ABSTRACT
The safety of human papillomavirus (HPV) vaccines, one of the major challenges to public vaccination, has been controversial. This study assessed the adverse reactions of 9-valent HPV (9vHPV) vaccines. This open-label, observational, multi-center, post-marketing study assessed the safety of 9vHPV administered according to local clinical practice. All post-marketing adverse events (AEs) reports received between December 2019 and November 2021 in Chongqing were analyzed. A total of 1000 individuals aged 16–26 years provided safety data post-vaccination; The most common AEs (60.1%) experienced by 9vHPV vaccine recipients were vaccination-site AEs (pain, swelling, induration) and non-vaccination-site AEs (dizzy, weak, fever). Vaccination-site AEs most were mild-to-moderate in intensity. Discontinuations and HPV 9-related serious AEs were rare (0.3% and 0.0%, respectively). Eight SAEs were reported during the study but none were considered as related to the study vaccine. The 9vHPV vaccine was generally well tolerated in subjects aged 16–26 years; Vaccination-site AEs were more common with 9vHPV.
Introduction
The human papillomavirus (HPV) is the most common sexually transmissible infection (STI) in the world. HPV is a deoxyribonucleic acid (DNA) virus classified into two categories: low-risk HPVs (LR-HPVs) and high-risk HPVs (HR-HPVs) that caused anogenital, cutaneous warts, and oropharyngeal cancers.Citation1–5 Among them, cervical cancer, the fourth most prevalent cancer in women, is an HPV-related disease that leads to high mortality in women.Citation6,Citation7
There were 604,000 new cervical cancer patients and 342,000 deaths worldwide in 2020, about 109,741 new cases and 59,060 deaths in China.Citation8 According to the prediction of epidemiological evidence if interventions are not taken, the number of new cervical cancer cases from 2010 to 2050 may increase at a rate of 40%–50% in China.Citation9 And the prevalence rate of HPV infection changes in different countries, but also varies in different regions of China.Citation10–14 The prevalence of HR-HPV infection and HPV genotype distribution in Chongqing women with abnormal cytological tests HPV52, HPV58, HPV16 were the most prevalent genotypes.Citation15
At present, the use of HPV vaccines is one of the most important ways to refrain from HPV infection. 9-Valent Human papillomavirus vaccine (9vHpv) is a prophylactic vaccine designed to prevent anogenital cancer and related precancerous or atypical lesions, genital warts and persistent HPV infection caused by the nine HPV types covered by the vaccine (HPV 6, 11, 16, 18, 31, 33, 45, 52 and 58).Citation16–22 Which includes the main HPV genotype popular in Chongqing? The 9vHPV vaccine was licensed in December of 2014 in the United States, in February of 2015 in Canada under the trade name GARDASIL 9. In 2018, the 9vHPV was approved in China,Citation23 preliminary demonstrating their effectiveness and safety.Citation24–26
However, issues related to loss of public confidence in vaccination and misperceptions surrounding vaccine safety have emerged in some regions,Citation27–29 despite the data from large epidemiological studies and active surveillance programs by national and international organizations, which overwhelmingly support the safety of HPV vaccination.Citation30–32 As misunderstandings regarding vaccine safety and clusters of anxiety-related immunization reactions can have damaging consequences for vaccination programs.Citation29,Citation33 Especially the HPV vaccine has just been licensed in China, and the vaccination program has not yet been carried out. Widespread concerns about the safety of vaccines existed. There were many rumors of adverse reactions to HPV vaccine circulating on the Internet, such as lifelong disability after vaccination and premature ovarian failure. For example, an article entitled “328 deaths in one year, 2,000 lifelong disabilities 50,000 adverse reactions! This HPV vaccine came to China, and millions of women were vaccinated at a high price…” it went viral on social networks. The article’s most seemingly convincing references to the Vaccine Adverse Event Reporting System (VAERS) surveillance data from the Centers for Disease Control and Prevention. However, this article directly equates the cases of health damage collected in the VAERS to health damage caused by vaccines. And on June of 2013, 2 months after formal inclusion in Japan’s national immunization program, proactive recommendations for the HPV vaccine were suspended following reports of AEs since found to be unrelated to vaccination, but which were extensively covered in the media.Citation34,Citation35 And these appalling news also caused concern among the domestic people. As of 2021, the coverage rate of 9vHpv in Chongqing was 5.7%, which was still at an extremely low level.
To investigate the safety of 9vHPV vaccine further in real world of China, we conducted a single-arm, open-label, observational, post-marketing, multi-center surveillance study involved healthy participants aged 16–26 years during its first 3 years of licensure in Chongqing. The project aims to complete safety monitoring of the vaccine within 5 years after the first import in accordance with relevant regulatory requirements. Sufficient native vaccine safety data lay the foundation for large-scale use of vaccines in the future.
Method
Study design
This single-arm, open-label, observational, post-marketing, multi-center surveillance study was conducted between December 2019 and November 2021 in Chongqing, Beijing, and Shanghai, and was designed to extensively collect and further monitor the safety of 9vHPV administered according to routine clinical practice in healthy participants aged 16–26 years. The total number of participants required for this surveillance study was approximately 3000 according to Chinese regulatory requirements, and the target sample size for the Chongqing surveillance site was 1000. Five urban clinics in Chongqing, special for injecting 9vHPV vaccine, were chosen to enroll subjects. Even though the study period has been across the coronavirus disease 2019 (COVID-19) pandemic, participants were recruited within 1 month at the beginning of the epidemic in 2019. And during the follow-up period, the incidence of COVID-19 in Chongqing was still at a low level, with a rate of 1.88/100000.
Subjects
Anyone who had finished the first-dose 9vHPV vaccine in the five clinics and had agreed to participate in this surveillance was enrolled in this study. Nevertheless, the 9vHPV vaccine was approved for female aged 16–26 years by Center For Drug Evaluation of National Medical Products Administration (CDE, NMPA) in China, so all subjects in this study were female aged 16–26 years who had excluded the contraindications according to the instructions anyone with an allergic reaction to a previous dose of 9vHPV, yeast, amorphous aluminum hydroxyphosphate sulfate, and polysorbate 80. CDE is the national agency responsible for clinical trials, marketing authorization application acceptance, and technical evaluation of drugs in China.
Instruments
This was a prospective surveillance combining self-administrated safety reporting and active follow-up. All subjects received a vaccination report card (VRC) after each dose 9vHPV. Two different measures were taken to monitor the following safety events: 1) Self-administrated observation using VRC at points of vaccination or home to record all safety-related events during the whole observation period. On the VRC, beginning after each study vaccination and for a total of 30 days including the day of vaccination, the subjects were asked to record all vaccination-site and non-vaccination-site AEs/SAEs on the VRC. Beyond 30 days and till next dose of vaccination, only SAE, 9vHPV-related AE, and other reportable safety events were reported; 2) In addition, a total of five active in-person interviews or by telephone follow-up visits were conducted by the investigators with specific safety questionnaire after each dose 9vHPV. The questionnaire used in the follow-up was a paper questionnaire that had been pre-tested and certified by experts.
Other reportable safety events during the observation period: including but not limited to pregnancy, exposure during lactation, overdose, under dose, lack of therapeutic effect, off-label use, medication error, drug interaction, misuse, abuse, occupational exposure, drug interaction, product confusion, unexpected therapeutic benefit/effect, and suspected transmission via a medicinal product of an infectious agent, even product quality complaint, etc. The other reportable safety events during the observation period were also collected.
To avoid possible bias, detailed informed consent was obtained from the subjects at the beginning of the study, and active follow-up was conducted after each dose of vaccination. In addition, in order to make up for the transportation cost of the subjects to participate in the program, a certain subsidy was given.
Vaccine
Gardasil 9 is directed at HPV serotypes 6, 11, 16, 18, 31, 33, 45, 52, and 58, and adsorbed on 500 μg of amorphous aluminum hydroxyphosphate sulfate adjuvant.
Statistical methodology
Descriptive statistics were used in this surveillance. Statistical descriptive methods: For continuous variables, summary statistics included number of non-missing subjects (n), number of missing subjects (nmiss), mean, standard deviation (SD), median. The median was kept in the same decimal place with the raw data. The mean and SD were rounded to 1 decimal place more than the raw data. For categorical variables, the number of subjects in each category and percentage is presented. Counts that were zero were displayed as “0.” Percentages were based on nonmissing data unless otherwise specified. Percentages were the one decimal place. Statistical programming was used SPSS 23.0.
Ethical approval and informed consent
The study was done in accordance with the principles of Good Clinical Practice (GCP), and the study was approved by the institutional review board (IRB) of Chongqing Center for Disease Control and Prevention, and CDE, NMPA. All participants provided written informed consent before study participation in accordance with local laws and regulations. We had strict quality control throughout the study, and the data of the subjects were archived by a special person.
Results
Demographics
A total of 1000 individuals were enrolled in the study with no protocol deviations: 982 completed study. Pregnancy and planned pregnancy were the main reasons for exclusion from the pre-protocol set ().
Table 1. Subject disposition.
The demographic characteristics of participants at enrollment are presented in . All of the subjects were female aged 16–26 years old, the mean age was 23.32 ± 1.82 years and 957 subjects (95.7%) were aged 20–26 years old.372 (37.2%) subjects had incomes of less than 5000 RMB, and 276 (27.6%) had no incomes. 824(82.4%) subjects were single, and 176 (17.6%) were married.546 (54.6%) with bachelor’s degree and 227 (22.7%) were students.
Table 2. Baseline characteristics of study participants.
Administration information
There were 1000 individuals received at least one dose of 9vHPV vaccine: 982 received three doses and 992 received two doses. And all 982 individuals received three doses within one year (). 18 persons did not complete the program study. Among them, nine cases were pregnant, three cases withdrew due to AE including menorrhagia, cold, and pneumonia, three cases were decided by the investigator because they did not want to continue to participate in the project after leaving Chongqing, two cases voluntarily withdrew consent for no reason, one case was lost to follow-up and could not be contacted by home visit or telephone. All pregnancies in study subjects occurring during the study were followed to outcome. Of the nine subjects with known pregnancy outcomes, eight were live births and one was elective pregnancy-induced abortion. Spontaneous abortion and fetal congenital anomalies were not reported.
Table 3. HPV 9 Administration information.
Safety data
During the surveillance period, a total of 2107 AEs within 30 days after any dose were reported in 685 study participants, with 68.5% of participants experiencing at least one AE. 1741 AEs were related to 9vHPV vaccine and eight SAEs were reported in four study participants. One subject reported two SAEs of anal abscess and hemorrhoids, one subject reported four SAEs of ovarian cyst, fallopian tube obstruction, abdominal adhesion, and uterine polyps, and the other two subjects reported a brain tumor and an infectious pneumonia, respectively. The above eight SAEs were judged by experts to be unrelated to vaccination.
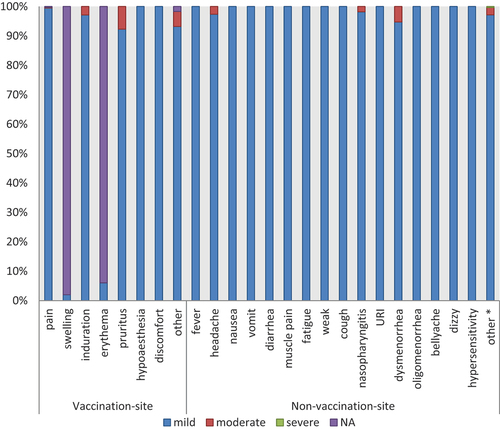
AEs of special interest all were hypersensitivity, included two cases of neck skin rashes and two cases of facial rashes recovered spontaneously within a short period of time (). The most frequently reported local AEs were vaccination-site pain in 547 subjects (54.70%), swelling in 193 subjects (19.3%) and induration in 90 subjects (9.0%) (). The most frequently reported non-vaccination-site AEs were nasopharyngitis in 50 subjects (5.0%), dysmenorrhea in 35 subjects (3.5%) and dizzy in 33 subjects (3.3%). The other 139 subjects (13.9%) were not listed in detail that included a variety of AEs that occurred only once, such as toothache, hair loss, dizziness, heat stroke, style, etc., most of which recovered spontaneously in a short time.
Table 4. The summary of AE.
Table 5. AEs occurred within 30 days following any HPV9 vaccination.
The most frequently reported non-vaccination-site AEs related to 9vHPV were dizzy in nine subjects (0.9%) and weak in eight subjects (0.8%) (). Among them oligomenorrhea, dysmenorrhea, opsomenorrhea, and menorrhagia were specifications not listed of 9vHPV-related AEs. And most recover or heal within a month.
Table 6. Non-vaccination-site AEs related to HPV 9 within 30 days.
The reported AEs were generally mild or moderate in nature. We classified the grade of AEs according to guiding principles for grading criteria for adverse reactions in clinical trials of prophylactic vaccines formulated by NMPA. 1828 (86.8%) of vaccination-site AEs were mild and moderate ( and ). There was only one SAE occurred in non-vaccination-site AEs, which was brain tumor and was judged not to be related to 9vHPV vaccine.
Table 7. Grade of adverse events after HPV9 vaccination.
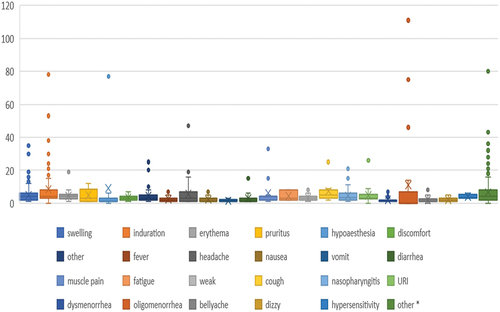
As shown in , the duration of reported AEs was generally short. The mean duration was 4.9 ± 6.3 days, with the longest 111 days and the shortest 1 day.
Discussion
The safety data observed in this post-marketing surveillance, observational study is consistent with those reported in pre-clinical trials and post-marketing case series.Citation34–38 Dizzy, weak, fever were the most common HPV9-related non-vaccination-site AEs occurred within 30 days following any vaccination. The 9vHPV recipients were slightly more likely to experience these AEs than tetravalent vaccine recipients were, and this possibly occurs due to the higher amounts of virus-like particles and adjuvants in the 9vHPV, as well as serotypes.Citation39
The 9vHPV vaccine was generally well tolerated by study participants. Vaccination-site AEs were the most frequently reported safety events in this study and were mostly mild. Compared with other 9vHPV vaccine recipients in global clinical trial programs, participants in Chongqing reported fewer vaccination-site and non-vaccination-site AEs (vaccination-site AEs: 60% vs. 84.8%; non-vaccination-site AEs: 5% vs. 51.9%).Citation38 Consistent with the lower incidence of AEs observed in Chongqing, vaccination-site and non-vaccination-site AEs were less common among Asian participants than among participants in the global study of 9vHPV vaccine (vaccination-site AEs, 85.5% vs. 90.7%; non-vaccination-site AEs: 43.8% vs. 55.8%).Citation40
The results of our study should be interpreted with some caution because it has limitations. First, the work has been conducted using post-marketing surveillance only, and the selected studies investigated just women with 16 to 26 age ranges. The applicable population of 9vHPV vaccine for other ages and genders has not been expanded. In 2022, the instructions for use of the 9vHPV in China broadened the age range from 9 to 45 years old, which was not covered by this study. It is necessary to monitor the safety data of use in a wider age group.
Secondly, in this program, AE of special interest were defined as autoimmune diseases, including Graves’ disease, Hashimoto’s disease, systemic lupus erythematosus, type 1 diabetes mellitus, multiple sclerosis, optic neuritis, uveitis, acute-disseminated encephalomyelitis, Guillain-Barre syndrome, and idiopathic thrombocytopenic purpura. With the exception of the VRC and five active follow-ups, there was no additional effort to capture neurological or cardiovascular disease in the vaccination-related hospital setting. Efforts to capture SAEs related to that will be the main content to be supplemented in our follow-up study. When a subject has a potentially relevant SAE, we will focus on investigating the subject’s relevant disease history, immunization history, medication history, and other information in detail, closely follow-up the whole process, and ask experts to judge its correlation with vaccines.
In addition, the surveillance program planned to enroll 3000 subjects, as required by regulation, this sample size was not calculated to estimate incidence or to test any scientific hypotheses. And the short follow-up period of the selected studies is also responsible for other potential weaknesses of the data. While the project has helped to understand the safety characteristics of 9vHPV in real-world use, or to identify some new safety signals, it has not been possible to validate these signals or to make firm/scientific conclusions.
Conclusions
The safety profile of 9vHPV observed in this project showed that 9vHPV had a good safety profile in Chinese women aged 16–26 years. No new confirmed safety signals were identified during the observational period of this program. Even though the project reported a few cases of specifications not listed of 9vHPV-related AEs, a review of the evidence in accordance with the World Health Organization Guidelines for the Classification of Causality for Adverse events after vaccination found that all of these AEs causal associations with vaccination were all judged to be unclassifiable or inconsistent. And most events were subject to confounding factors or the available information was insufficient to support a causal association with vaccination.
This study further confirmed the safety of 9-valent cervical cancer vaccine and provided a solid basis for the public to rest assured about vaccination. The Chongqing Municipal government has launched a campaign to promote the coverage of the first dose of HPV vaccine for girls in junior high schools in 2023. The local safety data of this study can also be used as an important basis for medical staff to promote the communication.
Abbreviations
| HPV | = | Human papillomavirus |
| STI | = | Sexually transmissible infection |
| LR-HPV | = | Low-risk HPV |
| HR-HPV | = | High-risk HPV |
| 9vHPV | = | 9-Valent Human papillomavirus vaccine |
| CDC | = | Center for Disease Control and Prevention |
| AE | = | Adverse Event |
| SAE | = | Serious Adverse Event |
| WHO | = | World Health Organization |
| VRC | = | Vaccination report card |
| SS | = | Safety Set |
| SD | = | Standard deviation |
| nmiss | = | number of missing subjects |
| CDE | = | Center For Drug Evaluation |
| NMPA | = | National Medical Products Administration |
| IRB | = | Institutional review board |
| COVID-19 | = | Coronavirus disease 2019 |
| VAERS | = | Vaccine Adverse Event Reporting System |
Acknowledgments
Thanks to the staff for conducting volunteer enrolment and questionnaire survey in three district CDCs of Chongqing.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- De Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D, Plummer M. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13:607–8. doi:10.1016/S1470-2045(12)70137-7.
- Forman D, de Martel C, Lacey CJ, Soerjomataram I, Lortet-Tieulent J, Bruni L, Vignat J, Ferlay J, Bray F, Plummer M, et al. Global burden of human papillomavirus and related diseases. Vaccine. 2012;30:F12–23. doi:10.1016/j.vaccine.2012.07.055.
- Buchanan TR, Graybill WS, Pierce JY. Morbidity and mortality of vulvar and vaginal cancers: impact of 2-, 4-, and 9-valent HPV vaccines. Hum Vaccines Immunother. 2016;12:1352–6. doi:10.1080/21645515.2016.1147634.
- Asiaf A, Ahmad ST, Mohammad SO, Zargar MA. Review of the current knowledge on the epidemiology, pathogenesis, and prevention of human papillomavirus infection. Eur J Cancer Prev. 2014;23:206–24. doi:10.1097/CEJ.0b013e328364f273.
- De Martel C, Plummer M, Vignat J, Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. 2017;141(4):664–70. doi:10.1002/ijc.30716.
- Bruni L, Albero G, Serrano B, Mena M, Gómez D, Muñoz J, Bosch FX, De Sanjosé S. ICO/IARC information centre on HPV and cancer (HPV information centre). Human papillomavirus and related diseases in the World. Summary Report 22 October 2021.
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. doi:10.3322/caac.21660.
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. doi:10.3322/caac.21660.
- Shi JF, Canfell K, Lew JB, Qiao YL. The burden of cervical cancer in China: synthesis of the evidence. Int J Cancer. 2012;130(3):641–52. doi:10.1002/ijc.26042.
- Wheeler CM, Hunt WC, Cuzick J, Langsfeld E, Pearse A, Montoya GD, Robertson M, Shearman CA, Castle PE. A population-based study of human papillomavirus genotype prevalence in the United States: baseline measures prior to mass human papillomavirus vaccination. Int J Cancer. 2013;132(1):198–207. doi:10.1002/ijc.27608.
- Dareng EO, Ma B, Famooto AO, Akarolo-Anthony SN, Offiong RA, Olaniyan O, Dakum PS, Wheeler CM, Fadrosh D, Yang H, et al. Prevalent high-risk HPV infection and vaginal microbiota in Nigerian women. Epidemiol Infect. 2016;144(1):123–37. doi:10.1017/S0950268815000965.
- Teka B, Gizaw M, Ruddies F, Addissie A, Chanyalew Z, Skof AS, Thies S, Mihret A, Kantelhardt EJ, Kaufmann AM, et al. Population-based human papillomavirus infection and genotype distribution among women in rural areas of south Central Ethiopia. Int J Cancer. 2021;148(3):723–30. doi:10.1002/ijc.33278.
- Zhu Y, Qian F, Zou W, Wu X, Liu C, Shen G, Lai S, Yang S. Prevalence and genotype distribution of human papillomavirus infection in Huzhou city, eastern China, 2018–2019. Trans Royal Soc Trop Med Hyg. 2021;115(1):30–7. doi:10.1093/trstmh/traa077.
- Chen X, Xu H, Xu W, Zeng W, Liu J, Wu Q, Zhao X, Jiang T. Prevalence and genotype distribution of human papillomavirus in 961,029 screening tests in Southeastern China (Zhejiang Province) between 2011 and 2015. Sci Rep. 2017;7(1):14813. doi:10.1038/s41598-017-13299-y.
- Luo Q, Lang L, Han N, Liang L, Shen L, Zhang H. Prevalence and genotype distribution of high-risk human papillomavirus infection among women with cervical cytological abnormalities in Chongqing, China, 2014–2020. Diagn Cytopathol. 2021;49(12):1237–43. doi:10.1002/dc.24891.
- De Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, Tous S, Felix A, Bravo LE, Shin H-R, et al. Human papillomavirus genotype attribution in invasive cervi-cal cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048–56. doi:10.1016/S1470-2045(10)70230-8.
- Serrano B, Alemany L, Tous S, Bruni L, Clifford GM, Weiss T, Bosch FX, de Sanjosé S. Potential impact of a nine-valent vaccine in human papillomavirus related cervical dis-ease. Infect Agents Cancer. 2012;7:38. doi:10.1186/1750-9378-7-38.
- de Sanjose S, Alemany L, Ordi J, Tous S, Alejo M, Bigby SM, Joura EA, Maldonado P, Laco J, Bravo IG, et al. Worldwide human papillomavirus genotype attribution in over 2000 cases of intraepithelial and invasive lesions of the vulva. Eur J Cancer. 2013;49:3450–61. doi:10.1016/j.ejca.2013.06.033.
- Alemany L, Saunier M, Alvarado-Cabrero I, Quiros B, Salmeron J, Shin HR, Pirog EC, Guimerà N, Hernandez-Suarez G, Felix A, et al. Human papillomavirus DNA prevalence and type distribution in anal carcino-mas worldwide. Int J Cancer. 2014;136:98–107. doi:10.1002/ijc.28963.
- Alemany L, Saunier M, Tinoco L, Quiros B, Alvarado-Cabrero I, Alejo M, Joura EA, Maldonado P, Klaustermeier J, Salmerón J, et al. Large contribution of human papillomavirus in vaginal neoplastic lesions: aworldwide study in 597 samples. Eur J Cancer. 2014;50(16):2846–54. doi:10.1016/j.ejca.2014.07.018.
- Lacey CJ, Lowndes CM, Shah KV. Chapter 4: burden and management of non-cancerous HPV-related conditions: HPV-6/11 disease. Vaccine. 2006;24:S35–41. doi:10.1016/j.vaccine.2006.06.015.
- Joura E, Giuliano A, Iversen OE, Bouchard C. 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372:711–23. doi:10.1056/NEJMoa1405044.
- Pan XF, Li R, Pan A, Larson H. Human papillomavirus vaccine approval in China: a major step forward but challenges ahead. Lancet Infect Dis. 2016;16(12):1322–3. doi:10.1016/S1473-3099(16)30450-9.
- Chen W, Zhao Y, Xie X, Liu J, Li J, Zhao C, Wang S, Liao X, Shou Q, Zheng M, et al. Safety of a quadrivalent human papillomavirus vaccine in a phase 3, randomized, double-blind, placebo-controlled clinical trial among Chinese women during 90 months of follow-up. Vaccine. 2019;37(6):889–97. doi:10.1016/j.vaccine.2018.12.030.
- Baloch Z, Yasmeen N, Li Y, Zhang W, Lu H, Wu X, Xia X, Yang S. Knowledge and awareness of cervical cancer, human papillomavirus (HPV), and HPV vaccine among HPV-infected Chinese women. Med Sci Monit. 2017;23:4269–77. doi:10.12659/MSM.903370.
- Chen R, Wong E. The feasibility of universal HPV vaccination program in Shenzhen of China: a health policy analysis. BMC Pub Health. 2019;19:781. doi:10.1186/s12889-019-7120-7.
- Hanley SJ, Yoshioka E, Ito Y, Kishi R. HPV vaccination crisis in Japan. Lancet. 2015;385(9987):2571. doi:10.1016/S0140-6736(15)61152-7.
- Tanaka Y, Ueda Y, Egawa-Takata T, Yagi A, Yoshino K, Kimura T. Outcomes for girls without HPV vaccination in Japan. Lancet Oncol. 2016;17(7):868–9. doi:10.1016/S1470-2045(16)00147-9.
- World Health Organization (WHO). Global advisory committee on vaccine safety, 2–3 December 2015. Wkly Epidemiol Rec. 2016;91:21–32.
- International Papillomavirus Society. IPVS policy statement on safety of HPV vaccines. Papillomavirus Res. 2016;2:9–10. doi:10.1016/j.pvr.2015.11.001.
- World Health Organization (WHO). Human papillomavirus vaccines: WHO position paper. Wkly Epidemiol Rec. 2017;92:241–68.
- Global Advisory Committee on Vaccine Safety, World Health Organization (WHO). Statement on safety of HPV vaccines.
- World Health Organization (WHO). Guide to introducing HPV vaccine into national immunization programmes.
- Hanley SJB, Yoshioka E, Ito Y, Kishi R. HPV vaccination crisis in Japan. Lancet. 2015;385(9987):2571. doi:10.1016/S0140-6736(15)61152-7.
- Tsuda K, Yamamoto K, Leppold C, Tanimoto T, Kusumi E, Komatsu T, Kami M. Trends of media coverage on human papillomavirus vaccination in Japanese newspapers. Clin Infect Dis. 2016;63:1634–8. doi:10.1093/cid/ciw647.
- Joura EA, Giuliano AR, Iversen OE, Bouchard C, Mao C, Mehlsen J, Moreira ED, Ngan Y, Petersen LK, Lazcano-Ponce E, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. New Engl J Med. 2015;372:711–23. doi:10.1056/NEJMoa1405044.
- Vesikari T, Brodszki N, Van Damme P, Diez-Domingo J, Icardi G, Petersen LK, Tran C, Thomas S, Luxembourg A, Baudin M. A randomized, Double-blind, phase III study of the immunogenicity and safety of a 9-valent human papillomavirus L1 virus-like particle vaccine (V503) versus Gardasil® in 9–15-year-old girls. Pediat Infect Dis J. 2015;34(9):992–8. doi:10.1097/INF.0000000000000773.
- Moreira ED Jr, Block SL, Ferris D, Giuliano AR, Iversen O-E, Joura EA, Kosalaraksa P, Schilling A, Van Damme P, Bornstein J, et al. Safety profile of the 9-valent HPV vaccine: a combined analysis of 7 phase III clinical trials. Pediatrics. 2016;138(2):e20154387. doi:10.1542/peds.2015-4387.
- Martínez-Lavín M, Amezcua-Guerra L. Serious adverse events after HPV vaccination: a critical review of randomized trials and post-marketing case series. Clin Rheumatol. 2017;36(10):2169–78. doi:10.1007/s10067-017-3768-5.
- Yang DY. Update on the new 9-valent vaccine for human papillomavirus prevention. Can Fam Phys. 2016;62:399–402.