ABSTRACT
Owing to both vaccine- and infection-induced immunity, the COVID-19 seroprevalence is ~90% in most countries. It is important to examine the protective role of booster vaccines and hybrid immunity in the COVID-endemic state. Utilizing a hospital information system for COVID-19, we conducted a cohort study by linking laboratory-confirmed COVID-19 case data to the national immunization records during the BA.5 omicron predominant period (1 August−31 December 2022) in Chiang Mai, Thailand. Out of 63,009 adults with COVID-19 included in the study, there were 125 (0.2%) severe COVID outcomes and 6.4% had a previous omicron infection. Protection against severe COVID-19 was highest among those with at least one booster vaccine (63%; aHR 0.37 [95%CI 0.19–0.73]) as compared to those without prior vaccination or natural infection. Hybrid immunity offered better protection (35%; aHR 0.65 [95%CI 0.09–4.73) than primary vaccine series alone or previous infection alone. Evaluating risk by age group, those aged 70 years or more had nearly 40 times (aHR 39.58 [95%CI 18.92–82.79]) the risk of severe-COVID-19 as compared to the 18–39-year age group. While booster vaccines remain the most effective way of protecting against severe COVID-19, particularly in the elderly, hybrid immunity may offer additional benefit.
Introduction
Cumulatively, the Coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has led to more than 770 million confirmed cases and nearly 7 million deaths globally, with almost 5 million cases and more than 34,000 deaths in Thailand alone (as of 1st November 2023).Citation1 Vaccines against SARS-CoV-2 have significantly reduced the impact of COVID-19 globally.Citation2,Citation3 As the pandemic evolved, newer SARS-CoV-2 variants emerged, and to address issues of both waning vaccine effectiveness and attenuated efficacy against newer variants, many countries have introduced booster vaccinations, with some countries recommending variant-specific COVID-19 vaccines.Citation4,Citation5 At present, the population-level COVID-19 seroprevalence is estimated to be ~90% in most countries and is contributed by both vaccine- and infection-induced immunity.Citation6 While WHO has declared the end of the global COVID-19 emergency, COVID-19 persists as an endemic disease and still represents a global threat as “It is still killing and it is still changing. The risk remains of new variants emerging that cause new surges in cases and deaths.”Citation7 As the pandemic continues to evolve, the nature of population-wide immunity against SARS-CoV-2 has become increasingly complex. Hybrid immunity, defined as the immunity achieved by a combination of natural COVID-19 infection with completion of appropriate immunization, has shown to provide equivalent or even superior protection to receiving a booster-vaccine.Citation8,Citation9 People who have recovered from COVID-19 and then received a vaccine were found to have higher SARS-CoV-2 Receptor Binding Domain-specific memory B-cells and variant-neutralizing antibodies and a distinct population of cytokine-expressing T cells as compared to previously naive individuals.Citation10 As many countries adapt to an endemic state of COVID-19, it is important to fully examine the role played by hybrid immunity at a population level.
The aim of this study was to examine the protective effect of booster vaccinations and hybrid immunity against severe outcomes with BA.5 omicron SARS-CoV-2 variant.
Materials and methods
We conducted a retrospective cohort study on Thai residents aged 18 years or older, with a laboratory confirmed SARS-CoV-2 infection between 1 August and 31 December 2022 using a unique hospital information system (HIS) established in Chiang Mai province, located in Northern Thailand, with a population of 1.6 million. Non-Thai residents and migrants were excluded from the study as the vaccination data and outcome capture for this group was incomplete. Date of first positive SARS-CoV-2 test served as the index date. Molecular testing from northern Thailand revealed that > 90% of samples sequenced from July onwards were of BA.5 sub-lineages.Citation11
We have previously published the details related to creating and implementing the information systems used in this studyCitation12 and the study methodology.Citation13,Citation14 In Chiang Mai province, all COVID-19 cases detected are reported into the web-based HIS of Chiang Mai Provincial Health Office. During the study period, mandatory reporting of all COVID-19 cases was enforced under the Communicable Disease Control Act. Each hospital’s information system records data on severity and progression of the disease including requirement of ventilatory support and these are linked with the province HIS. Similarly, deaths occurring within the province are routinely updated in the HIS following mortality reports to Chiang Mai Provincial Health Office. The Ministry of Public Health, Thailand captures and maintains all national vaccination records via the Ministry of Public Health Immunization Centre database. The baseline clinical characteristics and SARS-CoV-2 test details were extracted from the HIS and linked with the types of COVID-19 vaccines and dates of vaccination extracted from the national immunization database.
The study was conducted on routine data collected as part of the national COVID-19 response under the Communicable Disease ACT (B.E. 2558) and was exempted from ethics review and informed consent.
“Severe COVID-19” was defined as previously described,Citation13,Citation14 as requiring invasive mechanical ventilation (IMV) during hospital admission, or death within 30 days of a positive SARS-CoV-2 test. Records of all subjects were followed till death or up to 30 days from first positive test, whichever was earlier. “Hybrid immunity” was defined as a combination of previous infection and completing at least the primary COVID-19 vaccine series. “Previous infection” was defined as a positive SARS-CoV-2 test before 90 days prior to index date in line with previously published studies.Citation15 We only considered previous infection during the omicron predominant period beginning in January 2022 in Chiang Mai province of Thailand.Citation16
Descriptive statistics for the subjects with and without severe COVID-19 outcomes are reported separately to evaluate differences in baseline characteristics between the groups. Mean and standard deviation (SD) are reported for normally distributed data or median and interquartile range (IQR) for skewed data when analyzing continuous variables. Categorical variables are summarized as frequency and percentages. Between-group comparisons for continuous variables were done using t-test (normally distributed data) or Mann–Whitney U test (skewed data). Between-group comparisons for categorical variables were done using chi-squared test or fisher’s exact test, as appropriate.
To estimate the hazard ratios (HRs) for severe COVID-19, Cox proportional hazards regression was used. As per previous analyses,Citation13,Citation14 the follow-up period was taken from the first positive SARS-CoV-2 test date and censored at the earliest of date of first starting IMV, date of death, or 30 days from first positive test date. A number of factors were added in the regression model to estimate adjusted HRs (aHR) and the 95% confidence interval (95% CI) such as age, gender, calendar day of test (in weekly units), vaccination status, and combination of natural and vaccine induced immunity. All statistical analyses were be conducted using stata (version 15.0 SE, College station, TX: StataCorp LP). Significance tests are 2 sided and a p-value <.05 was considered statistically significant.
Results
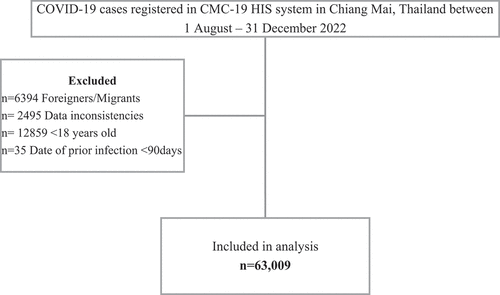
Between 1 August and 31 December 2022, there were 84,792 COVID-19 cases reported in Chiang Mai province, Thailand. There were 63,009 (74.31%) Thai residents above 18 years of age with complete data included in the final analysis after applying the exclusion criteria (). Severe COVID-19 outcomes and deaths were observed in 125 (0.2%) and 71 (0.1%) subjects, respectively. There were 4,064 (6.4%) subjects with a previous omicron infection since January 2022 in the cohort.
Figure 1. Flow chart of subject selection for adult COVID-19 patients who are residents of Chiang Mai, Thailand between 1 August−31 December 2022.
Subjects with severe COVID-19 outcomes were over 30 years older and ~60% were aged 70 years or older, compared to just ~10% among those without severe outcomes. As compared to those without severe outcomes, those with severe outcomes were more likely to be male. Only 7% of those with severe outcomes had at least one booster vaccine as compared to > 20% among those without severe outcomes. Interestingly, 16% of those with severe outcomes received only the primary series vaccine as compared to 9% among those without severe outcomes. This could be explained by the time lapse since last vaccine. The median time from last primary vaccine was 398 days (IQR 319-512) for the cohort suggesting that the protective benefit of the primary vaccination series has already waned by the index date.
Those with severe outcomes were more likely to be unvaccinated and not have a prior infection while those without severe outcomes were more likely to have received at least one booster dose or have hybrid immunity. Among subjects with a prior infection, those with severe outcomes were more likely to have had the infection >6 months prior to index date ().
Table 1. Comparison of clinical characteristics of adult COVID-19 patients with and without severe outcomes during BA.5 predominance (1 Aug 2022–31 Dec 2022) in Chiang Mai, Thailand.
In models examining the role of booster vaccination (irrespective of previous infection), after adjusting for age, gender, and calendar time of test, receiving one booster dose was associated with 58% (aHR 0.42 [95%CI 0.21–0.83]; ) risk reduction against severe COVID-19 as compared to unvaccinated group. No events were observed among those who received three or four booster doses, while two booster doses were associated with 84% (aHR 0.16 [95%CI 0.02–1.21]) risk reduction against severe COVID-19; statistical significance was not achieved due to low number of events ().
Table 2. Association between vaccination, natural immunity, age, and baseline characteristics with severe COVID-19 outcomes among adult COVID-19 cases during BA.5 predominance (1 Aug 2022–31 Dec 2022) in Chiang Mai, Thailand.
In models examining the role of previous infection in combination with vaccination, after adjusting for age, gender, and calendar time of test, receiving at least one booster vaccine only (no-previous infection) was associated with 63% (aHR 0.37 [95%CI 0.19–0.73], ) risk reduction against severe COVID-19 as compared to no prior vaccination or infection. Hybrid immunity was associated with a 35% (aHR 0.65 [95%CI 0.09–4.73], ) risk reduction against severe COVID-19; however, statistical significance was not achieved due to low number of events. Hybrid immunity provided better protection than either previous infection alone, or primary vaccination series alone ().
In models examining the role of age, the age groups 18–29 and 30–39 were combined for the Cox-PH analysis due to zero outcome events in the 18–29 year age group (). In separate models adjusting for gender, calendar time of test and vaccination status, and immunity from vaccination and infection, those in the older age groups remained at significantly higher risk of severe-COVID-19 outcomes. Despite correcting for vaccination and prior infection, those aged 70 years or more had nearly 40 times (aHR 39.58 [95%CI 18.92–82.79]; ) the risk of severe-COVID-19 as compared to the 18–39-year age group, suggesting that up-to date vaccination is critical to avoid adverse outcomes due to COVID-19 in this age group.
Discussion
The impact of COVID-19 in terms of the number of cases and deaths globally has been extremely high; however, the impact of vaccinations is undisputable, when they have been implemented appropriately. Two dose primary vaccination schedules were highly effective against early variants but by necessity have evolved to third, fourth and even fifth doses to manage new variants and concerns around waning immunity. In the current landscape of high vaccination-derived and natural immunity, more data are needed on the protective effect of COVID-19 booster vaccines against newer BA.5 sub-lineages in addition to assessing the role of hybrid immunity against severe COVID-19.
We found that receiving at least one booster vaccine offered greater than 60% protection against severe COVID-19 outcomes during a period of BA.5 predominance.Citation16 Using earlier datasets of the same HIS database, we have previously reported >90% effectiveness of booster vaccines during delta variant and ~85% effectiveness against earlier omicron lineages.Citation13,Citation17 In comparison, the effectiveness against BA.5 lineage appears lower. This could in part be due to waning of the vaccine effectiveness over time and lower effectiveness of the monovalent vaccines against newer SARS-CoV-2 variants. Bivalent vaccines were not yet available during the study period.
In our study, although hybrid immunity was associated with better protection as compared to infection induced immunity alone or primary series alone, booster vaccines, irrespective of previous infection, remained the most effective method of protecting against severe COVID-19 with BA.5. Our findings are similar to the observations by Altarawneh and colleagues who conducted a national, matched, test-negative, case-control study in Qatar and found booster vaccines with or without previous infection conferred very strong protection against severe or fatal BA.2 infection.Citation18 However, some previous studies have reported that hybrid immunity had higher effectiveness over booster vaccination alone.Citation9,Citation19 It is noteworthy that the study by Nordström and colleaguesCitation9 was limited to earlier variants (alpha and delta) while Carazo and colleagues assessed the protective role of only prior-BA.1 infection against BA.2,Citation19 which may explain the differences observed with our study.
Importantly, stratifying the analysis by age group demonstrates a significantly higher risk for severe COVID-19 outcomes in older age groups, but especially so in those over 70 years of age. Our data strongly suggest that increasing booster vaccine coverage throughout the population, but particularly among elderly, remains an important strategy to continue protection against severe COVID-19 outcomes. For vulnerable groups, two booster vaccinations may be necessary.
Limitations of our study included the fact that we were unable to examine other confounders such as chronic comorbidities which are important risk factors of severe COVID-19 outcomes. Additionally, the source population were those diagnosed with COVID-19, and testing could have been done for reasons other than clinical suspicion, and finally, we did not differentiate or control for incidental findings of COVID-19.
Conclusion
Booster vaccination remains the most optimal tool for protecting against severe COVID-19 outcomes, irrespective of previous infection status. Our study suggests that hybrid immunity may offer additional benefit over natural immunity or vaccine induced immunity alone, but larger cohorts are required to assess this benefit fully. A very important observation from this work confirms the significantly higher risks of severe COVID-19 outcomes in elderly populations, highlighting the need to ensure up to date booster coverage of this vulnerable group.
Declaration of generative AI and AI-assisted technologies in the writing process
Authors confirm that this manuscript is our original work and generative-AI and AI-assisted technologies were not used in the writing process.
Acknowledgments
We are grateful to the Chiang Mai Provincial Health Office and the Department of Disease Control Ministry of Public Health for the collaborative partnerships in managing health information of COVID-19 pandemic.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets generated and/or analyzed during the current study can be made available from the Faculty of Public Health, Chiang Mai University ([email protected]) on reasonable request.
Additional information
Funding
References
- Our World in Data. Coronavirus pandemic (COVID-19). [accessed 2023 Nov 16]. https://ourworldindata.org/coronavirus.
- Doroshenko A. The combined effect of vaccination and nonpharmaceutical public health interventions-ending the COVID-19 pandemic. JAMA Netw Open. 2021;4(6):e2111675. doi:10.1001/jamanetworkopen.2021.11675.
- Moore S, Hill EM, Tildesley MJ, Dyson L, Keeling MJ. Vaccination and non-pharmaceutical interventions for COVID-19: a mathematical modelling study. Lancet Infect Dis. 2021;21(6):793–6. doi:10.1016/S1473-3099(21)00143-2.
- Morbidity and Mortality Weekly Report (MMWR). Interim recommendations from the advisory committee on immunization practices for the use of bivalent booster doses of COVID-19 vaccines — United States; 2022 Oct [accessed 2023 Nov 16]. https://www.cdc.gov/mmwr/volumes/71/wr/mm7145a2.htm.
- ECDC-EMA statement on booster vaccination with omicron adapted bivalent COVID-19 vaccines. [accessed 2023 Nov 16]. https://www.ema.europa.eu/en/news/ecdc-ema-statement-booster-vaccination-omicron-adapted-bivalent-covid-19-vaccines.
- WHO SAGE roadmap for prioritizing uses of COVID-19 vaccines [accessed 2023 Nov 16]. https://www.who.int/publications/i/item/WHO-2019-nCoV-Vaccines-SAGE-Roadmap.
- WHO chief declares end to COVID-19 as a global health emergency [accessed 2023 Nov 16]. https://news.un.org/en/story/2023/05/1136367.
- Pilz S, Theiler-Schwetz V, Trummer C, Krause R, Ioannidis JPA. SARS-CoV-2 reinfections: overview of efficacy and duration of natural and hybrid immunity. Environ Res. 2022;209:112911. doi:10.1016/j.envres.2022.112911.
- Nordström P, Ballin M, Nordström A. Risk of SARS-CoV-2 reinfection and COVID-19 hospitalisation in individuals with natural and hybrid immunity: a retrospective, total population cohort study in Sweden. Lancet Infect Dis. 2022;22(6):781–90. doi:10.1016/S1473-3099(22)00143-8.
- Rodda LB, Morawski PA, Pruner KB, Fahning ML, Howard CA, Franko N, Logue J, Eggenberger J, Stokes C, Golez I, et al. Imprinted SARS-CoV-2-specific memory lymphocytes define hybrid immunity. Cell. 2022;185(9):1588–601.e14. doi:10.1016/j.cell.2022.03.018.
- GISAID. nCoV-19 variants dashboard. [accessed 2023 Nov 16]. https://gisaid.org/hcov-19-variants-dashboard/.
- Intawong K, Olson D, Chariyalertsak S. Application technology to fight the COVID-19 pandemic: lessons learned in Thailand. Biochem Biophys Res Commun. 2021;538:231–237. doi:10.1016/j.bbrc.2021.01.093.
- Intawong K, Chariyalertsak S, Chalom K, Wonghirundecha T, Kowatcharakul W, Ayood P, Thongprachum A, Chotirosniramit N, Noppakun K, Khwanngern K, et al. Reduction in severity and mortality in COVID-19 patients owing to heterologous third and fourth-dose vaccines during the periods of delta and omicron predominance in Thailand. Int J Inf Dis. 2022;126:31–38. doi:10.1016/j.ijid.2022.11.006.
- Intawong K, Chariyalertsak S, Chalom K, Wonghirundecha T, Kowatcharakul W, Thongprachum A, Chotirosniramit N, Noppakun K, Khwanngern K, Teacharak W, et al. Waning vaccine response to severe COVID-19 outcomes during omicron predominance in Thailand. PLoS One. 2023;18(5):e0284130. doi:10.1371/journal.pone.0284130.
- Yahav D, Yelin D, Eckerle I, Eberhardt CS, Wang J, Cao B, Kaiser L. Definitions for coronavirus disease 2019 reinfection, relapse and PCR re-positivity. Clin Microbiol Inf. 2021;27(3):315–8. doi:10.1016/j.cmi.2020.11.028.
- Puenpa J, Rattanakomol P, Saengdao N, Chansaenroj J, Yorsaeng R, Suwannakarn K, Thanasitthichai S, Vongpunsawad S, Poovorawan Y. Molecular characterisation and tracking of severe acute respiratory syndrome coronavirus 2 in Thailand, 2020–2022. Arch Virol. 2023;168(1):26. doi:10.1007/s00705-022-05666-6.
- Intawong K, Chariyalertsak S, Chalom K, Wonghirundecha T, Kowatcharakul W, Ayood P, Thongprachum A, Chotirosniramit N, Noppakun K, Khwanngern K, et al. Heterologous booster vaccines reduce severity and mortality in COVID-19 during BA.2 and BA.4/BA.5 omicron predominance in Thailand. J Microbiol Immunol Infect. 2023 Oct 18;S1684-1182(23):00192–5. doi:10.1016/j.jmii.2023.10.001.
- Altarawneh HN, Chemaitelly H, Ayoub HH, Tang P, Hasan MR, Yassine HM, Al-Khatib HA, Smatti MK, Coyle P, Al-Kanaani Z, et al. Effects of previous infection and vaccination on symptomatic omicron infections. N Engl J Med. 2022;387(1):21–34. doi:10.1056/NEJMoa2203965.
- Carazo S, Skowronski DM, Brisson M, Barkati S, Sauvageau C, Brousseau N, Gilca R, Fafard J, Talbot D, Ouakki M, et al. Protection against omicron (B.1.1.529) BA.2 reinfection conferred by primary omicron BA.1 or pre-omicron SARS-CoV-2 infection among health-care workers with and without mRNA vaccination: a test-negative case-control study. Lancet Inf Dis. 2023;23(1):45–55. doi:10.1016/S1473-3099(22)00578-3.
