ABSTRACT
This study aimed to investigate the relationship between complete pathological remission (PCR), tertiary lymphoid structure (TLS) maturation and expression and clinical outcomes of patients with resectable non-small cell lung cancer (NSCLC) receiving neoadjuvant chemoimmunotherapy. Totally 80 patients with resectable NSCLC (stage IB-IIIB) receiving neoadjuvant chemoimmunotherapy were analyzed. We used the Kaplan-Meier method to plot survival curves and the log-rank test to compare differences. Among all patients included, 45 patients (56.25%) achieved major pathological response (MPR), including 30 patients (37.50%) with PCR. The proportion of patients diagnosed with stage IB, II, IIIA and IIIB was 1.25%, 10.00%, 52.50% and 36.25%, respectively. We divided patients into PCR group and non-PCR group respectively according to whether they achieved PCR. We found that patients achieving PCR had significantly improved disease-free survival (DFS) (mDFS: NR vs. 20.24 months, P = .020). TLS expression was low in 43 cases (53.75%) and high in 37 cases (46.25%). TLS maturation was low in 55 cases (68.75%) and high in 25 cases (31.25%). The DFS of patients with TLS high-maturation (34.07 vs. 22.30 months, P = .024) and TLS high-expression (34.07 vs. 22.30 months, P = .041) was significantly longer. In most subgroups, the PCR, TLS high-maturation and TLS high-expression group respectively achieved a better clinical outcome relative to the non-PCR, TLS low-maturation and TLS low-expression group. In patients with resectable NSCLC receiving neoadjuvant chemoimmunotherapy, the acquirement of PCR may predict better DFS. In addition, high expression and maturation of TLS may be prognostic factors.
Introduction
Lung cancer is the second most common cancer globally, with non-small cell lung cancer (NSCLC) accounting for about 85%.Citation1 Recently, neoadjuvant immunotherapy combined with chemotherapy has become an important treatment strategy for locally advanced NSCLC patients.Citation2 This approach has demonstrated great potential in improving prognosis and survival in NSCLC.Citation3 Several studies have shown that neoadjuvant immunotherapy plus chemotherapy significantly improved survival and pathological remission in patients with NSCLC.Citation4 The follow-up time of operable patients is long, and prolonged follow-up time may lead to delays in the clinical dissemination of potentially effective therapeutic measures. Therefore, it is essential to explore alternative study endpoints. Neoadjuvant chemoimmunotherapy improves pathological regression in NSCLC, which may be associated with improved long-term outcomes. In resectable NSCLC, previous studies have reported a range of 24.0%–37.0% for the percentage of complete pathological remission (PCR).Citation5–7 The correlation of PCR with survival outcomes has been shown in some landmark neoadjuvant clinical trials.Citation7,Citation8 This study not only analyzed the relationship between PCR and survival, but also explored the correlation between clinicopathological features and PCR.
Currently, Programmed cell death-Ligand protein 1 (PD-L1) expression and tumor mutation burden (TMB) have been proved to differentially predict immunotherapy response in the neoadjuvant setting.Citation9,Citation10 However, there is a lack of studies exploring the role of tertiary lymphoid structures (TLS) in the prognosis of NSCLC patients treated with neoadjuvant chemoimmunotherapy.Citation11 TLSs are ectopic lymphoid organs which form in non-lymphoid tissues in response to chronic inflammation,Citation12 mainly composed of B cells, T cells and dendritic cells.Citation13 TLS is believed to play a role in triggering and activating T cells in the tumor microenvironment and is associated with a good prognosis and response to immunotherapy.Citation14 In tumor regression beds, the discovery of TLS is seen as an immune-associated pathological response.Citation15 TLS has been identified as as an independent prognostic factor for immunotherapy in lung cancer, with effective immunotherapy requiring the formation of CXCL13-dependent TLS.Citation16 Recent studies have confirmed that mature TLS was correlated with an increase in the number of cytotoxic lymphocytes and a decreased frequency of lymphocyte metastasis, suggesting that mature TLS may enhance anti-tumor immunotherapeutic efficacy through activation of lymphocytes.Citation17 The presence of TLS has also provided new insights into the mechanism by which neoadjuvant chemoimmunotherapy is preferable to chemotherapy from the perspective of the tumor microenvironment.Citation18 However, few studies have explored the exact relationship between TLS and clinical prognosis in terms of its expression and maturation.
This study aimed to explore the correlation between PCR and clinical prognosis in NSCLC patients treated with neoadjuvant chemoimmunotherapy. In addition, the prognostic role of TLS on the efficacy of neoadjuvant chemoimmunotherapy was also examined.
Materials and methods
Patients and ethical statement
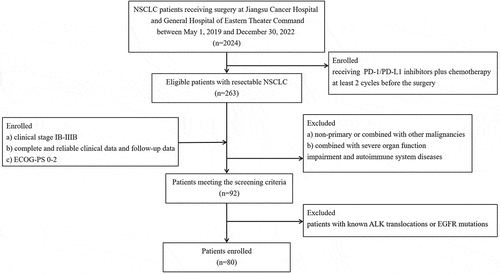
The selection process and design of this study is shown in . The data of patients with NSCLC receiving immune checkpoint inhibitors plus platinum-based chemotherapy before receiving surgery at Nanjing Medical University Affiliated Cancer Hospital and General Hospital of Eastern Theater Command from May 1, 2019 to December 30, 2022 were retrospectively reviewed in this study. The eligibility standards were as follows: (1) pathologically confirmed non small cell lung cancer; (2) receiving PD-1/PD-L1 inhibitors plus chemotherapy at least two cycles before receiving surgery; (3) clinical stage IB-IIIB (the 8th edition of the AJCC TNM staging system); (4) complete and reliable clinical data and follow-up data. The exclusion standards were as follows: (1) patients with small cell lung cancer; (2) combined with severe organ function impairment and autoimmune system diseases; (3) patients with known ALK translocations or EGFR mutations. Immune checkpoint inhibitors included PD-1 inhibitors (pembrolizumab, toripalimab, tislelizumab, sintilimab, camrelizumab, nivolumab) and PD-L1 inhibitors (atezolizumab). All included patients were treated with chemotherapy with a platinum-containing regimen, including platinum analogs (carboplatin, cisplatin, nedaplatin), pemetrexed and paclitaxel analogs (docetaxel, paclitaxel, albumin paclitaxel, paclitaxel liposomes).
This study received approval from the Ethics Committee of Jiangsu Cancer Hospital (2023-040). All procedures of this study were in accordance with the Declaration of Helsinki (as revised in 2013). The requirement for informed consent was waived due to the retrospective nature of the study.
Data collection and assessment
Clinical data were collected from medical records. Patients who had not visited the hospital for three months were followed up by telephone contact to get information including tumor recurrence and survival. Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 was applied to classify effectiveness into four categories: complete response (CR), partial response (PR), stable disease (SD) and disease progression (PD). Objective response rate (ORR) indicates CR and PR.
Tumor samples including the resected lung and lymph node tissues were obtained after patients receiving the surgery, which were assessed by senior pathologists in Nanjing Medical University Affiliated Cancer Hospital and General Hospital of Eastern Theater Command. PCR was defined as 0% viable tumor cells remaining in the dissected tissues and lymph nodes, with MPR defined as 10% or less on the postoperative pathological review.Citation19
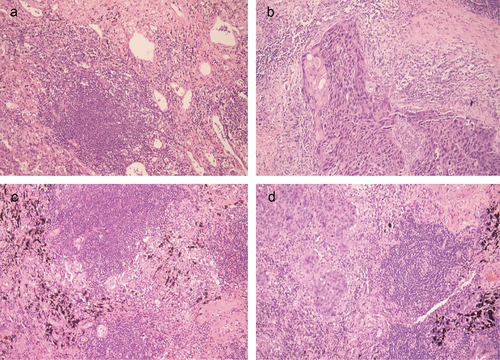
TLS was defined as aggregates of lymphocytes with high endothelial microvessels. Two experienced pathologists assessed TLS independently through a weak magnification (10 × 10) and 10 fields of view. In 10 fields of view, specimens with no more than 5 TLSs were defined as low expression, and those with more than 5 TLSs were defined as high expression. According to the previous study, the maturation of TLS was divided into three stages: early TLSs (E-TLS, dense aggregates of lymphocytes without follicle dendritic cells (FDC)), primary follicle-like TLSs (PFL-TLS, clusters of B-cells, FDC network without germinal centers), secondary follicle-like TLS (SFL-TLS, clusters of B cells, FDC network with germinal centers).Citation20 This classification used to be defined by immunohistochemistry, and in this study it was defined by HE, the rationality of which has been proved by specialized pathologists. In our study, patients with no TLS and E-TLS were classified into the low-maturation group and patients with PFL-TLS and SFL-TLS were classified into the high-maturation group ().
Statistical analysis
All statistical analyses were performed in SPSS (version 26.0), GraphPad Prism ((V.7.0; GraphPad Software) and R environment v.4.1.3. Continuous and categorical variables were described by median (interquartile range) and frequency (percentage) respectively. We used the Kaplan-Meier method to plot survival curves and the log-rank test to compare differences. Differences between groups were tested by χ2 or Fisher’s exact test (for categorical variables). We used subgroup analyses to assess the association between groups and the risk of disease progression in different subgroups. Cox regression was used for both univariate and multivariate analyses. Two-sided p values <.05 were considered statistically significant.
Results
Patient characteristics
A total of 80 patients were analyzed, with a mean age of 61.55 ± 8.80 years. Baseline characteristics of patients included gender, age, smoking history, tumor site and other clinical characteristics (). Among the patients, adenocarcinoma and squamous cell carcinoma accounted for 25.00% and 75.00%, respectively. After operation, 45 patients (56.25%) achieved MPR, including 30 patients (37.50%) with PCR (). TLS expression was low in 43 cases (53.75%) and high in 37 cases (46.25%). TLS maturation was low in 55 cases (68.75%) and high in 25 cases (31.25%). We found that the achievement of PCR tended to be correlated with the treatment cycles and TLS maturation. The status of pathological remission was not significantly associated with tumor size, tumor site and histological type. Among the patients, a total of 58 patients received adjuvant treatment, and we summarized the details in Supplementary Table S1.
Figure 3. Pathologic assessment of response to immune checkpoint inhibitors. The black dashed line indicates the threshold for a major pathological response (90% regression). PR, partial response; CR, complete response; SD, stable disease. (Evaluation of efficacy during neoadjuvant chemoimmunotherapy according to response Evaluation criteria in Solid tumors, RECIST).
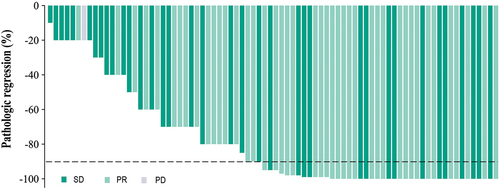
Table 1. Baseline clinical characteristics of patients.
The relationship of PCR, TLS maturation and expression with DFS
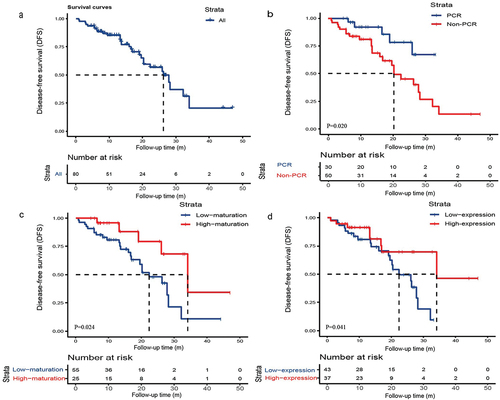
As data cutoff (June 1, 2023), the median follow-up time and DFS of all enrolled patients was 17.5 months (interquartile range: 9.7–27.0) and 26.3 months [95% confidence interval (CI): 19.7–32.9], respectively. 29 patients (36.25%) have relapsed, and Supplementary Table S2 showed the specific progression patterns. Of these recurrences, 13 patients (44.83%) had regional recurrence, 12 patients (41.38%) had distant metastases, and 4 patients (13.79%) had both regional recurrence and distant metastases. The sites of distant metastases included bone metastases, brain metastases and lymph node metastases in the clavicular region. We first analyzed the correlation between PCR and DFS. Kaplan-Meier analysis showed that patients with PCR had better DFS than patients with non-PCR (mPFS: NR vs. 20.24 months, P = .020). We further evaluated the maturation and expression of TLS and DFS, which showed that the DFS of patients with TLS high-maturation (34.07 vs. 22.30 months, P = .024) and TLS high-expression (34.07 vs. 22.30 months, P = .041) was significantly longer than that of low maturation and expression ().
Response status of patients in different groups
Among the enrolled patients, the number of people assessed for efficacy who achieved PR, SD and PD was 43 (53.75%), 36 (45.00%) and 1 (1.25%), respectively. The ORR and DCR of the total patients reached 53.75% and 98.75%. The response to treatment in each group is shown in and , and no patient was able to achieve CR. The PCR, high-maturation and high-expression group had higher ORR than the other three groups (63.33% vs. 48.00%, 60.00% vs. 50.91% and 54.05% vs. 53.49%), respectively.
Figure 5. Response status per RECIST depending on (a) all patients, (b) PCR, (c) maturation of TLS and (d) expression of TLS.
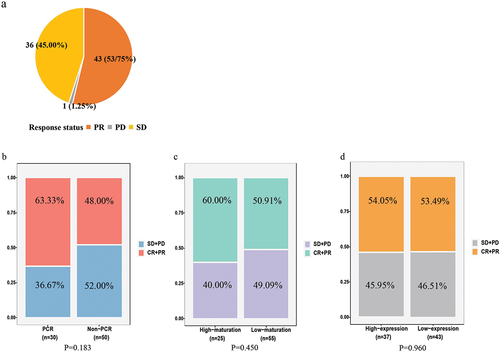
Figure 6. Forest plot of risk of disease progression for patients in (a) PCR group compared to non-PCR group in different subgroups, (b) TLS high-maturation group compared to TLS low-maturation group in different subgroups, and (c) TLS high-expression group compared to TLS low-expression group in different subgroups.
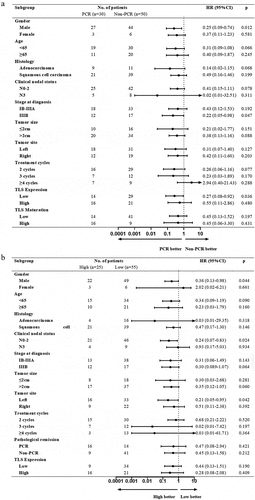
Table 2. Summary of confirmed response assessed by RECIST version 1.1.
The relationship of clinicopathological characteristics with PCR, TLS maturation and expression
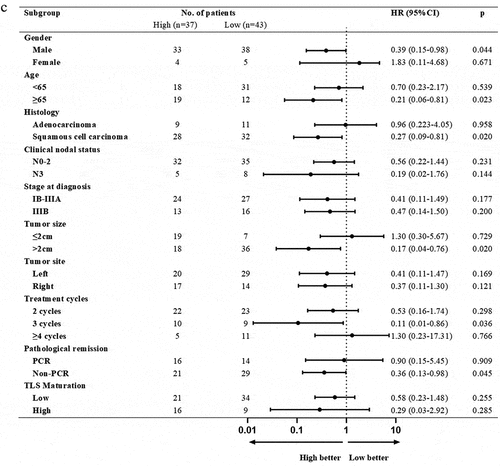
We used forest plots to present the risk of disease progression in the stratified analyses. In most subgroups, the PCR, TLS high-expression and TLS high-maturation group respectively achieved a better clinical outcome relative to the non-PCR, TLS low-expression and TLS low-maturation group. It is worth noting that in the male and TLS low-expression subgroup, PCR group tended to reduce the risk of disease progression compared to non-PCR group (). In the male, clinical nodal status with N0–2 and tumor site of left subgroup, patients with TLS high-maturation could benefit better from survival (). In the male, ≥65 years old, squamous cell carcinoma, treatment cycles of 3 and non-PCR subgroup, patients with high TLS-expression achieved better DFS ().
Cox proportional hazard regression analysis of clinical indicators to predict risk of disease progression
We evaluated clinical characteristics of the patients to determine their prognostic value for DFS. In the univariate analysis, Eastern Cooperative Oncology Group performance status (ECOG PS) (P < .001), smoking history (P = .005), TLS Expression (P = .041), TLS Maturation (P = .024), and PCR (P = .020) exhibited significant correlations with DFS. These factors were included in the multivariate analysis. The results revealed that ECOG PS (P < .001) and PCR (P = .017) remained as independent prognostic factors for DFS of patients receiving neoadjuvant chemoimmunotherapy ().
Table 3. Univariate and multivariate analysis of DFS in all patients.
Discussion
In this retrospective study, we analyzed the clinical outcomes and pathological characteristics of 80 patients with stage IB-IIIB NSCLC receiving neoadjuvant chemoimmunotherapy from two centers. Additionally, we evaluated surgical resection specimens of all 80 patients to assess pathological remission and the expression and maturation of TLS. By analyzing the relationship between pathological findings and survival, we drew the following conclusions: patients who obtained PCR had significantly longer DFS, and patients with high TLS expression and high maturation had better clinical benefit than those with low expression and low maturation, respectively.
Neoadjuvant immunotherapy plus chemotherapy has significantly improved clinical prognosis and increased the percentage of PCR in patients with resectable NSCLC.Citation8,Citation21 The previous conventional regimen of neoadjuvant chemotherapy obtained low rates of PCR (4%), which led to the limitation of PCR as an endpoint indicator in chemotherapy. Preoperative chemoimmunotherapy significantly increased the proportion of PCR, making it a potential alternative treatment endpoint. Checkmate 816 showed a significant correlation between PCR and event-free survival (EFS), the median EFS of PCR and non-PCR group was NR and 26.6 months (HR = 0.13, 95%CI: 0.05–0.37), respectively.Citation7 A large-scale meta-analysis suggested that PCR may be considered as a surrogate endpoint for survival benefit in resectable NSCLC and may serve as one of the predictors in clinical studies of neoadjuvant therapy.Citation22 In our study, we drew similar conclusions. We explored the relationship between PCR and DFS and found that PCR group achieved significantly better survival benefits compared to the non-PCR group (mDFS: NR vs. 20.24 months, P = .020). During neoadjuvant therapy, there was a total of one patient whose efficacy was assessed as PD, who was in the non-PCR group.
Immunotherapy response has heterogeneity, and the efficacy of it varies among different patients. Relying solely on traditional biomarkers such as PD-L1 and TMB makes it difficult to accurately predict the efficacy of neoadjuvant immunotherapy.Citation9,Citation10 Therefore, it is necessary to explore more effective biomarkers for predicting the prognosis of NSCLC with neoadjuvant immunotherapy. TLS is an ectopic lymphoid organ that develops in non-lymphoid tissues at sites of chronic inflammation. Previous studies have shown that the presence of TLS is associated with good prognosis and response to immunotherapy.Citation14 The presence of TLS not only improves the clinical outcome of cancer patients, but also provides a favorable site for the generation of anti-tumor humoral and cellular immune responses.Citation23 Tumors with abundant TLSs have a greater infiltration of CD8+ T cells, which may be depleted, explaining the greater likelihood of anti-tumor immune effects in TLS-rich tumors.Citation24 TLS has been shown to correlate with the efficacy of immunotherapy.Citation25 However, few studies explored the prognostic role of TLS in neoadjuvant chemoimmunotherapy for NSCLC, especially with regard to TLS expression and maturation. In our study, we evaluated the maturity and expression of TLS by HE staining. Currently TLS is strictly defined mainly by immunohistochemistry, whereas TLSs defined as lymphocyte aggregates can be assessed by HE staining. Several studies have demonstrated the correlation between TLS assessed by HE staining and survival in colorectal tumors.Citation26,Citation27 Different levels of TLS expression and maturity may have different effects on the outcome of immunotherapy in tumor patients, which may be related to the tumor type and metastatic sites. Our study found higher DFS rates in the high-expression group of TLS compared to the low-expression group (mDFS: 34.07 vs. 22.30 months, P = .024). In most subgroups, patients with high expression of TLS can achieve better prognosis. A retrospective study confirmed our conclusion.Citation28 However, a distinct advantage of ours is that all of our patients received preoperative chemoimmunotherapy, which is highly relevant for predicting prognosis in the neoadjuvant population.
TLS has different maturation stages and exerts different anticancer effects. One study demonstrated higher value of TLS maturity in cancer prognosis.Citation29 We categorized TLS into low-maturation and high-maturation group based on their maturity, respectively. In our study, the TLS high-maturation group had significantly better DFS, confirming that TLS maturity is a favorable prognostic factor for neoadjuvant chemoimmunotherapy for NSCLC, which is similar to the findings of previous studies.Citation11 TLS is a specific biomarker predictive of immunotherapy,Citation30 and the conclusions of studies on whether neoadjuvant chemotherapy induces or impairs the effect of TLS are inconsistent.Citation31,Citation32 Therefore, the role of neoadjuvant chemoimmunotherapy in TLS remains to be further explored.
We found that ECOG PS and PCR were independent prognostic factors for DFS of patients receiving neoadjuvant chemoimmunotherapy by univariate and multivariate Cox regression analyses. Several studies found that stage, nodal status or tumor size appeared as significant contributors for DFS.Citation7,Citation33,Citation34 However, we did not draw similar conclusions, which could be due to the small sample size. Of note, although our preliminary analysis suggested that high expression and maturation of TLS may be prognostic factors, multivariate cox analyses did not support the expression and maturation of TLS as independent prognostic factors. Therefore, larger-scale clinical studies are needed to further explore the relationship between clinicopathological characteristics and survival of patients receiving neoadjuvant chemoimmunotherapy.
Our study explored the relationship between PCR and DFS, providing evidence for supporting PCR as a surrogate endpoint. Although TLS has been proved to be correlated with the efficacy of immunotherapy, in most of these studies, patients received immunotherapy postoperatively. Few studies have been conducted on the relationship between TLS and the prognosis of NSCLC patients receiving neoadjuvant chemoimmunotherapy. In our study, all patients received immunotherapy combined with chemotherapy before surgery. In addition, most of the previous studies on TLS have explored the relationship between its density, location and abundance and prognosis. Our study of the relationship between TLS expression and maturation and the prognosis of NSCLC patients provides more dimensions for studies on TLS in the future. Few studies assessed TLS expression and maturation based solely on HE staining, which is a major novelty of our study. It provides a reference for simplifying the assessment of TLS in the future clinic. Our group initially explored this assessment method, the results of which were promising. In the future, we will combine more assessment methods to further explore the feasibility and accuracy of this method. In addition, our study is a double-center study, with a pathology assessment team consisting of pathologists from both centers of expertise, in an attempt to minimize subjective errors to some extent.
As this study is a retrospective study, following the neoadjuvant chemoimmunotherapy since its clinical application, the follow-up time is lower than that of prospective studies, which is a limitation of retrospective studies. Despite the shorter follow-up time, the PCR and tertiary lymphoid structures still reflect positive features at a follow-up time of 17 months, which further demonstrates their value in prognosis. The short follow-up time is a common limitation of current retrospective studies related to neoadjuvant chemoimmunotherapy, and in some retrospective studies, the follow-up time was even around 1 year. However, instead of studying the survival difference between regimens alone, which would require a much longer follow-up time, we explored the prognostic value of PCR and TLSs. The current follow-up time is somewhat reasonable. But we do acknowledge that this is indeed a limitation for us, and with the widespread use of neoadjuvant chemoimmunotherapy regimens, the follow-up time in subsequent relevant studies is expected to be further extended.
This study has the inherent flaws of a retrospective study. Firstly, the gender distribution was unequal, which may affect the results analyzed in this study. Secondly, the information of PD-L1 expression was insufficient and the percentage of PCR/MPR rate was not representative of the overall neoadjuvant population. In addition, we did not have enough samples, which affected the percentage of PCR/MPR rates. And because of sample size limitations, it was not possible to develop a more refined TLS scoring system. Currently, TLSs are evaluated on surgical specimens and the criteria are not yet standardized. In the future, larger sample sizes, more simplified assessment methods and more complete scoring systems are needed to analyze the prognostic role of TLS in neoadjuvant chemoimmunotherapy for NSCLC.
In conclusion, In patients with resectable NSCLC receiving neoadjuvant chemoimmunotherapy, the acquirement of PCR may predict better DFS. In addition, high expression and maturation of TLS may be prognostic factors.
Data sharing statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethical statement
This study received approval from the Ethics Committee of Jiangsu Cancer Hospital (2023-040). The requirement for informed consent was waived due to the retrospective nature of the study.
Supplementary Table.docx
Download MS Word (20.9 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary data
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2285902.
Additional information
Funding
References
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–12. doi:10.3322/caac.21763.
- Felip E, Rosell R, Maestre JA, Rodríguez-Paniagua JM, Morán T, Astudillo J, Alonso G, Borro JM, González-Larriba JL, Torres A, et al. Preoperative chemotherapy plus surgery versus surgery plus adjuvant chemotherapy versus surgery alone in early-stage non–small-cell lung cancer. J Clin Oncol. 2010;28(19):3138–45. doi:10.1200/jco.2009.27.6204.
- Zhang B, Zhong H, Han B. Neoadjuvant immunotherapy for patients with non-small cell lung cancer-is a new era coming? JAMA Oncol. 2023;9(3):301–2. doi:10.1001/jamaoncol.2022.6898.
- Conroy M, Forde PM. Advancing neoadjuvant immunotherapy for lung cancer. Nat Med. 2023;29(3):533–4. doi:10.1038/s41591-023-02246-2.
- Provencio M, Nadal E, González-Larriba JL, Martínez-Martí A, Bernabé R, Bosch-Barrera J, Casal-Rubio J, Calvo V, Insa A, Ponce S, et al. Perioperative nivolumab and chemotherapy in stage III non–small-cell lung cancer. N Engl J Med. 2023;389:504–13. doi:10.1056/NEJMoa2215530.
- Provencio M, Serna-Blasco R, Nadal E, Insa A, García-Campelo MR, Casal Rubio J, Dómine M, Majem M, Rodríguez-Abreu D, Martínez-Martí A, et al. Overall survival and biomarker analysis of neoadjuvant nivolumab plus chemotherapy in operable stage IIIA non–small-cell lung cancer (NADIM phase II trial). J Clin Oncol. 2022;40(25):2924–33. doi:10.1200/jco.21.02660.
- Forde PM, Spicer J, Lu S, Provencio M, Mitsudomi T, Awad MM, Felip E, Broderick SR, Brahmer JR, Swanson SJ, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med. 2022;386(21):1973–85. doi:10.1056/NEJMoa2202170.
- Heymach JV, Mitsudomi T, Harpole D, Aperghis M, Jones S, Mann H, Fouad TM, Reck M. Design and rationale for a phase III, Double-blind, placebo-controlled study of neoadjuvant durvalumab + chemotherapy followed by adjuvant durvalumab for the treatment of patients with resectable stages II and III non-small-cell lung cancer: the AEGEAN trial. Clin Lung Cancer. 2022;23(3):e247–e51. doi:10.1016/j.cllc.2021.09.010.
- Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, Chen L, Pardoll DM, Topalian SL, Anders RA. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20(19):5064–74. doi:10.1158/1078-0432.ccr-13-3271.
- Yarchoan M, Hopkins A, Jaffee EM, Jaffee, EM. Tumor mutational burden and response rate to PD-1 inhibition. N Engl J Med. 2017;377(25):2500–1. doi:10.1056/NEJMc1713444.
- Sun X, Liu W, Sun L, Mo H, Feng Y, Wu X, Li C, Chen C, Li J, Xin Y, et al. Maturation and abundance of tertiary lymphoid structures are associated with the efficacy of neoadjuvant chemoimmunotherapy in resectable non-small cell lung cancer. J Immunother Cancer. 2022;10(11):e005531. doi:10.1136/jitc-2022-005531.
- Sautès-Fridman C, Petitprez F, Calderaro J, Fridman WH. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat Rev Cancer. 2019;19(6):307–25. doi:10.1038/s41568-019-0144-6.
- Fridman WH, Meylan M, Petitprez F, Sun CM, Italiano A, Sautès-Fridman C. B cells and tertiary lymphoid structures as determinants of tumour immune contexture and clinical outcome. Nat Rev Clin Oncol. 2022;19(7):441–57. doi:10.1038/s41571-022-00619-z.
- Schumacher TN, Thommen DS. Tertiary lymphoid structures in cancer. Science ( New York, N.Y.). 2022;375(6576):eabf9419. doi: 10.1126/science.abf9419.
- Cottrell TR, Thompson ED, Forde PM, Stein JE, Duffield AS, Anagnostou V, Rekhtman N, Anders RA, Cuda JD, Illei PB, et al. Pathologic features of response to neoadjuvant anti-PD-1 in resected non-small-cell lung carcinoma: a proposal for quantitative immune-related pathologic response criteria (irPRC). Ann Oncol. 2018;29(8):1853–60. doi:10.1093/annonc/mdy218.
- Tamiya Y, Nakai T, Suzuki A, Mimaki S, Tsuchihara K, Sato K, Yoh K, Matsumoto S, Zenke Y, Nosaki K, et al. The impact of tertiary lymphoid structures on clinicopathological, genetic and gene expression characteristics in lung adenocarcinoma. Lung Cancer ( Amsterdam, Netherlands). 2022;174:125–32. doi:10.1016/j.lungcan.2022.11.001.
- Wakasu S, Tagawa T, Haratake N, Kinoshita F, Oku Y, Ono Y, Takenaka T, Oda Y, Shimokawa M, Mori M. Preventive effect of tertiary lymphoid structures on lymph node metastasis of lung adenocarcinoma. Cancer Immunol Immunother. 2023;72(6):1823–34. doi:10.1007/s00262-022-03353-8.
- Cai W, Jing M, Gu Y, Bei T, Zhao X, Chen S, Wen J, Gao J, Wu C, Xue Z. Tumor microenvironment features decipher the outperformance of neoadjuvant immunochemotherapy over chemotherapy in resectable non-small cell lung cancer. Front Immunol. 2022;13:984666. doi:10.3389/fimmu.2022.984666.
- Pataer A, Kalhor N, Correa AM, Raso MG, Erasmus JJ, Kim ES, Behrens C, Lee JJ, Roth JA, Stewart DJ, et al. Histopathologic response criteria predict survival of patients with resected lung cancer after neoadjuvant chemotherapy. J Thorac Oncol. 2012;7(5):825–32. doi:10.1097/JTO.0b013e318247504a.
- Posch F, Silina K, Leibl S, Mündlein A, Moch H, Siebenhüner A, Samaras P, Riedl J, Stotz M, Szkandera J, et al. Maturation of tertiary lymphoid structures and recurrence of stage II and III colorectal cancer. Oncoimmunology. 2018;7(2):e1378844. doi:10.1080/2162402x.2017.1378844.
- Felip E, Altorki N, Zhou C, Csőszi T, Vynnychenko I, Goloborodko O, Luft A, Akopov A, Martinez-Marti A, Kenmotsu H, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB–IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet. 2021;398(10308):1344–57. doi:10.1016/s0140-6736(21)02098-5. London, England.
- Waser NA, Adam A, Schweikert B, Vo L, McKenna M, Breckenridge M, Penrod JR, Goring S. Pathologic response as early endpoint for survival following neoadjuvant therapy (NEO-AT) in resectable non-small cell lung cancer (rNSCLC): systematic literature review and meta-analysis. Ann Oncol. 2020;31:S806–S806. doi:10.1016/j.annonc.2020.08.116.
- Petitprez F, de Reyniès A, Keung EZ, Chen TW, Sun CM, Calderaro J, Jeng YM, Hsiao LP, Lacroix L, Bougoüin A, et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature. 2020;577(7791):556–60. doi:10.1038/s41586-019-1906-8.
- Lauss M, Donia M, Svane IM, Jönsson G. B cells and tertiary lymphoid structures: friends or foes in cancer immunotherapy? Clin Cancer Res. 2022;28(9):1751–8. doi:10.1158/1078-0432.ccr-21-1130.
- Vanhersecke L, Brunet M, Guégan JP, Rey C, Bougouin A, Cousin S, Moulec SL, Besse B, Loriot Y, Larroquette M, et al. Mature tertiary lymphoid structures predict immune checkpoint inhibitor efficacy in solid tumors independently of PD-L1 expression. Nat Cancer. 2021;2(8):794–802. doi:10.1038/s43018-021-00232-6.
- Väyrynen JP, Sajanti SA, Klintrup K, Mäkelä J, Herzig KH, Karttunen TJ, Tuomisto A, Mäkinen MJ. Characteristics and significance of colorectal cancer associated lymphoid reaction. Int J Cancer. 2014;134(9):2126–35. doi:10.1002/ijc.28533.
- Ueno H, Hashiguchi Y, Shimazaki H, Shinto E, Kajiwara Y, Nakanishi K, Kato K, Maekawa K, Miyai K, Nakamura T, et al. Objective criteria for crohn-like lymphoid reaction in colorectal cancer. Am J Clin Pathol. 2013;139(4):434–41. doi:10.1309/ajcpwhueftgbwke4.
- Fukuhara M, Muto S, Inomata S, Yamaguchi H, Mine H, Takagi H, Ozaki Y, Watanabe M, Inoue T, Yamaura T, et al. The clinical significance of tertiary lymphoid structure and its relationship with peripheral blood characteristics in patients with surgically resected non-small cell lung cancer: a single-center, retrospective study. Cancer Immunol Immunother. 2022;71(5):1129–37. doi:10.1007/s00262-021-03067-3.
- Goc J, Germain C, Vo-Bourgais TK, Lupo A, Klein C, Knockaert S, de Chaisemartin L, Ouakrim H, Becht E, Alifano M, et al. Dendritic cells in tumor-associated tertiary lymphoid structures signal a Th1 cytotoxic immune contexture and license the positive prognostic value of infiltrating CD8+ T cells. Cancer Res. 2014;74(3):705–15. doi:10.1158/0008-5472.can-13-1342.
- Brunet M, Crombé A, Cousin S, Vanhersecke L, Le Loarer F, Bessede A, Italiano A. Mature tertiary lymphoid structure is a specific biomarker of cancer immunotherapy and does not predict outcome to chemotherapy in non-small-cell lung cancer. Ann Oncol. 2022;33(10):1084–5. doi:10.1016/j.annonc.2022.06.007.
- Remark R, Lupo A, Alifano M, Biton J, Ouakrim H, Stefani A, Cremer I, Goc J, Régnard JF, Dieu-Nosjean MC, et al. Immune contexture and histological response after neoadjuvant chemotherapy predict clinical outcome of lung cancer patients. Oncoimmunology. 2016;5(12):e1255394. doi:10.1080/2162402x.2016.1255394.
- Siliņa K, Soltermann A, Attar FM, Casanova R, Uckeley ZM, Thut H, Wandres M, Isajevs S, Cheng P, Curioni-Fontecedro A, et al. Germinal centers determine the prognostic relevance of tertiary lymphoid structures and are impaired by corticosteroids in lung squamous cell carcinoma. Cancer Res. 2018;78(5):1308–20. doi:10.1158/0008-5472.can-17-1987.
- Chaft JE, Oezkan F, Kris MG, Bunn PA, Wistuba II, Kwiatkowski DJ, Owen DH, Tang Y, Johnson BE, Lee JM, et al. Neoadjuvant atezolizumab for resectable non-small cell lung cancer: an open-label, single-arm phase II trial. Nat Med. 2022;28(10):2155–61. doi:10.1038/s41591-022-01962-5.
- Deutsch JS, Cimino-Mathews A, Thompson E, Provencio M, Forde PM, Spicer J, Girard N, Wang D, Anders RA, Gabrielson E, et al. Association between pathologic response and survival after neoadjuvant therapy in lung cancer. Nat Med. 2023. doi:10.1038/s41591-023-02660-6.