive integrated moving average ARIMA model. All analysis were performed using STATA 14® software was used for the analysis.
Ethics
The study was approved by the Hospital del Niño Jose Renán Esquivel Bioethics Committee in Panama City.
Results
Descriptive results
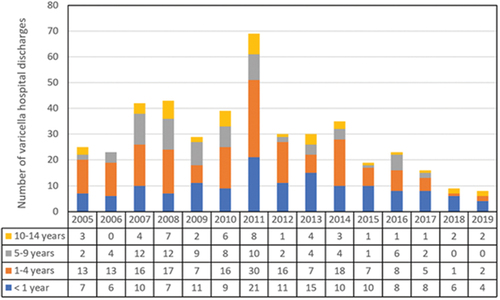
From 2005 to 2019, there were 440 varicella hospital discharges at the HNDJRE (), of which we evaluated 293 clinical records. presents the characteristics of these 293 patients.
Table 1. Characteristics of patients hospitalized with varicella in the hospital del Niño Dr José Renán Esquivel (2005–2019).
Of the 293 patients for whom clinical records were evaluated, 59% (n = 174) complications related to varicella were recorded. These included: secondary skin and soft tissue infection, possibly due to bacterial superinfection in 87.4% (n = 152), pneumonia due to varicella in 3.4% (n = 6), encephalitis due to varicella and febrile convulsion, both in 2.9% (n = 5), aseptic meningitis, post-varicellar cerebellar ataxia, secondary facial paralysis, post-infectious glomerulonephritis due to varicella, Guillain Barré syndrome, septic shock, each corresponding to 0.57% (n = 1) of the patient’s clinical records. The distribution of complications for these patients by age and by period is presented in .
Table 2. Distribution of complications due to varicella by age group in the hospital del Niño Dr José Renán Esquivel patients (2005–2019).
The median number of days of hospitalization at the HNDJRE according to the different periods was 5 days. Prolonged hospitalizations observed in this study were associated with chronic concomitant pathologies such as oncologic conditions and chronic disease (acute lymphoblastic leukemia, human immunodeficiency virus infection, burns, neoplasia, multiple sclerosis, and congenital heart disease).
In terms of deaths, there were 11, representing 2.5% of the total of varicella hospital discharges from the HNDJRE in the 2005–2019 period. None of the patients who died in the post-vaccination period (n = 4) had the vaccine. Death-related complications included septic shock, pneumonia, encephalitis, and secondary bacterial infections. All these were related to the initial varicella infection.
The cumulative number of varicella hospital discharges in the pre-vaccination period was 270 and in the post-vaccination period was 75, corresponding to a 72.7% reduction. Meanwhile, the mean number of varicella hospital discharges during the pre-vaccination period (2005–2011) was 38.6; during the transition period (2012–2014), was 31.7 and in the post-vaccination period (2015–2019) was 15.2, corresponding to a 47% reduction.
The hospitalization rate due to varicella in the pre-vaccine era was, on average, 24.99 per each 10,000 discharged patients (CI 17.04–32.57), 21.14 (CI 18.44–23.83) in the transition period and decreased to an average hospitalization rate of 13.08 per each 10,000 discharged patients after the introduction of universal varicella vaccination (CI 6.21–19.95). The reduction in the mean number of varicella hospital discharges from the pre-vaccination to the post-vaccination period was 47.7%.
In terms of gender, the male was generally predominant, with 58.4.% varicella hospital discharges in the 2005–2019 period.
In the pre-vaccination period (2005–2011), it was observed that the predominant age group was 1–4 years of age, with 41.5% (n = 112) of hospitalizations due to varicella at the HNDJRE and a mean age was 3.4 years, while from 2014, the predominant age group was under 1 year of age, with 48% (n = 36) and a mean age of 4 months. The overall mean age of the total patients with varicella was 2.92 years (range, 0–14 years) ().
Varicella vaccination analysis
National vaccination coverage against varicella in Panama was 42.6% in 2014 (first dose: 15 months and a booster dose at 4 years of age), reaching a coverage of 91.4% in 2019. The booster dose was introduced in 2018, with a coverage of 40% in 2020, according to data obtained from Panama’s EPI program.
Interrupted time series analysis
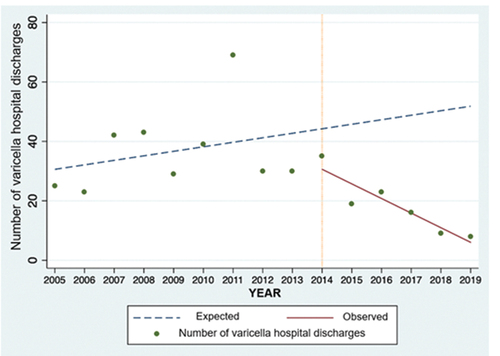
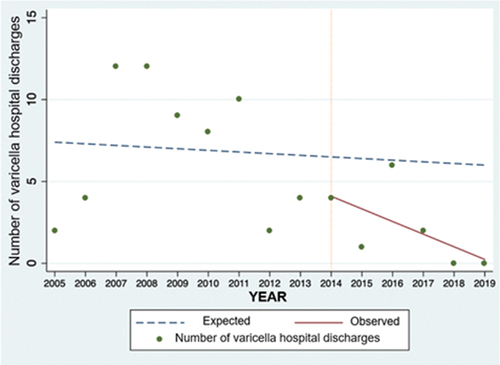
The interrupted time series analysis shows a progressive decrease in varicella hospital discharges in children under 14 years of age after 2014. () Comparing the observed to the expected number of discharges, the reduction observed after the intervention is 88.3% (CI 95%: 80.7–91.6%) ().
Table 3. Estimated level and trend changes of reported varicella incidence before and after vaccine introduction by age group.
For children under 1 year of age, the interrupted time series analysis estimated a reduction of 79.4% (CI 95%: 75.9–81.9%) in observed varicella hospital discharges compared to the expected number of discharges for 2019.
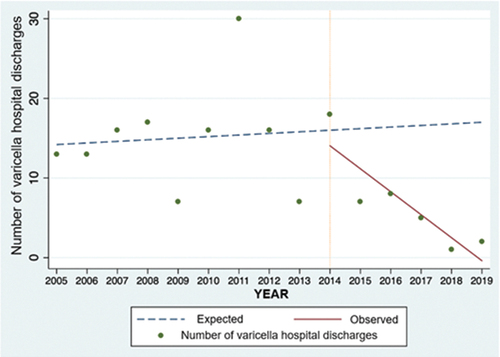
For children in the 1–4 years of age group, the interrupted time series analysis estimated a reduction of 93.7% (CI 95%: 82.6–96.2%) in observed varicella hospital discharges compared to the expected number of discharges for 2019 ().
A similar trend was observed in the 5–9 years and 10–14 years of age groups, in which the number of varicella hospital discharges observed was lower than expected. However, a decreasing trend was observed for the 5–9 years of age group before the start of vaccination ().
Discussion
The introduction of the varicella vaccine in the national vaccination schedules with either one or two doses, which started in 1995 in the USA,Citation4 has shown a significant decrease in the incidence of infections, hospitalizations, hospital costs, and associated complications.Citation3,Citation13,Citation14
A reduction in the number of patients discharged with varicella diagnosis from the HNDJRE since 2015 was evidenced after the start of the universal varicella vaccination in 2014 and the administration of the first dose at 15 months of age, also impacting the reduction in the incidence of patients from the 2015–2019 period. The data are more evident for 2018 and 2019, when, in addition, more than 90% of vaccination coverage against varicella was achieved. This was also documented in the interrupted time series analysis, which showed a descending trend when comparing the number of varicella hospital discharges observed against the expected number of discharges during the years in the post-vaccination period.
In an analysis performed for the years 1993–2004, Shah et al. found a 53.1% decrease in the incidence of varicella in hospitalized patients in the USA.Citation7 Studies of the impact of varicella vaccination in the USA, Canada, Costa Rica, Brazil, and Uruguay have shown a reduction in the notification of varicella cases and a decrease in hospitalization (between 70% and 90% respectively), at the age group 1–4 years of age, showing a significant reduction in the number of varicella cases.Citation3,Citation7,Citation8,Citation14–18 Our study observed a 47% decrease in the mean number of varicella hospital discharges, with children under 5 years of age being the age group where a more significant impact of the varicella vaccination was observed, which was also observed in reports of some authors where children under 6 years of age are the age group where varicella is most common and, therefore, the group which would get a more significant benefit from the vaccine. It is considered that the reduction in the occurrence of varicella takes 4–5 years after vaccine implementationCitation6,Citation19; thus, the impact of varicella vaccination in Panama should be observed from 2018, which coincides with our findings.
A study conducted in Australia by Quinn et al. showed that including a single-dose vaccine schedule reduced disease in children and adults, including hospitalizations (64–69% vaccine effectiveness for the prevention of hospitalizations due to varicella.) However, there are still reports of varicella virus circulation cases in children.Citation20
In contrast to other studies,Citation4 our study did not show a shift of infections to older age groups. The age group with the highest hospitalizations was children under 5 years of age, showing a subsequent reduction of 56.9% after introducing the varicella vaccine.
The mean age was 2.92 years, which coincides with other studies, such as the one conducted in Costa Rica by Ávila-Aguero et al., where the mean age was 3.5 years.Citation14
The interrupted time series analysis showed a progressive reduction of 88.3% in the number of varicella hospital discharges in patients under 14 years of age since 2014. The under 5 years of age group has the highest impact of the vaccine, which agrees with the findings of a study conducted in Tucuman, Argentina, by Barrenechea and his team after the vaccine's introduction in 2015, where a reduction in the incidence of varicella cases since 2016 was observed, being more evident in 2019.Citation21
The reduction of disease incidence is related to a decrease in complications.Citation9 The main complication found was secondary skin and soft tissue infection, possibly due to bacterial superinfection, causing infection in the skin and soft tissues (87.4%). This finding is consistent with the report by Sáez-Llorens et al. in a study conducted at the HNDJRE from January 1991 to December 2000, where the most frequent complications were skin and subcutaneous infections (45%), respiratory infections (25%), and neurological disorders (7%).Citation22
Regarding complications, there was no significant change in mortality due to varicella neither in the pre-vaccination, transition, and post-vaccination period, with a mean annual death of 1 case, representing 2.5% of the total varicella hospital discharges from the HNDJRE, similar to the data reported by Ávila-Aguero et al. with 4% of deaths due to varicella. In the study by Sáez-Llorens et al., deaths represented 2.5%, consistent with our findings.
In a study by Latasa et al. in Madrid, Spain, no severe varicella cases were reported in vaccinated patients. In contrast, the cases of patients previously vaccinated showed mild cases of the illness.Citation23 Our findings show that for the vaccinated patients, even though complications occurred, such as secondary skin and soft tissue infection possibly due to bacterial superinfection, these were not lethal. Also, most of the hospitalizations and complications occurred in unvaccinated patients.
The median lenght of hospitalization was 5 days. In the study by Sáez-Llorens et al., the mean was 8.9 days, and prolonged hospitalizations were associated with patients with complications.Citation22 In our findings, some prolonged hospitalizations were also associated with chronic concomitant pathologies such as neoplasia, acquired immunodeficiencies, and chronic diseases, and in 2018 and 2019, an increase in patients under 1 year of age hospitalized with varicella, with prolonged hospitalizations.
Since 2018, the impact of varicella vaccination has been evident at the HNDJRE, with a 47% reduction in the mean number of varicella hospital discharges.
After introducing the first dose of the varicella vaccine in 2014, inpatient hospitalizations of children under 5 years of age were reduced by 56.9%.
Along with the decrease in the incidence of hospitalizations due to varicella, there is a decrease in complications occurrence, with the most frequent in hospitalized patients being secondary skin and soft tissue infection and secondary skin and soft tissue infection possibly due to bacterial superinfections.
Acknowledgments
We would like to thank the Hospital del Niño Dr José Renán Esquivel for the access to the clinical records, the Panamanian Expanded Program on Immunization for the immunization data, other colleagues for guidance in the analysis of the results, and Ogmas Writers for editorial assistance.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data supporting this study’s findings are available from the corresponding author DE upon reasonable request.
Additional information
Funding
References
- Seward JF, Zhang JX, Maupin TJ, Mascola L, Jumaan AO. Contagiousness of varicella in vaccinated cases. JAMA. 2004;292(6):704. doi:10.1001/jama.292.6.704.
- Ayoade F, Kumar S. Varicella-zoster virus (Chickenpox). 2023.
- Varela FH, Pinto LA, Scotta MC. Global impact of varicella vaccination programs. Hum Vaccin Immunother. 2019;15(3):645–8. doi:10.1080/21645515.2018.1546525.
- World Health Organization. Varicella and herpes zoster vaccines: WHO position paper, June 2014. Wkly Epidemiol Rec. 2014;89(25):265–87. [accessed 2023 Sep 23]. https://pubmed.ncbi.nlm.nih.gov/24983077/.
- Gabutti G, Franchi M, Maniscalco L, Stefanati A. Varicella-zoster virus: pathogenesis, incidence patterns and vaccination programs. Minerva Pediatr. 2016;68:213–25.
- Marin M, Meissner HC, Seward JF. Varicella prevention in the United States: a review of successes and challenges. Pediatrics. 2008;122(3):e744–e51. doi:10.1542/peds.2008-0567.
- Shah SS, Wood SM, Luan X, Ratner AJ. Decline in varicella-related ambulatory visits and hospitalizations in the United States since routine immunization against varicella. Pediatr Infect Dis J. 2010;29(3):199–204. doi:10.1097/INF.0b013e3181bbf2a0.
- Lopez AS, Zhang J, Marin M. Epidemiology of varicella during the 2-dose varicella vaccination program — United States, 2005–2014. MMWR Morb Mortal Wkly Rep. 2016;65(34):902–5. doi:10.15585/mmwr.mm6534a4.
- Leung J, Harpaz R. Impact of the maturing varicella vaccination Program on varicella and related outcomes in the United States: 1994–2012. J Pediatric Infect Dis Soc. 2016;5(4):395–402. doi:10.1093/jpids/piv044.
- World Health Organization. Vaccine introduction in Panama. [accessed 2023 Sep 23]. https://immunizationdata.who.int/pages/vaccine-intro-by-country/pan.html?YEAR=.
- Boletín Estadístico 2018. Ministerio de Salud de Panamá. Published 2018. [accessed 2023 June 6]. https://www.minsa.gob.pa/sites/default/files/programas/boletin_2018.pdf.
- Linden A. Conducting interrupted time-series analysis for single- and multiple-group comparisons. Stata J. 2015;15(2):480–500. doi:10.1177/1536867X1501500208.
- Hirose M, Gilio AE, Ferronato AE, Ragazzi SLB. Impacto da vacina varicela nas taxas de internações relacionadas à varicela: revisão de dados mundiais. Revista Paulista de Pediatria. 2016;34(3):359–66. doi:10.1016/j.rpped.2015.12.006.
- Avila-Aguero ML, Ulloa-Gutierrez R, Camacho-Badilla K, Soriano-Fallas A, Arroba-Tijerino R, Morice-Trejos A. Varicella prevention in Costa Rica: impact of a one-dose schedule universal vaccination. Expert Rev Vaccines. 2017;16(3):229–34. doi:10.1080/14760584.2017.1247700.
- Giachetto G. Varicela: Situación Epidemiológica y Actualización de Las Medidas de Prevención. 2013 [accessed 2023 June 6]. http://www.scielo.edu.uy/scielo.php?script=sci_arttext&pid=S1688-12492013000400011.
- Centers for Disease Control and Prevention. Prevention of varicella: recommendations of the advisory committee on immunization practices (ACIP). Centers for disease control and prevention. MMWR Recomm Rep. 1996;45(RR–11):1–36.
- Waye A, Jacobs P, Tan B. The impact of the universal infant varicella immunization strategy on Canadian varicella-related hospitalization rates. Vaccine. 2013;31(42):4744–8. doi:10.1016/j.vaccine.2013.08.022.
- de Martino Mota A, Carvalho-Costa FA. Varicella zoster virus related deaths and hospitalizations before the introduction of universal vaccination with the tetraviral vaccine. J Pediatr (Rio J). 2016;92(4):361–6. doi:10.1016/j.jped.2015.10.003.
- Jung J, Ko YJ, Kim YE, Huh K, Park BJ, Yoon SJ. Epidemiological impact of the Korean national immunization program on varicella incidence. J Korean Med Sci. 2019;34(7). doi:10.3346/jkms.2019.34.e53.
- Quinn HE, Gidding HF, Marshall HS, Booy R, Elliott EJ, Richmond P, Crawford N, McIntyre PB, Macartney KK. Varicella vaccine effectiveness over 10 years in Australia; moderate protection from 1-dose program. J Infect. 2019;78(3):220–5. doi:10.1016/j.jinf.2018.11.009.
- Barrenechea GGCREPM& SBL. Análisis por series temporales de la incidencia de varicela y el impacto de la implementación de la vacuna en Tucumán. Rev Argent Salud Publica. 2020;12(7):1–7.
- Sáez-Llorens X, Suman OD, Morós DD, Pilar Rubio MD. Complicaciones y costos asociados a la varicela en niños inmunocompetentes. Revista Panamericana de Salud Pública. 2002;12(2). doi:10.1590/S1020-49892002000800006.
- Latasa P, Gil de Miguel A, Barranco Ordoñez MD, Rodero Garduño I, Sanz Moreno JC, Ordobás Gavín M, Esteban Vasallo M, Garrido-Estepa M, García-Comas L. Effectiveness and impact of a single-dose vaccine against chickenpox in the community of Madrid between 2001 and 2015. Hum Vaccin Immunother. 2018;14(9):2274–80. doi:10.1080/21645515.2018.1475813.