ABSTRACT
Heterologous vaccination with inactivated vaccine followed by adenoviral vector-based vaccine has shown superiority in enhancing immune response compared to homologous primary series. However, data comparing immunity decline after a third booster following heterologous CoronaVac/ChAdOx-1nCov-19 has been limited. Here, we assessed neutralizing activity against omicron variant and T cell response at 3 months monitoring in 96 individuals who received ChAdOx-1nCov-19, BNT162b2, or mRNA-1273 as a third dose following heterologous CoronaVac/ChAdOx-1nCov-19. Comparing the antibody levels at 3 and 1 month(s) after the third booster, the results showed a persistence of anti-RBD IgG in all vaccine regimens, with the IgG level waning slower in the ChAdOx-1nCov-19 boosted group (geometric mean ratio (GMR): 0.64 (95%CI: 0.59–0.70)) compared to the BNT162b2 (0.34 (95%CI:0.31–0.38)) and mRNA-1273 boosted groups (0.32 (95%CI: 0.29–0.36)). Neutralizing activity against omicron BA.2 and BA.4/5 dropped by 1.2 to 1.5-fold but remained detectable, with the highest level observed in the mRNA-1273 group, followed by BNT162b2 and ChAdOx-1nCov-19 groups, respectively. Furthermore, the number of individuals with T cell reactivity decreased in BNT162b2 and mRNA-1273 groups, while it increased in ChAdOx-1nCov-19 group at 3-month post-boost compared to 1 month. Data on the durability of immune response could help comprehensively optimize the booster vaccine strategy.
Introduction
Since the emergence of SARS-CoV-2, the pandemic has continued to claim the lives of numerous people and has caused significant economic, social, and public health disruptions. Several COVID-19 vaccines have been rolled out, expanding and demonstrating their effectiveness against infection and disease severity. A previous study showed that immunization with a two-dose COVID-19 vaccine provided limited protection against symptomatic disease caused by emerging SARS-CoV-2 variants.Citation1 However, booster vaccinations significantly increased protection against both mild and severe disease.Citation1,Citation2 Furthermore, a third dose could exhibit cross-neutralization with SARS-CoV-2 variants and demonstrate superiority in both the quantity and quality of IgG antibodies compared to a second dose.Citation3,Citation4 Although booster vaccinations have been implemented in several countries, long-term data on the immune response after receiving an additional vaccine dose remains limited.
The waning of the immune response has been observed over time since vaccination. Studies on antibody kinetics after the third dose of vaccination indicate a peak in the immune response at approximately 3 weeks, followed by a subsequent decline.Citation5,Citation6 However, only a few studies have reported on the longitudinal immune response after the heterologous booster dose.Citation5,Citation7,Citation8 Additionally, this coincided with the emergence of new omicron subvariants, which highly evade immunity, raising concerns about the durability of the immune response after the third dose booster and its cross-reactivity in neutralizing the omicron subvariant.
In Thailand, heterologous prime/boost vaccination with CoronaVac followed by ChAdOx-1nCov-19 (the mix-and-match vaccine) was implemented in July 2021. This approach has demonstrated the potential to elicit a stronger immune response compared to a two-dose inactivated vaccine and to provide a response comparable to a two-dose ChAdOx-1nCov-19 vaccine.Citation9 Additionally, the heterologous CoronaVac/ChAdOx-1nCov-19 vaccination offers the advantage of reducing the interval between the first and second doses of the ChAdOx-1nCov-19 vaccine from 10 weeks to 4 weeks. By late 2021, Thailand had authorized several monovalent COVID-19 vaccines, including inactivated vaccines (CoronaVac (Sinovac) and BBIBP-CorV (Sinopharm)), non-replicating viral vector vaccines (ChAdOx-1nCov-19 (Oxford/AstraZeneca) and Ad26.COV2.S (Johnson & Johnson)), as well as mRNA-based vaccines (BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna)).Citation10
Due to concerns about waning antibody titers and in response to the emergence of the new omicron variant, third booster vaccinations following heterologous CoronaVac/ChAdOx-1nCov-19 regimen were subsequently administered to combat the new emerging omicron variant. Our previous study demonstrated that booster vaccination could rapidly increase the immune response and enhance the neutralizing capacity against omicron at 1 month after the boost.Citation11 However, updated data comparing the decline in immunity after a third booster dose following a heterologous primary series, as well as an assessment of the sustained ability to neutralize the omicron variant, are needed.
To address this concern, we extended our previous reportCitation11 to assess the persistence of anti-RBD IgG, neutralizing antibodies against omicron BA.2 and BA.4/5, and T cell response among individuals who received ChAdOx-1nCov-19, BNT162b2, or mRNA-1273 as a third dose following heterologous CoronaVac/ChAdOx-1nCov-19 vaccination. Our focus was on gathering immune responses at the three-month follow-up point after the third booster vaccination with different vaccines, which expands upon the work presented in our previous report.
Materials and methods
Study cohort
This study is an extension of our previous report,Citation11 which enrolled participants previously vaccinated with the heterologous CoronaVac/ChAdOx-1nCov-19 regimen to receive a monovalent vaccine as a third dose. The original vaccines from AstraZeneca (ChAdOx-1nCov-19; hereafter referred to as ChAdOx1), Pfizer-BioNTech (BNT162b2), and Moderna (mRNA-1273), which were developed based on the ancestral strain, were used as booster vaccinations. The cohort study began between November 30, 2021, and April 5, 2022, at the Center of Excellence in Clinical Virology, Faculty of Medicine, Chulalongkorn University. In this study, our focus was to gather additional information at the three-month follow-up point after the third booster vaccination. Exclusion criteria included individuals who provided clinical reports of infections or were seropositive (seroconversion) for anti-nucleocapsid (N) IgG. The study protocol was reviewed and approved by the Institutional Review Board of the Faculty of Medicine, Chulalongkorn University (IRB numbers 871/64) and conducted in accordance with the Declaration of Helsinki and Good Clinical Practice principles. This study was registered with the Thai Clinical Trials Registry (TCTR20211120002). All participants provided written consent before enrolling in this study.
Serological analysis and neutralizing activity
Sera samples were collected 3 months after the third dose vaccination and were tested for anti-nucleocapsid (N) IgG and anti-receptor binding domain (RBD) IgG against the ancestral strain using the commercially available automated ARCHITECT system (Abbott Diagnostics, Abbott Park, IL) through a chemiluminescent microparticle immunoassay (CMIA). Anti-N IgG was tested in all individuals to confirm breakthrough COVID-19 infection, with the seropositivity threshold considered as ≥ 1.4. The testing of anti-RBD IgG was conducted using the SARS-CoV-2 IgG II Quant assay (Abbott Diagnostics, Abbott Park, IL) and reported as AU/mL according to the manufacturer’s instructions. To convert the concentration into binding antibody units per milliliter (BAU/mL), the numerical AU/mL value was multiplied by a factor of 0.142. Anti-RBD IgG with a value ≥ 7.1 BAU/mL was considered positive. Neutralizing activity was tested against omicron BA.2 and BA.4/5 using a cPassTM SAR-CoV-2 neutralizing antibody detection kit through an ELISA-based surrogate virus neutralization test (sVNT) following the manufacturer’s recommendations (GenScript Biotech, Piscataway, NJ).Citation12 Briefly, sera samples, along with positive and negative controls, were diluted 1:10 with sample buffer and incubated with the recombinant SARS-CoV-2 RBD protein conjugated with horseradish peroxidase for 30 min at 37°C. Subsequently, 100 µL of the reaction mixture was transferred to ELISA plates coated with human angiotensin-converting enzyme 2 protein and incubated for 15 min at 37°C. Afterward, the ELISA plates were washed three times with wash buffer, and 100 µL of substrate solution was added. This mixture was incubated in the dark for 15 min at 20°C to 25°C. Finally, 50 µL of stop solution was added, and the absorbance was immediately measured at 450 nm. The neutralizing activity was calculated as follows: Inhibition (%) = (1 − OD value of the sample/average OD of the negative control) × 100, with a value ≥ 30% indicating the presence of neutralizing antibodies.
Quantification of total IFN-γ response
In addition, the SARS-CoV-2 specific T-cell response was assessed by measuring the total IFN-γ response in whole blood, following the manufacturer’s instructions (QuantiFERON, Qiagen, Hilden, Germany). Heparinized whole blood was transferred to blood collection tubes, consisting of two SARS-CoV-2 antigen tubes, a Mitogen tube (positive control), and a Nil tube (negative control). The antigen tube was coated with either S1 (RBD) peptides for CD4+ epitopes (Ag1) or S1+S2 peptides for CD4+ and CD8+ epitopes (Ag2) from the ancestral strain. Briefly, 0.8–1.2 mL of whole blood was added to collection tubes and incubated for 24 h at 37°C. Afterward, the collection tubes were centrifuged to harvest the plasma. Plasma samples were diluted at 1:2 with diluent and subjected to IFN-γ detection using ELISA, following the manufacturer’s guidelines (Qiagen, cat. no. 626410). Following the ELISA, the interferon-gamma concentration was quantified based on the eight-point standard (0.125 to 8 IU/mL) and calculated as IU/mL by QuantiFERON RD (v5.03) software. The lower detection limit was 0.065 IU/mL, and IFN-γ values ≥ 10 IU/mL were defined as 10 IU/mL. The final values of IFN-γ related to the SARS-CoV-2 specific T-cell stimulation were estimated by subtracting the value from the Nil tube. A value of IFN-γ (Ag1-Nil or Ag2 -Nil) ≥ 0.15 IU/mL and ≥ 25% of Nil after subtraction is considered a positive response, according to the manufacturer’s instruction.Citation11
Statistical analysis
Anti-RBD IgG was presented as geometric mean titers (GMT) with a 95% confidence interval (CI). Neutralizing activity and T cell response were presented as medians with interquartile ranges (IQR). The immune response at one-month post-boost, as published elsewhere,Citation11 was obtained as a comparator. The geometric mean ratio (GMR) was calculated to compare the changes over time in the antibody response between 3-month and one-month post-boost. If the GMR was higher, it meant the decay rate was slower. The GMR and 95% CI were estimated using ageneral linear model analysis. Additionally, the fold decrease in neutralizing activity against omicron was determined by comparing the results observed at 1 month with those observed at 3 months. Paired T-test and one-way analysis of variance (ANOVA) with Bonferroni adjustment were used to compute the difference in immune response for normally distributed data, while the Wilcoxon signed-rank test and Kruskal – Wallis test with Dunn’s post hoc correction were employed for non-normally distributed data. The statistical analysis was conducted using IBM SPSS Statistics v21.0 (IBM Corp., Armonk, NY) and GraphPad Prism v9.0 (GraphPad, San Diego, CA). Statistical significance was determined based on a p-value < .05.
Results
This study was an extension of our previous report,Citation11 in which we followed up with 167 participants who were primed with heterologous CoronaVac/ChAdOx1 and received a third booster with ChAdOx1, BNT162b2, or mRNA-1273 (). At 3 months after booster vaccination, 64 individuals were lost to follow-up, and seven individuals who had anti-N IgG seropositivity (seroconversion) indicating previous infection, were excluded from the analysis. Therefore, the remaining 96 participants who provided paired serum samples at 28 and 90 days after booster vaccination were enrolled (49.5% female). Of these 96 individuals, 32 received ChAdOx1 (mean (SD) age, 44.4 (9.0) years), 25 received BNT162b2 (mean (SD) age, 39.2 (10.1) years), and 39 received mRNA-1273 (mean (SD) age, 42.9 (9.8) years) as a third booster. There was no difference in age and sex distribution between the groups (). The median (IQR) interval between the 3rd dose vaccination and blood collection was 98 (93–99) days.
Figure 1. Study design and measurement of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-specific binding antibody responses and neutralizing activity against omicron BA.2 and BA.4/5. Participants who primed with heterologous CoronaVac/ChAdOx1 were received third dose of ChAdOx1, BNT162b2 and mRNA-1273 and followed-up at three months after vaccination (panel a). The anti-receptor binding domain (RBD) IgG (BAU/mL) in sera from boosted individuals with ChAdOx1, BNT162b2 and mRNA-1273 at one (1 M) and three months (3 M) after vaccination were compared (panel b). Error bars in panel b indicate the geometric mean titers (GMT) and the number above the graph indicates geometric mean ratio (GMR). Neutralizing activity against omicron BA.2 (panel c) and BA.4/5 (panel d) were compared between one and three months after booster vaccination. Numbers above the bar graph in panels c and d indicate the fold decrease in neutralizing activity, median values with interquartile ranges (IQRs) are shown as horizontal bars. Dotted lines indicate cutoff values 7.1 BAU/mL in panel b and 30% inhibition in panel c and d. The comparison was perform using Wilcoxon signed-rank test (two-tailed). *, p < .05, **, p < .01,***, p < .001; BAU-binding antibody unit; sVNT : surrogate virus neutralization test; CV: CoronaVac; AZ: ChAdOx1; BNT: BNT162b2; MN:mRNA-1273.
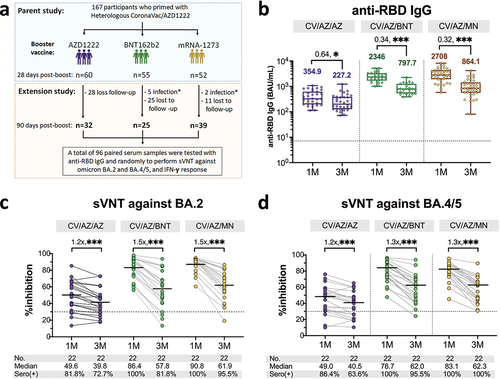
Table 1. Characteristics of participants in the study.
We observed a significant drop in anti-RBD IgG at three-month post-third booster in all vaccine groups (). For individuals who received ChAdOx1 as a third dose, the geometric mean ratio (GMR) of anti-RBD IgG was 0.64, which their geometric mean titers (GMTs) decreasing from 354.9 BAU/mL in the first month to 227.2 BAU/mL at 3 months after the booster. In the case of mRNA vaccine booster vaccination, the GMT of BNT162b2 boosted individuals dropped from 2346 BAU/mL in the first month to 797.7 BAU/mL at 3 months after the third dose, resulting in a GMR of 0.34. Similarly, individuals who received mRNA-1273 as a third dose had a GMR of 0.32, decreasing their GMT from 2708 BAU/mL at 1 month to 864.1 BAU/mL at three-month post-boost. These results indicate that the decrease in anti-RBD IgG levels was faster in individuals who received the mRNA vaccine as a booster than those who received ChAdOx1 (Figure S1).
Neutralizing activity against omicron BA.2 and BA.4/5 was detected. A comparison of neutralizing activity against omicron between the 3rd and 1st month(s) after the booster dose revealed that there was a 1.2-fold lower in the ChAdOx1 boosted group and 1.5-fold lower for the BNT162b2, and mRNA-1273 boosted groups (). Moreover, we found that 72.7% (16/22), 81.8% (18/22), and 95.5% (21/22) had detectable neutralizing antibodies against omicron BA.2 at 3 months after the third dose of ChAdOx1, BNT162b2, and mRNA-1273, respectively. At 3 months after the third dose, the neutralizing activity against omicron BA.4/5 showed a 1.2-fold drop for ChAdOx1 boosted group and a 1.3-fold decline in BNT162b2 and mRNA-1273 boosted groups compared to 1 month after booster vaccination (). Additionally, the majority of individuals who received BNT162b2 (95.5% (21/22) or mRNA-1273 (100% (22/22)) remained seropositive against omicron BA.4/5 while 63.6% (14/22) of individuals who received ChAdOx1 were seropositive against omicron BA.4/5.
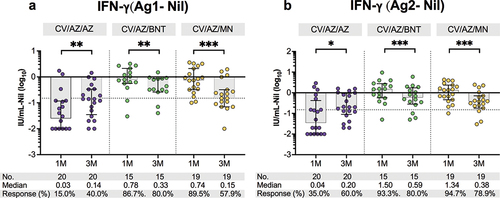
We proceeded to evaluate the T cell response by stimulating with specific CD4+ epitopes (Ag1) at three months after the booster vaccination. Among individuals who received BNT162b2, 80% (12/15) showed T cell reactivity, while 57.9% (11/19) of those who received mRNA-1273 and 40% (8/20) of those who received ChAdOx1 exhibited a response, as indicated by the production of interferon-gamma (). Additionally, we found that 80% (12/15) of individuals who received BNT162b2, 78.9% (15/19) of those who received mRNA-1273, and 60% (12/20) of those who received ChAdOx1 produced an interferon-gamma response when stimulated with CD4+ and CD8+ epitopes (Ag2) (). When comparing with the first month, individuals who received BNT162b2 and mRNA-1273 as booster doses showed significantly lower levels of secreted interferon-gamma and a decrease in the number of individuals with T cell reactivity at 3 months post-boost. In contrast, individuals who received ChAdOx1 as a booster exhibited an increase in interferon-gamma levels and the number of T cell-reactive individuals after 3 months, compared to 1 month after the booster.
Figure 2. Total IFN-γ responses against SARS-CoV-2 antigens in heterologous CoronaVac/ChAdOx1 vaccinated individuals at one (1 M) and three months (3 M) after booster vaccination. Heparinized whole blood from boosted individuals with ChAdOx1, BNT162b2, or mRNA-1273 were stimulated by (a) Ag1, which is a CD4+ epitope derived from RBD, minus negative control (Nil), and (b) Ag2, which is CD4+ and CD8+ epitopes derived from S1 and S2 subunits, minus negative control (Nil). T cell responses were considered positive if the IFN-γ level was above cutoff values (0.15 IU/mL and ≥ 25% of Nil). Horizontal bars indicate the median with interquartile range (IQR). The cutoff values were represented by horizontal dotted line. Statistical analysis was performed using Wilcoxon signed rank test (two-tailed). ns indicates no significant difference;*, p < .05; **, p < .01; ***, p < .001. CV: CoronaVac; AZ: ChAdOx1; BNT: BNT162b2; MN:mRNA-1273.
Discussion
This study extended the findings of our previous studyCitation11 by providing immunogenicity data from 3 months of follow-up after mRNA and adenoviral-vector booster vaccines in individuals who had previously received heterologous CoronaVac/ChAdOx1 vaccination. We observed sustained detectable immunogenicity through 3-month post-third boost, although a decline in antibody response was observed in all vaccination regimens. This finding corroborates a previously conducted studyCitation7 that reported on the durability of the immune response at 3 months after booster vaccination in participants who received either an adenoviral-vector or mRNA vaccine as a third dose following two doses of CoronaVac vaccines. Additionally, a previous reportCitation13 has summarized the benefits of heterologous vaccination with primary series and/or booster doses, showing a better immune response than homologous vaccination. Our study presents data on immunogenicity after a heterologous booster following a heterologous inactivated and adenoviral vector vaccine as the primary regimen, which supports the use of a mix-and-match vaccine strategy.
Overall, the decay rate of anti-RBD IgG in individuals who received BNT162b2 and mRNA-1273 as a third dose showed similar results. Additionally, the waning of IgG in individuals boosted with the ChAdOx1 vaccine occurred at a slower rate compared to those boosted with mRNA vaccines. This finding was supported by an immunogenicity study conducted at three months after receiving a different type of vaccine as a third dose in individuals who were initially vaccinated with two doses of ChAdOx1 and two doses of BNT162b2.Citation5 Furthermore, this finding highlights the different kinetics of antibody levels after mRNA booster vaccination compared to ChAdOx1, with a higher level of antibody response in the initial months after the booster but a much faster waning afterward.
In our previous study,Citation11 we reported cross-neutralization against omicron BA.1 and BA.2 at 28 days after vaccination, with lower neutralizing antibody titers found in participants boosted with ChAdOx1 and the highest response found in those given mRNA-1273 as a third dose. In this current study, we observed the persistence of neutralizing activity against omicron BA.2 and BA.4/5 at 3 months after vaccination, particularly in people who received the mRNA booster. This finding is supported by a previous reportCitation14 indicating that boosting with a third dose of mRNA vaccine based on the ancestral strain could increase variant-neutralizing antibody levels and generate memory B cells that maintain cross-reactivity against omicron subvariants.
In line with the previous report,Citation11 spike-specific T-cell response was lower in individuals who received adenoviral vector vaccines as a third booster at 1-month post-boost compared to those who received mRNA vaccines. Our results showed a general decrease in T-cell response in individuals who received mRNA vaccination at 3 months after the boost compared to 1 month. This finding aligns with previous studiesCitation5,Citation15 that used different techniques to evaluate T-cell response and also showed a decrease in the level of secreted IFN-γ at 3 months after mRNA booster vaccination. Additionally, we found that the decline rate of secreted IFN-γ level after BNT162b2 booster vaccination was similar to mRNA-1273 vaccine. This result is consistent with a previous reportCitation5 on the T-cell response after boosting with BNT162b2 or mRNA-1273 following two doses of viral vector vaccine or two doses of BNT162b2 vaccine.
In contrast, spike-specific T cell response in individuals who received ChAdOx1 as a third boost was more reactive at 3 months compared to 1 month after booster vaccination. This indicates a delay in the peak T-cell response following the ChAdOx1 booster compared to mRNA booster, particularly in individuals who initially received the heterologous CoronaVac/ChAdOx1 vaccination as their primary regimen. Induction of a spike-specific T cell response was observed 55–137 days after a single dose of ChAdOX1 and 9–12 days after a single dose of BNT162b2.Citation16 Consistent with a phase 2 trial of an adenoviral-vectored vaccine, the T cell peak was reported 2 weeks after two doses of ChAdOx-1nCov-19, with the peak of T cells also observed on day 56, marking the last point of data collection.Citation17 A kinetic study of T cell responses following an adenoviral vector expressing the SARS-CoV-2 nucleoprotein showed that the breadth and magnitude of CD8+ and CD4+ T cell responses were significantly higher at 3 months after a single dose compared to 2 weeks.Citation18 An increase in the frequency of IFN-γ+/CD8+ T cells at a later time point compared to 1 month has also been observed after a single dose of Ad26.COV2.S immunization.Citation19 The delayed increase in IFN-γ-producing T cell responses after ChAdOX1 vaccination may be related to the persistence of transcriptionally active adenoviral vectors at a low level, where a small amount of antigen continues to drive and maintain the activation of effector T cells.Citation20 However, this does not fully explain the delayed peak of T cell responses observed in adenoviral-vectored vaccines. Further investigations are needed to explore the underlying molecular mechanisms.
This study has some limitations. First, the small number of participants within each vaccine group was due to a high number of participants lost to follow-up from the parent study. Additionally, we did not determine the neutralizing antibody titer against omicron using live virus. Instead, we used a surrogate neutralizing method that imitates the host-virus binding interaction by using human angiotensin-converting enzyme 2 (ACE-2) and SARS-CoV-2 receptor binding domain proteins. Nevertheless, it is important to note that this study was conducted as a short-term follow-up, assessing immune response only at 3 months after booster vaccination. Therefore, longer follow-up studies are needed to determine the durability of the immune response.
This study provides valuable insights into the difference in the decay rates of immune response between adenoviral vector and mRNA vaccines when used as a booster dose following heterologous prime/boost vaccination as the primary series. The utilization of heterologous vaccine combinations represents a relatively novel approach that requires more immunogenicity data for support. Therefore, the study of the longevity and sustainability of immunity following heterologous COVID-19 vaccine combinations is needed to inform vaccine recommendations and decisions.
Author contributions
Conceptualization, N.S. (Nungruthai Suntronwong) and Y.P.; data collection, T.T. (Thaksaporn Thatsanathorn), N.S. (Natthinee Sudhinaraset), NW and Y.P.; formal analysis, N.S. (Nungruthai Suntronwong); methodology, N.S. (Nungruthai Suntronwong), T.T. (Thanunrat Thongmee), and S.K.; project administration, Y.P.; writing – original draft, N.S. (Nungruthai Suntronwong); writing – review and editing, N.S. (Nungruthai Suntronwong) and Y.P.; All authors have read and agreed to the published version of the manuscript.
0. Supplementary file.docx
Download MS Word (2.1 MB)Acknowledgments
We would like to thank all the Center of Excellence in Clinical Virology personnel and all participants for contributing to and supporting this project.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data generated during this study are contained within this manuscript and its Supplementary Information file https://doi.org/10.1080/21645515.2023.2283916.
Supplementary data
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2283916.
Additional information
Funding
References
- Andrews N, Stowe J, Kirsebom F, Toffa S, Rickeard T, Gallagher E, Gower C, Kall M, Groves N, O’Connell AM, et al. COVID-19 vaccine effectiveness against the omicron (B.1.1.529) variant. N Engl J Med. 2022 Apr 21;386(16):1532–7. doi:10.1056/NEJMoa2119451.
- Andrews N, Stowe J, Kirsebom F, Toffa S, Sachdeva R, Gower C, Ramsay M, Lopez Bernal J. Effectiveness of COVID-19 booster vaccines against COVID-19-related symptoms, hospitalization and death in England. Nat Med. 2022 Apr;28(4):831–7. doi:10.1038/s41591-022-01699-1. Epub 2022 Jan 14. PMID: 35045566; PMCID: PMC9018410.
- Lustig Y, Gonen T, Meltzer L, Gilboa M, Indenbaum V, Cohen C, Amit S, Jaber H, Doolman R, Asraf K, et al. Superior immunogenicity and effectiveness of the third compared to the second BNT162b2 vaccine dose. Nat Immunol. 2022 Jun;23(6):940–6. doi:10.1038/s41590-022-01212-3.
- Zhou R, Liu N, Li X, Peng Q, Yiu CK, Huang H, Yang D, Du Z, Kwok HY, Au KK, et al. Three-dose vaccination-induced immune responses protect against SARS-CoV-2 omicron BA.2: a population-based study in Hong Kong. Lancet Reg Health West Pac. 2023 Mar;32:100660. doi:10.1016/j.lanwpc.2022.100660.
- Liu X, Munro APS, Feng S, Janani L, Aley PK, Babbage G, Baxter D, Bula M, Cathie K, Chatterjee K, et al. Persistence of immunogenicity after seven COVID-19 vaccines given as third dose boosters following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK: three month analyses of the COV-BOOST trial. J Infect. 2022 Jun;84(6):795–813. doi:10.1016/j.jinf.2022.04.018.
- Qu P, Faraone JN, Evans JP, Zheng YM, Yu L, Ma Q, Carlin C, Lozanski G, Saif LJ, Oltz EM, et al. Durability of booster mRNA vaccine against SARS-CoV-2 BA.2.12.1, BA.4, and BA.5 subvariants. N Engl J Med. 2022 Oct 6;387(14):1329–31. doi:10.1056/NEJMc2210546.
- Assawakosri S, Kanokudom S, Suntronwong N, Chansaenroj J, Auphimai C, Nilyanimit P, Vichaiwattana P, Thongmee T, Duangchinda T, Chantima W, et al. Immunogenicity and durability against omicron BA.1, BA.2 and BA.4/5 variants at 3 to 4 months after a heterologous COVID-19 booster vaccine in healthy adults with two-doses CoronaVac vaccination. MedRxiv. 2022. doi:10.1101/2022.11.24.22282735.
- Cowling BJ, Cheng SMS, Martín-Sánchez M, Au NYM, Chan KCK, Li JKC, Fung LWC, Luk LLH, Tsang LCH, Ip DKM, et al. Slow waning of antibodies following BNT162b2 as a third dose in adults who had Previously received 2 doses of inactivated vaccine. J Infect Dis. 2023 Jan 11;227(2):251–5. doi:10.1093/infdis/jiac380.
- Wanlapakorn N, Suntronwong N, Phowatthanasathian H, Yorsaeng R, Vichaiwattana P, Thongmee T, Auphimai C, Srimuan D, Thatsanatorn T, Assawakosri S, et al. Safety and immunogenicity of heterologous and homologous inactivated and adenoviral-vectored COVID-19 vaccine regimens in healthy adults: a prospective cohort study. Hum Vaccin Immunother. 2022 Dec 31;18(1):2029111. doi:10.1080/21645515.2022.2029111.
- World Health Organization. COVID-19 vaccines; 2023 [accessed 2023 Oct 29]. https://www.who.int/westernpacific/emergencies/covid-19/covid-19-vaccines.
- Suntronwong N, Kanokudom S, Auphimai C, Assawakosri S, Thongmee T, Vichaiwattana P, Duangchinda T, Chantima W, Pakchotanon P, Chansaenroj J, et al. Effects of boosted mRNA and adenoviral-vectored vaccines on immune responses to omicron BA.1 and BA.2 following the heterologous CoronaVac/AZD1222 vaccination. J Med Virol. 2022 Dec;94(12):5713–22. doi:10.1002/jmv.28044.
- GenScript. SARS-CoV-2 surrogate virus neutralization test (sVNT); [accessed 2023 Oct 29]. https://www.genscript.com/gsfiles/techfiles/Application-Note-SARS-CoV-2-Surrogate-Virus-Neutralization-Test-sVNT.pdf?21885562.
- European Medicines Agency. Heterologous primary and booster COVID-19 vaccination: evidence based regulatory considerations; 2021 [accessed 2023 Aug 3]. https://www.ema.europa.eu/en/documents/report/heterologous-primary-booster-covid-19-vaccination-evidence-based-regulatory-considerations_en.pdf.
- Goel RR, Painter MM, Lundgreen KA, Apostolidis SA, Baxter AE, Giles JR, Mathew D, Pattekar A, Reynaldi A, Khoury DS, et al. Efficient recall of omicron-reactive B cell memory after a third dose of SARS-CoV-2 mRNA vaccine. Cell. 2022 May 26;185(11):1875–1887.e8. doi:10.1016/j.cell.2022.04.009.
- Gilboa M, Regev-Yochay G, Mandelboim M, Indenbaum V, Asraf K, Fluss R, Amit S, Mendelson E, Doolman R, Afek A, et al. Durability of immune response after COVID-19 booster vaccination and association with COVID-19 omicron infection. JAMA Netw Open. 2022 Sep 1;5(9):e2231778. doi:10.1001/jamanetworkopen.2022.31778.
- Vogel E, Kocher K, Priller A, Cheng CC, Steininger P, Liao BH, Körber N, Willmann A, Irrgang P, Held J, et al. Dynamics of humoral and cellular immune responses after homologous and heterologous SARS-CoV-2 vaccination with ChAdOx1 nCoV-19 and BNT162b2. EBioMedicine. 2022 Nov;85:104294. doi:10.1016/j.ebiom.2022.104294.
- Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S, Bellamy D, Bibi S, Bittaye M, Clutterbuck EA, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467–78. doi:10.1016/S0140-6736(20)31604-4.
- Hasanpourghadi M, Novikov M, Ambrose R, Chekaoui A, Newman D, Zhou XY, Ertl HCJ. T cell responses to adenoviral vectors expressing the SARS-CoV-2 nucleoprotein. Curr Trends Microbiol. 2021;15:1–28 .
- Liu J, Chandrashekar A, Sellers D, Barrett J, Jacob-Dolan C, Lifton M, McMahan K, Sciacca M, VanWyk H, Wu C, et al. Vaccines elicit highly conserved cellular immunity to SARS-CoV-2 omicron. Nature. 2022 Mar;603(7901):493–6. doi:10.1038/s41586-022-04465-y.
- Tatsis N, Fitzgerald JC, Reyes-Sandoval A, Harris-McCoy KC, Hensley SE, Zhou D, Lin SW, Bian A, Xiang ZQ, Iparraguirre A, et al. Adenoviral vectors persist in vivo and maintain activated CD8+ T cells: implications for their use as vaccines. Blood. 2007 Sep 15;110(6):1916–23. doi:10.1182/blood-2007-02-062117.
