ABSTRACT
Long-term follow-up of a cohort of unmarried girls who received one, two, or three doses of quadrivalent HPV vaccine, between 10 and 18 years of age, in an Indian multi-centric study allowed us to compare antibody responses between the younger and older age cohorts at 10-years post-vaccination, and study the impact of initiation of sexual activity and cervical HPV infections on antibody levels. Among the younger (10–14 years) recipients of a single dose, 97.7% and 98.2% had detectable binding antibody titers against HPV 16 and HPV 18 respectively at ten years post-vaccination. The proportions among those receiving a single dose at age 15–18 years were 92.3% and 94.2% against HPV 16 and HPV 18 respectively. Mean HPV 16 binding antibody titers were 2.1 folds (95%CI 1.4 to 3.3) higher in those vaccinated at ages 10–14 years, and 1.9 folds (95%CI 1.2 to 3.0) higher in those vaccinated at 15–18 years compared to mean titers seen in the unvaccinated women. Compared to previous timepoints of 36 or 48 months, binding antibodies against HPV 16 and neutralizing antibodies against both HPV 16 and HPV 18 were significantly higher at 10 years. This rise was more pronounced in participants vaccinated at 15–18 years. No association of marital status or cervical HPV infections was observed with the rise in titer. Durability of antibody response in single dose recipients correlated well with the high efficacy of a single dose against persistent HPV 16/18 infections irrespective of age at vaccination, as we reported earlier.
Introduction
The Strategic Advisory Group of Experts convened by the World Health Organization (WHO) in 2022 recommended an off-label use of a single dose schedule for Human Papillomavirus (HPV) vaccine in girls (and boys) aged 9 to 20 years.Citation1 Man et al. using a modeling study demonstrated that single dose vaccination with catch-up extended to age 20 years will have more significant impact in reducing the lifetime risk of cervical cancer and accelerating elimination of the disease compared to two dose vaccination limited to pre-adolescent girls.Citation2 Durability of immune response following a single dose of HPV vaccine is a crucial factor to guide policies, especially when the upper age for vaccination is extended to 20 years. The humoral response is dependent on age at vaccination; the antibody titers after two doses of bivalent vaccine in 18–25 year old females was documented to be only about half those achieved in aged 10–17 years.Citation3 Consequently, whether protective immune responses would last throughout the active sexual life of a woman following a single dose vaccination at age beyond 15 years is a question of paramount public health importance and will inform any future decision on requirement of a booster in these young adult women.
From the point of view of natural history of immune-mediated protection offered by HPV vaccine there is reassuring evidence favoring long-term protection. The virus like particle (VLP), the antigenic component of HPV vaccine with its particulate 55 nm structure displaying a repetitive array of surface epitopes, can robustly stimulate the long-lived plasma cells (LLPCs) in the bone marrow.Citation4,Citation5 The stimulated LLPCs continue to produce high-quality neutralizing antibodies against the targeted HPV types for many years and may even do so for a lifetime.Citation6,Citation7 This probably happens independent of additional antigenic exposure from natural infections though the evidence is not yet clear.
Long-term (>10 years post-vaccination) immunogenicity outcomes comparing seropositivity and antibody levels in single dose recipients and recipients of two or three doses were reported only by the Costa Rica HPV vaccine trial (CVT) and the Indian cohort study conducted by the International Agency for Research on Cancer (IARC), France.Citation8,Citation9 While the former is evaluating a bivalent HPV vaccine (CervarixTM, GlaxoSmithKline Biologicals, Belgium) administered to females aged 18–25 years the latter is evaluating a quadrivalent one (GardasilTM; Merck Sharp & Dohme, NJ, USA) in girls aged 10–18 years. The IARC study has the advantage of being able to compare the antibody responses between the young age (vaccinated at 10–14 years of age) and older age (vaccinated at 15–18 years of age) cohorts. In our earlier publication from the IARC study we reported the comparative immunogenicity between the two age groups after two and three doses of HPV vaccine at various time points with longest follow up being at 48 months post-vaccination.Citation10
In the present manuscript based on the IARC Indian study, we have compared the L1 binding and neutralizing antibody responses between the young and older age cohorts at 10-years post-vaccination and have reported any possible impact of marriage and cervical infection with type-specific and any HPV infections. Though the main focus of the article is long-term antibody response after a single dose, we have reported data on two and three dose recipients as well.
Methods
Study design
Details of the study initiating recruitment in September 2009 have been previously published.Citation9 In brief, this study was originally planned as a randomized control trial (RCT) aimed at comparison of the efficacy of two-dose (administered on days 1 and 180) and three-dose (administered on days 1, 60 and 180) regimens of GardasilTM. With the Indian Government ordering stoppage of HPV vaccination in all trials in April 2010 due to reasons not related to this study, it was converted to a cohort study with longitudinal follow up of four groups of participants receiving either three doses at days 1, 60 and ≥ 180 (three-dose group), two doses at days 1 and ≥ 180 (two-dose group), two doses by default at day 1 and 60 (two-dose default group), and one dose (single-dose group).
Study participants
The original RCT planned to recruit 20,000 unmarried girls aged 10 to 18 years, equally randomized to the two arms. The abrupt stoppage of vaccination resulted in 4,348 girls in the three-dose, 4,979 in the two-dose, 3,452 in the two-dose default and 4,950 girls in the single-dose groups. A post-hoc group of 1,484 unvaccinated married women was recruited as an additional comparison group with age- and place of residence-matched to the vaccinated 18- to 21-year-old married participants. All five groups (cohorts) are having annual follow-up that is expected to continue at least until August 2026. Blood samples were collected from a convenient group of participants for immunogenicity study at 10 years post-vaccination (details given below). In addition, cervical samples are collected yearly for four consecutive years during follow-up of the married vaccinated participants and all unvaccinated participants to estimate incident and persistent HPV infections. The cervical samples are analyzed with Luminex assay capable of individually detecting 21 HPV genotypes (including all 14 oncogenic types, HPV 6 and 11).
Blood sample collections to assess immune responses
Blood samples were obtained from a convenient sample of study participants (all study ages represented) at day 1 (pre-vaccination), months 7 (two- and three-dose groups only), months 12 and 24 (two-doses default and single-dose groups only), and at months 18, 36, 48 and 60 (for all four vaccinated cohorts). Ten-years after the first vaccine dose, an additional blood sample was collected from 324 recipients in the single-dose cohort randomly selected among those who provided at least a baseline and a 12-month blood sample. Blood samples at 10-years were also collected from 190 three-dose recipients and 167 two-dose recipients. One-time blood sample was collected from a random selection of 352 unvaccinated women at the same time when samples were collected at 10 years in vaccinated participants.
Measurement of binding antibodies using multiplex VLP-based IgG ELISA (M9ELISA)
The samples were tested using the multiplex HPV virus-like particle-based IgG ELISA on Meso Scale Discovery platform (M9ELISA)Citation11 at Centers for Disease Control and Prevention (CDC), Atlanta, GA, USA, laboratory to detect and quantify HPV 16 and 18 binding antibodies. To quantify the amount of antibodies present in the tested serum compared to the reference (standard) serum, a parallel line (PLL) value was obtained using the methods described in the World Health Organization (WHO) HPV Labnet Manual.Citation12 For each sample, raw relative light units (RLU) from three dilutions were used in the lab analysis. For a tested sample to be valid, the following PLL criteria had to be fulfilled: correlation ≥ 0.9, slope ≤-0.4, slope ratio ≥ 0.5 and at least two of the dilutions’ data points within linear range relative to the standard sample. International Units per mL (IU/mL) were used to report the total binding antibody titers for HPV 16 and 18. The lower limit of quantitation (LLOQ) (depicting the cutoffs for detectable antibodies) values for HPV types 16 and 18 were 1.0 IU/mL and 0.3 IU/mL, respectively.
Measurement of neutralizing antibodies using high throughput pseudovirion-based neutralization assay (HT-PBNA)
The samples were additionally tested with the automated, HT-PBNA at the joint Chemical Biology Core Facility of the European Molecular Biology Laboratory (EMBL) and the German Cancer Research Center (DKFZ) Heidelberg, Germany. HT-PBNA was used to measure neutralizing antibodies against the HPV-L1 protein of HPV 16 and 18. International Units per mL (IU/mL) were used to report the neutralization antibody titers for HPV 16 and 18. The cutoff value depicting the lowest detectable antibodies for both HPV 16 and 18 was 40 IU/mL.
Statistical analysis
In this analysis, the two-dose default cohort was not considered, as the dose regimen is of no practical significance. The participant characteristics among the different study cohorts at 10-year time-point were shown as proportions. The distribution of both total binding (assessed by M9ELISA) and neutralization (assessed by HT-PBNA) immune responses was presented as geometric mean titers (GMT) together with their 95% confidence intervals (CIs). To assess the effect of age at vaccination, marital status and previous HPV infection on immune responses, log-transformed GMT were used in linear regression models to obtain GMT ratios and their corresponding 95% CIs. The comparisons for age (15–18 versus 10–14 years within the same vaccine dose group; and either vaccinated age group versus unvaccinated were done for months 0, 7, 12, 18, 36 and 120 (total binding antibodies assessment) and months 18, 24, 36, 48, 60 and 120 (neutralization titers assessment). Similar comparisons for marital status (never- versus ever-married, within the same dose-group; and never- or ever-married vaccinated versus unvaccinated) were carried out for months 36 and 120 (total binding antibodies) and months 48 and 120 (neutralization titers).
In the regression models evaluating the effect of time-age (or time-marital status) combinations, the total binding titer comparisons were done between months 36 and 120, and neutralization antibody comparisons between months 48 and 120.
To assess if HPV infection prior to blood sample collection had an impact on the 10-year immune responses, a variable including the following three categories was created: never-married (a proxy for those unexposed to HPV infection, that is, not initiated sexual activity in the Indian context), married and not infected with specific HPV types, and married and infected with specific HPV types (detailed below). For these analyses, we separately considered infection with either HPV 16 or 18 or with any HPV type.
For comparisons with the unvaccinated cohort, statistical significance was inferred when the GMT ratio 95% CI did not include one. Stata 17.0 (StataCorp LP, Texas, USA) was used for all statistical analyses.
The study was approved by the ethics committee at IARC and at each individual site. The trial is registered with ISRCTN (ISRCTN98283094), and ClinicalTrials.gov (NCT00923702).
Results
The characteristics of participants providing serology samples for the unvaccinated and at 10-year time-point after first dose for the vaccinated cohorts are shown in . Serology samples at 10 years were obtained from 344, 324, 190, and 167 unvaccinated women, recipients of a single dose, two doses, and three doses, respectively. Over 60% of these recipients were vaccinated between ages 10–14 years. At the time of serology sample collection, 32.6% (n = 112) of unvaccinated, 24.7% (n = 80) of single-dose recipients, 31.1% (n = 59) of two-dose recipients and 30.5% (n = 51) of three-dose group were aged 26 years or older. All participants in the unvaccinated, 71.0% (n = 230) single-dose, 61.1% (n = 116) two-dose, and 69.5% (n = 116) three-dose recipients were married when the blood sample was collected.
Table 1. Women characteristics at 10-year serology sample collection.
Comparison of binding antibody titres
Comparison of the binding antibody titers assessed with M9ELISA between the 10–14 year and 15–18 year age cohorts overall and stratified by vaccination dose status at month 120 is presented in . Evolution of total binding GMT at different time points against HPV 16 and 18 stratified by different dose and age groups (in comparison with natural immunity observed in unvaccinated women) is shown in . Binding antibody levels for the two dose recipients were available only at month 120.
Figure 1. Evolution of binding and neutralization immune responses for vaccine-targeted HPV types 16 and 18 over time in the recipients of a single-dose or three doses of the HPV vaccine stratified by age.
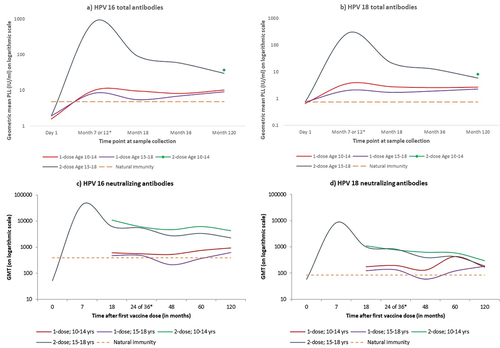
Table 2. Age-stratified distribution and comparison of immune responses against HPV types 16 and 18 measured by M9ELISA by dose received and time of sample collection.
Ten years after vaccination, a high proportion of single-dose recipients still had detectable binding antibody titers against HPV types 16 and 18 in both age cohorts; the proportion being slightly higher in the young compared to the older age cohort (97.7% versus 92.3% for HPV 16 and 98.2% versus 94.2% for HPV 18). All women in both age cohorts had detectable titers against HPV 16 and 18 in the three-dose group, and against HPV 16 in the two-dose group. The proportion of detectable titers against HPV 18 was higher in the 10–14 compared to the 15–18 year age cohort in the two-dose group (99.2% versus 95.8%).
Ten years after vaccination in the single dose recipients, the HPV 16 GMT for binding antibodies were 2.1 folds (95% CI 1.4 to 3.3) higher in those vaccinated at ages 10–14 years, and 1.9 folds (95% CI 1.2 to 3.0) higher for those vaccinated at 15–18 years compared to mean titer seen in the unvaccinated women. Among the single-dose recipients, participants aged 15–18 years had an 11% non-significant lower HPV 16 GMT compared to those aged 10–14 years at vaccination (GMT ratio 0.9; 95% CI 0.7 to 1.1). The evolution of single-dose GMTs against HPV 16 at different time points was similar between the two age cohorts except at month 18 when the older participants had significantly lower GMT (GMT ratio 0.6; 95%CI 0.4 to 0.9) than the younger age cohort (). The mean GMTs in both the age groups among single dose recipients at 10 years post-vaccination were significantly inferior to those of the two dose recipients at 10–14 years and three dose recipients at 15–18 years ().
The pattern of evolution of the binding antibody GMT kinetics against HPV types 18 stratified by the two age cohorts mirrored that of HPV 16 (). The 10-year GMT against HPV 18 after a single vaccine dose was 3.7 times (95% CI 2.8 to 4.8) higher among those vaccinated at 10–14 years, and 3.1 times (95% CI 2.3 to 4.2) higher among those vaccinated at 15–18 years compared to the titers in the unvaccinated women. The titers in the older recipients of the single dose were 14% lower (statistically non-significant) than those observed in the younger single dose recipients (GMT ratio 0.8; 95% CI 0.6 to 1.2). These titers among the two age cohorts were significantly inferior to those of their counterparts in the three- and two-dose groups ().
Comparison of neutralizing antibody titres
Patterns observed in the neutralization GMT at 10 years after vaccination against HPV 16 are described in and the antibody kinetics over time in . The proportion of single dose recipients having detectable neutralizing antibodies at ten years was high in the two age cohorts against HPV 16 (98.2% in young versus 97.1% in older participants), but much lower against HPV 18 (54.1% in younger and only 38.5% in older participants).
Table 3. Age stratified distribution and comparison of geometric mean neutralization titers against HPV types 16 and 18 measured by PBNA by dose received and time of sample collection.
At 10 years post-vaccination with a single dose, compared to unvaccinated women the HPV 16 neutralization GMTs were 2.4 times (95% CI 1.4 to 4.4) higher in the younger, and 1.6 times (95% CI 0.8 to 3.1) higher in the older age cohorts. The mean titer against HPV 16 was significantly higher in younger single dose recipients compared to older ones (GMT ratio 0.7; 95% CI 0.5 to 0.9).
The neutralizing titers against HPV 18 were at the 10-year time-point 2.0 (95% CI 1.1 to 3.8) and 2.2 (95% CI 1.0 to 4.5) times higher in young and older single dose recipients respectively, compared to unvaccinated women. However, no significant difference was observed in the mean neutralizing titer against HPV 18 (GMT ratio 1.1; 95% CI 0.6 to 1.8) between the age groups ().
Among those vaccinated at 15–18 year there was no significant difference in the HPV 18 neutralizing GMT at 10 years post-vaccination between those receiving a single dose (182; 95%CI 126 to 264), two doses (183; 95%CI 136 to 245) and three doses (188; 95%CI 139 to 254). The titers for the 10–14 year old recipients were lower in the single dose (169; 95% CI 140 to 204) compared to the two doses (296; 95% CI 240 to 364) and the three doses (305; 95% CI 236 to 394) groups.
Binding and neutralizing antibody titres at 10 years compared to earlier time-points
The binding antibody titers at months 36 and 120 and neutralizing antibody titers at months 48 and 120 are described in respectively. While the mean GMT of both antibody types against either HPV 16 or HPV 18 in the three-dose group was significantly reduced at month 120 compared to earlier time-points irrespective of the age groups, the titers either remained stable or even increased in the single dose recipients. The mean GMT against HPV 16 induced by a single dose was significantly higher at month 120 compared to month 36 for binding antibodies (adjusted GMT ratio 1.4; 95% CI 1.0 to 1.8) and at month 48 for neutralizing antibodies (adjusted GMT ratio 2.3; 95%CI 1.4 to 3.5). Though the rise in titer against HPV 18 (1.1-times; 95% CI 0.7–1.9) at 120 months was not statistically significant for binding antibody, a significant 1.7-time (95% CI 1.1 to 2.6) rise was observed for neutralizing antibodies. Compared to their younger counterparts the older participants had more pronounced and statistically significant rise in neutralizing antibody titers but not in binding antibody titers against either HPV type ().
Table 4. Age-distribution and comparison of immune responses at months 36 and 120 against HPV types 16 and 18 obtained using M9ELISA.
Table 5. Age-distribution and comparison of neutralization immune responses at months 48 and 120 against HPV types 16 and 18 obtained using PBNA.
Comparison by marital status and cervical HPV infection
The findings from our study cohorts for both the binding and neutralization antibody titers when marital status was used (in place of age stratification) generally mimicked those observed for age stratification (Supplementary tables S1a and S1b, and ). The married women had lower but statistically non-significant antibody titres against HPV 16 and HPV 18 compared to same dose unmarried recipients (except neutralizing antibodies against HPV 16 in the two dose recipients in whom the married women had significantly lower titre) (Supplementary Tables S1a and S1b). This might be because women vaccinated at ages 15–18 were more likely to be married at the time of 120-month blood sample collection compared to those vaccinated at 10–14 years (Supplementary table S2). Likewise, when we compared the month 120 to the earlier time points and analyzed separately for the never and ever married, both binding and neutralization antibodies induced by the single dose against HPV 16 and 18 were still elevated at month 120, except for those against HPV 18 in the never married participants ().
Table 6. Distribution and comparison of immune responses at months 36 and 120 by marital status against HPV types 16 and 18 obtained using M9ELISA.
Table 7. Distribution and comparison of neutralization immune responses at months 48 and 120 by marital status against HPV types 16 and 18 obtained using PBNA.
We additionally assessed the impact of HPV infections (HPV 16, HPV 18, any HPV) being detected during or prior to 10-year blood draw on the immune responses (Supplementary Tables S3a and S3b). The findings were not consistent across dose groups (e.g., the married recipients of two doses who were infected with HPV 16 had significantly lower binding and neutralizing antibody titer against HPV 16 compared to those unmarried, which was not the case for single or three dose recipients). No definite correlation was observed between cervical HPV infection status and the binding or neutralizing titers against either HPV 16 or 18 in any of the dose groups.
Discussion
Based on serial analysis of a convenient sample of participants we have reported earlier that the binding and the neutralizing antibody titers against HPV 16 and 18 stabilize at 18 months post-vaccination in the single dose recipients and remain constant at a level that is two to four-fold higher compared to that in unvaccinated sexually exposed women at least up to 10-year post-vaccination.Citation9 In the present manuscript we demonstrate that age at vaccination up to 18 years has little impact on durability of immune response. Earlier studies demonstrated that females receiving HPV vaccine at 10–17 years had at least twice the geometric mean titer of IgG compared to the females receiving same number of doses at 18–25 years few months after vaccination.Citation3 Inferior immune responses in older age group led the WHO to recommend three doses of the vaccine in females older than 14 years compared to two doses in the younger age groups.Citation13 The phenomenon that girls vaccinated at a younger age having significantly higher induced titers than those vaccinated at later ages was not seen in our study at the 10-year time-point at least up to the age of 18 years. Furthermore, the proportion of single dose recipients having detectable binding and neutralizing antibodies against HPV 16 or HPV 18 at 10 years post-vaccination was almost similar and exceeded 90% in both the young and older age groups except neutralizing titers against HPV 18. HPV 18 neutralizing antibodies were not detectable in 45.9% and 61.5% of younger and older recipients respectively. Nonetheless, for single dose recipients, there was no difference between the age groups as far as the geometric mean of either binding antibody against HPV 16/18 or neutralizing antibody against HPV 18 at 10-year post-vaccination was concerned although the HPV 18 neutralizing antibody titer in the older age group was significantly lower compared to their younger counterparts. The clinical significance of the few vaccine recipients with undetectable or low levels of anti-HPV antibody especially against HPV 18 is unclear. Earlier studies vaccinating 16–23-year-old women with Gardasil have also observed significant decline in anti-HPV 18 GMT at 9 years post vaccination (only 60% of the three-dose recipients remaining seropositive) without any waning of protection.Citation14 In our study no HPV 16/18 persistent infection was detected in the few women with waning or undetectable antibody titers (data not shown). They seem to remain protected, since very low levels of antibodies appear to be sufficient to protect cervicovaginal tissue against infection.Citation4
It was reassuring to see that the mean titer of antibodies (both binding and neutralizing) against HPV 16 or 18 in the single dose recipients remained stable between two and ten years and was two to four times higher at 10 years compared to that observed through natural immunity irrespective of age at vaccination. Our results are very much aligned with the CVT assessing 11-year immunogenicity of a single dose of CervarixTM in women aged 18 to 25 years at vaccination; 96.7% and 92.9% of the recipients were seropositive for binding antibodies against HPV 16 and HPV 18 respectively.Citation8 Like our study, the CVT also documented stable antibody levels (both binding and neutralizing) between years four and eleven in young adult women receiving a single dose.
Though the immune correlate of protection of the HPV vaccine is yet unknown, it is well recognized that a small amount of antibody is adequate to neutralize the virus at the point to entry to the basal layer of epithelium.Citation15,Citation16 The low yet stable antibody titer observed in the single dose recipients is providing durable protection is obvious from the high vaccine efficacy (VE) reported from our trial earlier. The VE of single dose of GardasilTM in our study against persistent HPV 16/18 infections based on assessment of 2135 women at a median follow up of 9 years was 95.4% (95% CI 85.0–99.9).Citation17 The high protection even in those receiving the vaccine at an older age is obvious from the fact that only a single case of persistent HPV 16 (and no HPV 18) was detected in the single dose cohort as opposed to 2.5% of the unvaccinated women being detected with persistent HPV 16/18 infections.Citation17
CVT observed a small but statistically significant increase in the mean titer of HPV 18 (but not HPV 16) in the single dose recipients between seven to 11 years, while for two or three dose recipients only a small decline was noted. After careful analysis of the qualitative change in the antibodies over time the authors concluded that boosting effect ascribed to natural exposure to HPV 16/18 could not explain the stable immune response in the single dose recipients. We observed a significant upward trend in neutralizing antibodies against both HPV 16 and 18 (and also binding antibodies against HPV 16) at 10 years post-vaccination in the single-dose recipients but not in the two or three dose recipients. If this phenomenon was associated with natural infection, we would have observed higher antibody titers at 10 years in the married women and especially the women who had incident HPV 16/18 infections at any time point. However, our detailed analysis failed to demonstrate any specific pattern and the married or infected women tended to have lower antibody titers (statistically non-significant) essentially because majority of them were vaccinated at an older age (15–18 years). Not all participants contributed samples at each time point so it is possible that this could impact the observed titers. Further follow up of our cohort and the antibody response assessment planned at 15 years post-vaccination will throw more light on the impact of natural infection on the protective immunity offered by the vaccines.
While the single dose antibody titers are stable at long term follow up, the titers following two or three doses are still showing a downward slope. It is possible that when the antibody levels stabilize ultimately there will be very little difference between the dose and age groups (as we have already seen for HPV 18 neutralizing antibody). A continued protection against persistent HPV 18 infections irrespective of doses of vaccine received or age at vaccination reinforces the hypothesis that very little antibody is required to neutralize the virus at the point of their entry into the basal layers of cervical epithelial cells.Citation18
To conclude, our study supports the WHO single dose recommendation extended up to 20 years of age at least from immunologic point of view. Earlier recommendations restricted lower dose of the vaccine (2 doses) to only girls and boys below 15 years of age while recommending three doses for the higher age groups. Based on our evidence reinforcing the latest WHO recommendations countries may safely consider expanding the catch-up cohort up to 20 years using a single dose. Taking advantages of the resources saved and simplified logistics associated with a single dose vaccine regimen, HPV vaccination programmes may cover larger proportions of their adolescent female (and male when resources permit) populations. Such a pragmatic strategy will in turn increase herd immunity and accelerate the elimination of cervical cancer as has been demonstrated in our modeling study.Citation2
Disclaimer
Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, or the Centers for Disease Control and Prevention, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the official position, decisions, policy or views of the International Agency for Research on Cancer/World Health Organization or the Centers for Disease Control and Prevention.
Supplemental Material
Download MS Word (38.8 KB)Acknowledgments
We are very grateful to the Bill & Melinda Gates Foundation for their generous financial port, Peter Dull and Carolyn Wendell (Integrated Clinical Vaccine Development, Bill & Melinda Gates Foundation) for their valuable support, encouragement, and scientific inputs; current and past members of the data safety monitoring board: Lynette Denny, John Schiller, Peter Sasieni, Thangarajan Rajkumar, Doreen Ramogola-Masire, Gina Ogilvy, Lutz Gissmann and Raul Murillo for their valuable advice and monitoring of the study safety and outcomes; We thank Ms Krittika Guinot, IARC, for help in preparation of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary data
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2289242.
Additional information
Funding
References
- World Health Organization. Human papillomavirus vaccines: WHO position paper (2022 update). Wkly Epidemiol Rec. 2022;97(50):645–10. https://apps.who.int/iris/handle/10665/365350.
- Man I, Georges D, de Carvalho TM, Ray Saraswati L, Bhandari P, Kataria I, Siddiqui M, Muwonge R, Lucas E, Berkhof J, et al. Evidence-based impact projections of single-dose human papillomavirus vaccination in India: a modelling study. Lancet Oncol. 2022 Nov;23(11):1419–1429. doi:10.1016/S1470-2045(22)00543-5.
- Schwarz TF, Leo O. Immune response to human papillomavirus after prophylactic vaccination with AS04-adjuvanted HPV-16/18 vaccine: improving upon nature. Gynecol Oncol. 2008 Sep;110(3 Suppl 1):S1–10. doi:10.1016/j.ygyno.2008.05.036.
- Stanley M. HPV - immune response to infection and vaccination. Infect Agent Cancer. 2010 Oct 20;5(1):19. doi:10.1186/1750-9378-5-19.
- Amanna IJ, Slifka MK. Mechanisms that determine plasma cell lifespan and the duration of humoral immunity. Immunol Rev. 2010 Jul;236(1):125–38. doi:10.1111/j.1600-065X.2010.00912.x.
- Khodadadi L, Cheng Q, Radbruch A, Hiepe F. The maintenance of memory plasma cells. Front Immunol. 2019 Apr 5;10:721. doi:10.3389/fimmu.2019.00721.
- Single-Dose HPV Vaccine Evaluation Consortium. Review of the current published evidence on single-dose HPV vaccination. 4th Edition. 2022 [accessed 2023 Aug 8]. 20220328_SDHPV_Evidence_Review_Edition_4_Final_L2.pdf(path.org).
- Kreimer AR, Sampson JN, Porras C, Schiller JT, Kemp T, Herrero R, Wagner S, Boland J, Schussler J, Lowy DR, et al. Evaluation of durability of a single dose of the bivalent HPV vaccine: the CVT trial. J Natl Cancer Inst. 2020 Oct 1;112(10):1038–46. doi:10.1093/jnci/djaa011.
- Joshi S, Anantharaman D, Muwonge R, Bhatla N, Panicker G, Butt J, Rani Reddy Poli U, Malvi SG, Esmy PO, Lucas E, et al. Evaluation of immune response to single dose of quadrivalent HPV vaccine at 10-year post-vaccination. Vaccine. 2023 Jan 4;41(1):236–45. doi:10.1016/j.vaccine.2022.11.044.
- Bhatla N, Nene BM, Joshi S, Esmy PO, Poli URR, Joshi G, Verma Y, Zomawia E, Pimple S, Prabhu PR, et al. Are two doses of human papillomavirus vaccine sufficient for girls aged 15-18 years? Results from a cohort study in India. Papilloma Res. 2018 Jun;5:163–171. doi:10.1016/j.pvr.2018.03.008.
- Panicker G, Rajbhandari I, Pathak HN, Brady AM, Unger ER. Multiplex immunoassay to measure antibody response to nine HPV vaccine types. J Immunol Methods. 2021 Nov;498:113136. doi:10.1016/j.jim.2021.113136.
- WHO. Human papillomavirus laboratory manual, 1st ed., 2009. World Health Organization; 2010. https://www.who.int/immunization/hpv/learn/hpv_laboratory_manual__who_ivb_2009_2010.pdf:.
- World Health Organization. Human papillomavirus vaccines: WHO position paper, May 2017. Wkly Epidemiol Rec. 2017;92(19):241–268. https://iris.who.int/handle/10665/255354.
- Nygård M, Saah A, Munk C, Tryggvadottir L, Enerly E, Hortlund M, Sigurdardottir LG, Vuocolo S, Kjaer SK, Dillner J. Evaluation of the long-term anti-human papillomavirus 6 (HPV6), 11, 16, and 18 immune responses generated by the quadrivalent HPV vaccine. Clin Vaccine Immunol. 2015 Aug;22(8):943–8. doi:10.1128/CVI.00133-15.
- Roden RBS, Stern PL. Opportunities and challenges for human papillomavirus vaccination in cancer. Nat Rev Cancer. 2018 Apr;18(4):240–54. doi:10.1038/nrc.2018.13.
- Kines RC, Thompson CD, Lowy DR, Schiller JT, Day PM. The initial steps leading to papillomavirus infection occur on the basement membrane prior to cell surface binding. Proc Natl Acad Sci USA. 2009 Dec 1; 106(48):20458–63. doi:10.1073/pnas.0908502106.
- Basu P, Carvalho AL, Almonte M, Chajès V, Weiderpass E. Pulling the investment levers on implementation research in oncology. Lancet Oncol. 2022 Apr;23(4):451–2. doi:10.1016/S1470-2045(22)00025-0.
- Longet S, Schiller JT, Bobst M, Jichlinski P, Nardelli-Haefliger D. A murine genital-challenge model is a sensitive measure of protective antibodies against human papillomavirus infection. J Virol. 2011 Dec;85(24):13253–9. doi:10.1128/JVI.06093-11.
