ABSTRACT
CAR-T cell therapy has demonstrated efficacy in treating certain hematological malignancies. However, the administration of CAR-T cells is accompanied by the occurrence of adverse events. Among these, cytokine release syndrome (CRS) has garnered significant attention. In this descriptive study, we set the search criteria to retrieve and obtain articles regarding CAR-T cell-related CRS from the Web of Science Core Collection (WoSCC). The bibliometric and knowledge-map analysis of these documents was conducted using Microsoft Excel 2019, GraphPad Prism 8, CtieSpace, and VOSviewer. 6,623 authors from 295 institutions in 49 countries coauthored a total of 1,001 publications. The leading country in this field was the United States. The most productive institution was the University of Pennsylvania. Carl H. June had the most citations, while Daniel W. Lee had the most co-citations. Research hotspots primarily concentrated on the pathogenesis, serum biomarkers, management, and therapeutic drugs of CRS, alongside neurotoxicity. Emerging topics within this discipline encompassed the following: a. Drugs for effective treatment and intervention of CRS; b. Conducting pertinent clinical trials to acquire real-world data; c. Management of toxicity (CRS and neurotoxicity) associated with CAR-T cell therapy; d. The study of BCMA-CAR-T cells in multiple myeloma (MM); e. Optimizing the CAR framework structure to enhance the effectiveness and safety of CAR-T cells. A bibliometric and scientific knowledge-map analysis provided a unique and objective perspective for exploring the field of CAR-T cell-related CRS, and may provide some new clues and valuable references for researchers.
Introduction
The ultimate goal of CAR-T cell therapy is to apply it to clinical treatment. Therefore, the efficacy and safety are important parameters to evaluate this treatment. Evidence has demonstrated its remarkable effectiveness in managing certain hematological malignancies. Nevertheless, adverse events can occur during the process of receiving CAR-T cells,Citation1–3 such as CRS, immune effector cell-associated neurotoxicity syndrome (ICANS), and hematological toxicity. In some cases, these adverse events can be severe and potentially life-threatening. Researchers have made a lot of efforts to improve the anti-tumor effect of CAR-T cells and reduce their adverse events. These measures include finding tumor-specific antigens (TSAs) as targets,Citation4 optimizing the structure of CARs (e.g., synNotch CARsCitation5,Citation6 and ON/OFF-switch CARsCitation7,Citation8), constructing special CARs (e.g., CARs that need special lightCitation9 or ultrasoundCitation10 to be activated), adding suicide genes,Citation11 and designing CD8α hinge and/or transmembrane domain with the best length.Citation12,Citation13
A better understanding of the underlying mechanism of CAR-T cell-related adverse events contributes to effective prevention and treatment. In recent years, numerous scholars have conducted in-depth research and summary on the diagnosis, mechanism and management of these adverse events from multifaceted perspectives.Citation1,Citation3,Citation14 CRS has garnered significant attention as the most prevalent adverse event.Citation15,Citation16 It is a super physiological reaction caused by excessive release of cytokines in the body.Citation17 The primary clinical presentations encompass fever, fatigue, headache, and joint pain. In more severe cases, hypotension, shock, capillary leakage, and multiple organ dysfunction syndrome (MODS) may transpire, posing a potential threat to life.Citation17,Citation18 Presently, the relevant mechanism of CRS remains unclear. A multitude of investigations have been conducted to explore various facets of CRS, including pathogenesis, prediction, diagnosis, classification, and management.Citation19–24
The study of CAR-T cells has emerged as a hot field in recent years.Citation25 The study and new knowledge of CAR-T cell-related CRS are also increasing rapidly. Bibliometrics can not only reflect the development trends and research hotspots in various fields, but also help researchers to obtain important information in a certain field by comprehensively and systematically analyzing the scientific literature in the field.Citation26,Citation27 Bibliometric analysis plays a crucial role in academia by facilitating various aspects of research. Firstly, it aids researchers in identifying research hotspots, frontier directions, and development trends within a specific field. This enables researchers to gain a comprehensive understanding of the research direction, avoid redundant work, and enhance research efficiency. Secondly, it serves as a tool for evaluating the quality and impact of research outcomes. Thirdly, it enables researchers to explore potential collaboration opportunities, partners, and institutions through the author cooperation network. Lastly, it provides a solid foundation and support for scientific research planning and decision-making processes. Currently, This field lacks bibliometric studies. This paper was the first to use a combination of bibliometrics and knowledge-map to analyze articles in this field. It aims to explore and seek the research overview, development trends, research hotspots, and new topics in this field.
Materials and methods
Data collection
The pertinent data was obtained and downloaded from WoSCC. The search strategy, inclusion, and exclusion criteria of this study are obtained in Annexes 1. A total of 1001 eligible articles were identified.
Data analysis and visualization
CiteSpace provides a test platform to study new ideas and compare existing approaches.Citation28 VOSviewer is a software for bibliometric mapping, which pays more attention to the visualization of scientific knowledge.Citation29 GraphPad Prism 8 and Microsoft Excel 2019 were used for data management and analysis. Furthermore, CiteSpace (version 6.2.R4) and VOSviewer (version 1.6.17) were used for bibliometric and visual analyses. Two researchers independently analyzed and extracted the required data.
Results
The annual growth trend of publication outputs
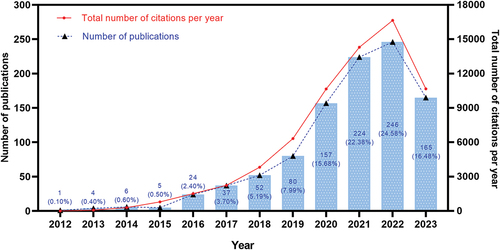
Based on the aforementioned retrieval strategy, 1001 related articles were identified. The number of articles published each year reflects the development trend of the field. shows that the number of articles and citations in this field is increasing annually. From 2012 to 2015, publication output was extremely low and almost stagnant. There were only 16 papers (accounting for 1.60%) in this stage. From 2016 to 2018, the number of publications showed a slowly increasing trend. Publications increased rapidly from 2019 to 2022. In this period, 707 papers (70.63%) were published. By August 25, 2023, 165 articles (16.48%) had been published. Only nine months of articles could be counted in 2023, so the current volume of publications was lower than in 2022. These articles were cited 67,374 times, with an average of 67.31 citations per paper. The H-index of these publications was 106. Based on the fitting equation (y = 22.56*x − 45423, correlation coefficient R2 = 0.8067) derived from the annual publication data, it is projected that the publication volume in 2023 will exceed that of 2022.
Figure 1. The growth trend and citation trend of publications related to CAR-T cell-related CRS.
Countries/regions and institutions
1001 articles were coauthored by 295 institutions from 49 countries (one paper may be completed by several countries or organizations, so it will be counted more than once). According to , the country with the most publications was the United States (n = 521, accounting for 35.06%), far exceeding other countries, followed by China (n = 336, 22.61%) and Germany (n = 73, 4.91%). Centrality refers to the intermediary role of a node in information transmission between other nodes. The higher the centrality of a node, the more important the node plays in information transmission. France, Italy, and Canada had a relatively high centrality of 0.46, 0.45, and 0.36, respectively. It is suggested that these countries have a strong bridge role in this field. The most productive institution was the University of Pennsylvania (n = 98, 2.85%). Notably, the top 10 institutions were all from the United States. illustrates the limited connections between the United States and China with other nations. Conversely, Austria has exhibited greater cooperation with certain countries over the preceding two years. In , it is evident that despite the close collaboration among numerous institutions, overall cooperation has been scarce during the aforementioned period.
Figure 2. The co-occurrence map of countries (a) and institutions (b) in the CAR-T cell-related CRS field.
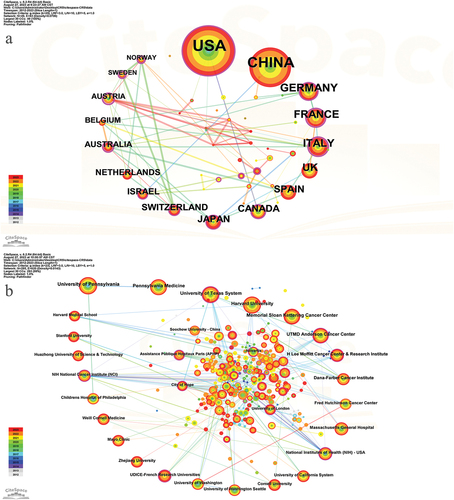
Table 1. The top 10 countries and institutions involved in CAR-T cell-related CRS.
Journals and co-cited journals
1001 articles were published in 303 academic journals. The most cited journal was the New England Journal of Medicine (n = 19950), far more than any other journal, followed by Blood (n = 4894) and Journal of Clinical Oncology (n = 4234) (). 80% of the top 10 journals were in the Q1 Journal Citation Reports (JCR) section, with the New England Journal of Medicine (IF = 158.499) having the highest impact factor. The journal with the most publications was Frontier in Immunology (n = 45, 4.5%), followed by Blood (n = 38, 3.8%) and Journal for Immunotherapy of Cancer (n = 37, 3.7%). 8 journals had over 20 publications. The density map of the publication volume of these journals is shown in . Through it, we can find all the journals with more than five articles published, which provides convenience for selecting journals.
Figure 3. The density map of journals (a) and co-cited journals (b) in the CAR-T cell-related CRS field.
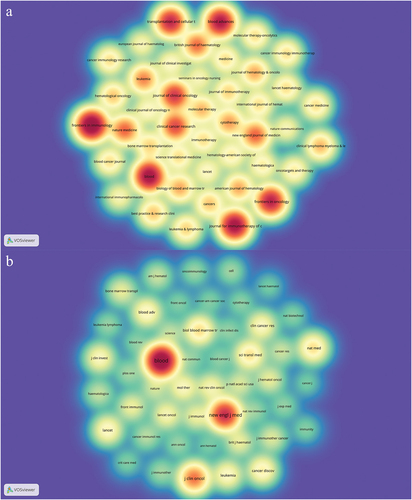
Table 2. The top 10 journals and co-cited journals related to CAR-T cell-related CRS.
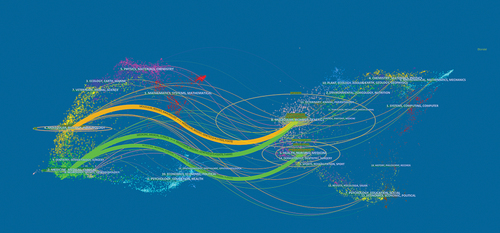
When two (or more) journals are cited by one or more papers simultaneously, they are considered to have a co-citation relationship. The most co-cited journal was Blood (n= 4991), followed by the New England Journal of Medicine (n = 3077) and Journal of Clinical Oncology (n = 1838). All three journals have been cited more than 1,000 times (). The density map of the co-cited amount of these journals is shown in . Through it, we can quickly find high-impact journals in this field. To determine the distribution of academic journals in this field, we constructed the dual-map overlay of journals ().Citation30
Figure 4. The dual-map overlay of journals on CAR-T cell-related CRS.
Authors and co-cited authors
A total of 6623 authors participated in the publication of these articles. As shown in , Carl H. June (n = 11151) was cited the most, followed by Bruce L. Levine (n = 10473) and Stephan A. Grupp (n = 9960). He Huang (n = 30) published the most articles. Include the authors who have contributed to a minimum of five scholarly articles, thereby establishing a cooperative network of these authors (). It shows that many researchers formed different collaborative groups.
Figure 5. The visualization map of authors (a) co-cited authors (b) involved in CAR-T cell-related CRS.
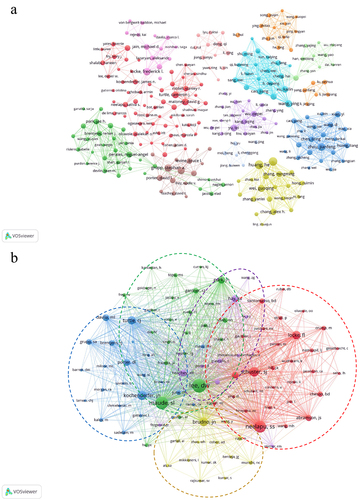
Table 3. The top 10 authors and co-cited authors of CAR-T cell-related CRS research.
If different authors are cited in one or more papers, these authors are called co-cited authors. The most co-cited author was Daniel W. Lee (n = 1016), followed by Shannon L. Maude (n = 844) and Sattva S. Neelapu (n = 758) (). The researchers who had been cited together at a minimum of 40 instances were combined, resulting in the creation of a network that shows their co-citation relationships. The co-citation network diagram of co-cited authors and their co-citation relationships is presented in .
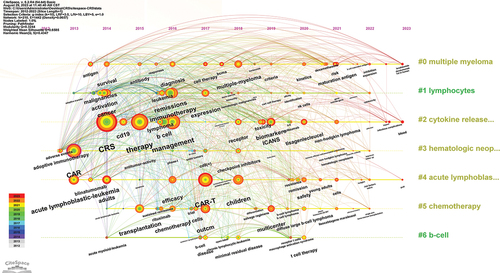
Keyword co-occurrence, clusters, and evolution
Keywords reflect the research focus and direction of a paper. To ensure accuracy and relevance, the collected keywords underwent a series of procedures, such as merging synonymous terms and eliminating insignificant ones. Ultimately, we obtained 2429 keywords. shows the top 20 keywords, all of which appeared more than 60 times. The most frequent keywords were CRS (n = 378), followed by CAR-T (n = 322) and immunotherapy (n = 199). A density map of keywords was constructed ().
Figure 6. The co-occurrence density map (a) and network (b) of keywords involved in CAR-T cell-related CRS.
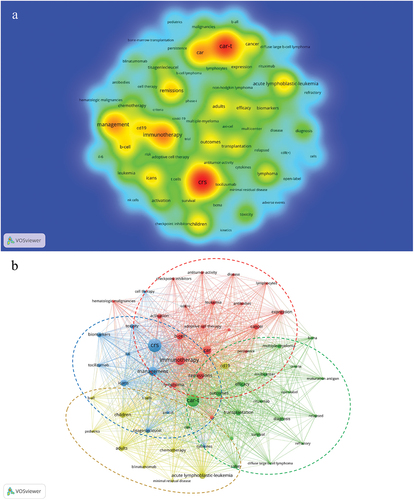
Table 4. Top 20 keywords related to CAR-T cell-related CRS.
The network clustering analysis of the keywords revealed 4 clusters, which represented 4 research scopes. As shown in , the largest cluster was cluster 1 (red), followed by clusters 2 (green), 3 (blue), and 4 (yellow). Cluster 1 had 20 keywords, including immunotherapy, CAR, adoptive cell therapy, cancer, hematologic malignancies, leukemia, lymphoma, antitumor-activity, and checkpoint inhibitors. Cluster 2 had 19 keywords, including CAR-T, outcomes, efficacy, survival, safety, transplantation, diffuse large B-cell lymphoma, non-Hodgkin lymphoma, multiple myeloma, BCMA, and rituximab. Cluster 3 had 11 keywords, including CRS, ICANS, toxicity, management, biomarkers, cytokines, IL-6, tisagenlecleucel, axi-cel, and tocilizumab. Cluster 4 had 10 keywords, including acute lymphoblastic leukemia (ALL), CD19, blinatumomab, minimal residual disease, children, adults, and chemotherapy.
The timeline viewer of keywords can cluster the keywords in chronological order. It can display the year when a keyword in a certain research field first appeared, and help to track the evolution of the keyword. The evolution track, important topics, and research hotspots in each stage of the field are shown in .
Co-cited references and reference burst
When two (or more) references are cited by one or more references simultaneously, they establish a co-citation relationship. The function of co-cited literature is to help researchers better understand the development trend, research direction, and discover new research hotspots in the subject field. It can also help researchers evaluate the importance of literature and help screen literature. shows the top 10 most co-cited articles in this field. These articles were co-cited more than 100 times, with the top 3 references co-cited over 300 times. The most co-cited article was published by Neelapu et al.Citation31 in 2017, with 334 citations. Notably, 40% of the top 10 most co-cited articles were from the New England Journal of Medicine.
Table 5. The top 10 co-cited articles related to CAR-T cell-related CRS.
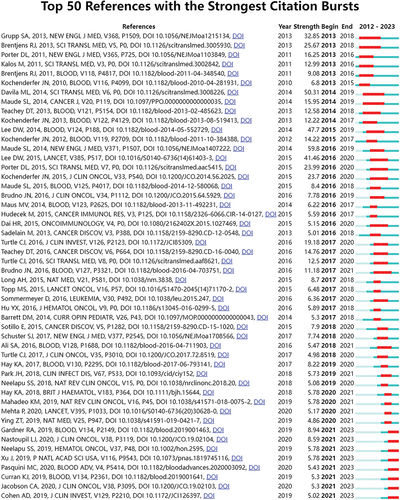
78 references with the strongest citation bursts were identified by CiteSpace. shows the top 50 among them. The number one reference was “Chimeric antigen receptor T cells for sustained remissions in leukemiaCitation41” (strength: 59.78). These 50 papers were published between 2013 and 2023, and 19 of them (38%) were published between 2018 and 2023. Currently, there are still eight papers in the state of citation burst.
Discussion
General information
The statistical number of annual publications can reflect the development trend of a certain field. The number of papers related to this field is increasing annually. Very few papers were published during the stagnation period. It was primarily because CAR-T technology was still in its infancy at this time, attracting less attention. The number of publications increased slowly during the slow growth period, indicating that the field has begun to attract attention from scientists. The effectiveness of CAR-T cells is a crucial parameter for evaluation, and the assessment of their safety and toxicity are equally significant indicators that warrant attention. As the therapy has been extensively investigated, certain adverse events have gradually surfaced, garnering significant attention. Since 2016, there have been more and more articles in this field, indicating that it is becoming a research hotspot. The fitting model of the annual publication volume we constructed suggests that this research field will continue to receive substantial attention in the future.
Our research found that, at the national level, there has been little new cooperation between countries other than Austria during the last two years. Similarly, at the institutional level, there is a lack of new cooperation among institutions. Generally, there has been a lack of close cooperation between countries or institutions in recent years. We consider that this may be because there are still some barriers to cooperation in this field, and relevant information and technology cannot be well shared. We call for strengthening communication and cooperation to better promote the development of this field and benefit more patients.
Despite publishing only 18 articles, the New England Journal of Medicine has been cited the most, far exceeding any other journal. The main reason is that most of these articles published in this journal are clinical experimental articles, which have very high clinical guidance and reference value. Currently, some relevant clinical trial data are continuously generated, and it is believed that there will be more high-quality literature published in the future. implies that research related to this field is currently focused on basic research and clinical transformation. Our analysis identified Carl H. June as the most-cited author, Daniel W. Lee as the most-co-cited author, and He Huang as the most-published author. By searching and reading the relevant papers of these authors, we can quickly understand the research hotspots, the research directions, and the research degree in this field.
Knowledge base
Co-citation means that two (or more) papers are cited by one or more papers.Citation42 It can measure the relationship between papers. This study included 10 articles with the most co-citations in the fieldCitation31–40 ().
By analyzing these articles, we found that these articles mainly focused on the research of CAR-T cell therapy in hematological malignancies. Although CAR-T cell research in solid tumors has developed rapidly in recent years, the clinical practice information provided by CAR-T cell research in hematological malignancies still has extremely important guidance and reference value. The main reason is that the curative effect of CAR-T cell therapy in the treatment of hematological malignancies has been widely recognized, whereas its effectiveness in treating solid tumors remains unsatisfactory,Citation43 and the absence of large-scale clinical trials is evident.
Neelapu et al.Citation31 published an important study in 2017. In this multicenter phase 2 clinical trial, 101 patients with refractory large B-cell lymphoma received axicabtagene ciloleucel (axi-cel, CD19-CAR-T cells), and achieved substantial clinical benefits. The results showed that the objective remission rate was 82% and the complete remission rate was 54%. The incidence of total adverse events was 100%, and the common adverse events included myelosuppression (neutropenia (84%), anemia (66%), and thrombocytopenia (58%)), CRS (93%), and ICANS (64%). Furthermore, this study explored CRS-related serum biomarkers, including IL-6, IL-10, IL-15, and IL-2 Rα. Another five studiesCitation32,Citation35,Citation38 focused on investigating the application of CD19-CAR-T cell therapy in hematological malignancies, encompassing the assessment of its efficacy, adverse events, and safety. These studies have yielded significant clinical trial data of utmost importance. Two studiesCitation31,Citation40 explored serum biomarkers (for diagnosis and prediction) related to CRS and neurotoxicity. One studyCitation36 discussed the kinetics, independent predictors, and predictive biomarkers of severe CRS (sCRS) after CD19-CAR-T cell therapy. These biomarkers included IFN-γ, IL-6, IL-8, IL-10, IL-15, MCP-1 and TNFRp55. Additionally, angiopoietin-2 and von Willebrand factor (VWF) were identified as biomarkers of endothelial activation. Neurotoxicity following CD19 CAR-T cells was detailed clinically, radiologically, and pathologically (endothelial activation and vascular disruption) in one study,Citation37 and the risk factors were identified. In another study,Citation40 researchers found that CRS was prevented by depletion of monocytes or blocking IL-6 receptors with tocilizumab; Anakinra, an IL-1 receptor blocker, could alleviate both CRS and neurotoxicity.
Generally, these 10 articles represent the most popular research topics, and the research scope mainly focuses on the following aspects:
Research on CAR-T cells in the treatment of hematological malignancies, including evaluation of efficacy, adverse events, and safety;
Studies on serum biomarkers related to CRS, neurotoxicity, and sCRS, such as IL-6, IL-8, IL-10, IL-15, IL-2 Rα, IFNγ, sgp130, sIL6R, ferritin, CRP, MCP-1 (sCRS; sensitivity, 100%; specificity, 95%) and TNFRp55;
Pathogenesis and diagnosis associated with CRS and neurotoxicity.
Drugs for CAR-T cell-related CRS and neurotoxicity;
Studies on the long-term follow-up and long-term toxicity of CD19-CAR-T cell therapy for patients with hematological malignancies.
Currently, the primary areas of research that garner significant attention in this domain encompass the aforementioned five facets. These perspectives are substantiated by recent pertinent literature.Citation44–51 Serious adverse events hinder the wide clinical application of this therapy. It is inevitable that the toxic reaction will occur when CAR-T cells exert anti-tumor effects for various reasons, such as the specificity of the target and the structure of the cells.Citation2 Before the conquest of CRS, the primary focus of forthcoming research in this field continued to revolve around the efficient prevention of CRS, hindering its advancement, alleviating its adverse consequences, and improving its prognosis. With the advancement of related clinical experiments, more and more important clinical data will be published in the future, which will help us to further clarify and conquer CRS.
Hotspot evolution, knowledge structure, and emerging topics
According to the richness of keywords and the frequency of each keyword (), the period from 2008 to 2022 can be divided into three stages, namely, 2012–2013, 2013–2019, and 2019–2023. During the second stage, the most keywords appeared, followed by the third stage. In the first stage, there were the fewest keywords. This is because, during this period, the CAR-T cell-related CRS field received little attention. Most keywords, especially the top 20 keywords all appeared in the second stage, suggesting that this field has begun to receive widespread attention. At this stage, the mechanism of CRS and improving the safety of CAR-T cells are important research topics. Furthermore, cluster # 3 shows that researchers have been paying attention to the adverse events of this therapy since 2012. As can be seen from cluster # 2, this research field has received extensive attention since 2014, and its related treatment, management, biomarkers, and ICANS have gradually become important research topics in various stages with the progress of time.
The representative keywords include CRS, CAR-T, immunotherapy, CAR, CD19, cancer, ALL, lymphoma, ICANS, biomarkers, management, remissions, outcomes, and efficacy (). Based on these representative keywords, the key aspects of this field can be summarized as follows:Citation1 As anti-tumor immunotherapy, CAR-T cell therapy is mainly used in the treatment of hematological malignancies, with CD 19 as the most commonly used targetCitation2–Citation52–55 the diagnosis (serum biomarkers) and management of CRS and ICANS are research hotspots;Citation56–58 andCitation3 the efficacy and safety of CAR-T cells are research priorities.Citation59–61
The network clustering analysis of the keywords identified four clusters (). Cluster 1 keywords are mainly about the research of CAR-T cells in malignant tumors. Cluster 2 keywords are about the research on CAR-T cells in hematological malignancies, including curative effect, safety, and prognosis. Cluster 3 keywords are mainly about the pathogenesis, diagnosis, management, and treatment of CAR-T cell-related adverse events. Cluster 4 keywords are related to research on the treatment of ALL, including CD19-CAR-T cell therapy, targeted therapy,Citation62–65 and chemotherapy.
References with strong citation bursts refer to a sudden increase in the number of citations for some references within a given period of time. Analysis of such references can reveal emerging topics for a given field.Citation66 By analyzing these articles in this study, we found that these articles were frequently cited within 10 years and 5 years. Furthermore, 8 articles remain in citation burst status. All this means that this area may continue to receive attention in the future. We ranked the eight articles according to the citation burst intensity (). They may represent the emerging topics that have received much attention in this field.
Table 6. The references (in the state of citation burstness) related to CAR-T cell related CRS.
Through the analysis and summary of eight articlesCitation67–74 that are in an explosive state, we can learn about the current emerging topics related to this field:
Drugs for effective treatment and intervention of CRS;Citation67
To explore and analyze the efficacy, toxicity, safety, prognosis, and drug resistance mechanism of CD19-CAR-T cells in hematological malignancies through real-world evidence;Citation68,Citation71–73
Management of toxicity (CRS and neurotoxicity) associated with CAR-T cell therapy;Citation69
The study of BCMA-CAR-T cells in multiple myeloma (MM);Citation70,Citation74
Optimizing the CAR framework structure to enhance the effectiveness and safety of CAR-T cells.Citation70
Despite the fact that glucocorticoids, tocilizumab, and anakinra can be used for the prevention and treatment of CRS, they cannot completely eliminate the occurrence of CRS or block its progress.Citation44 Consequently, the development of specific drugs remains important. According to recent research, researchers have also employed other strategies. Gong et al.Citation75 PEGylated CAR-T cells and attached a layer of spacer to their surfaces. As CAR-T cells proliferated in vivo, this attachment gradually decreased, and CAR-T cells gradually recovered their antitumor effect, which can alleviate the symptoms related to CRS. Li et al.Citation76 reduced the incidence of CRS by subcutaneous injection of hydrogel that can adsorb IL-6. Relevant large-scale clinical trials have provided us with real and reliable clinical data, which is of great significance in exploring the efficacy, toxicity, safety, and prognosis of CAR-T cell therapy. Furthermore, these clinical trials will continue to generate new data in the future, which will play an important role in promoting the development of this field. Although neurotoxicity is often discussed with CRS, it is now being paid attention to as a separate topic.Citation77,Citation78 BCMA is an important therapeutic target for MM. The study of BCMA-CAR-T cells in MM has emerged as a prominent area of research. Recently, the latest related research results have been published.Citation79–82 Besides finding new targets, optimizing and improving the structure of CAR-T cells to further enhance their anti-tumor effects and safety of CAR-T cells is also a research hotspot.Citation83
With the research and application of CAR-T cells in malignant tumors, its efficacy and prospects have been recognized and affirmed by more and more researchers. As a new type of tumor immunotherapy, its ultimate goal is to be applied to clinical treatment. Thus, while further improving the anti-tumor effect of CAR-T cells, minimizing its toxic reaction is still the research focus. CRS, as one of the most common adverse events of CAR-T cells, has undoubtedly become a research hotspot in this field. It is necessary to have a further and more comprehensive understanding of CAR-T cell-related CRS, which will help researchers overcome it.
Limitations
As a bibliometric analysis, the data used were all from the WoSCC database. For more comprehensive analyses, we obtained all relevant literature as much as possible. However, this study has some limitations. First of all, although the WoSCC database contains most literature, a few kinds of literature are not contained in it. Second, the quality of the collected literature was uneven, which may have led to a certain degree of bias in the analysis.
Conclusion
To our knowledge, this is the first bibliometric analysis of CAR-T cell-associated CRS. Research hotspots primarily concentrated on the pathogenesis, serum biomarkers, management, and therapeutic drugs of CRS, alongside neurotoxicity. Emerging topics within this discipline encompassed the following: a. Drugs for effective treatment and intervention of CRS; b. Conducting pertinent clinical trials to acquire real-world data; c. Management of toxicity (CRS and neurotoxicity) associated with CAR-T cell therapy; d. The study of BCMA-CAR-T cells in multiple myeloma (MM); e. Optimizing the CAR framework structure to enhance the effectiveness and safety of CAR-T cells. In conclusion, this study provides a unique and objective perspective on the field and may provide some new clues and useful references for researchers.
Author contributions
H.L.: Writing-Original draft preparation, manuscript, investigation, and figure preparation. Q.H.: Investigation, figure preparation, manuscript. Y.Z.: Conceptualization, Methodology, Supervision, manuscript. All authors approved the final version of the manuscript and agreed to be accountable for all specs of the work.
Annexes 1.pdf
Download PDF (135.9 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2291900
Additional information
Funding
References
- Larson RC, Maus MV. Recent advances and discoveries in the mechanisms and functions of CAR T cells. Nat Rev Cancer. 2021;21(3):145–16. doi:10.1038/s41568-020-00323-z. Epub 2021/01/242. PubMed PMID: 33483715; PubMed Central PMCID: PMC8353572.
- Miao L, Zhang Z, Ren Z, Li Y. Reactions related to CAR-T cell therapy. Front Immunol. 2021;12:663201. doi:10.3389/fimmu.2021.663201. Epub 2021/05/18. PMID: 33995389; PMCID: PMC8113953.
- Schubert ML, Schmitt M, Wang L, Ramos CA, Jordan K, Müller-Tidow C, Dreger P. Side-effect management of chimeric antigen receptor (CAR) T-cell therapy. Ann Oncol. 2021;32(1):34–48. doi:10.1016/j.annonc.2020.10.478. Epub 2020/10/26.
- Miao L, Zhang Z, Ren Z, Tang F, Li Y. Obstacles and coping strategies of CAR-T cell immunotherapy in solid tumors. Front Immunol. 2021;12:687822. doi:10.3389/fimmu.2021.687822. Epub 2021/06/08. PubMed PMID: 34093592; PubMed Central PMCID: PMCPMC8170155.
- Choe JH, Watchmaker PB, Simic MS, Gilbert RD, Li AW, Krasnow NA, Downey KM, Yu W, Carrera DA, Celli A, et al. SynNotch-CAR T cells overcome challenges of specificity, heterogeneity, and persistence in treating glioblastoma. Sci Transl Med. 2021;13(591):eabe7378. doi:10.1126/scitranslmed.abe7378. Epub 2021/04/30. PubMed PMID: 33910979; PubMed Central PMCID: PMCPMC8362330.
- Hyrenius-Wittsten A, Su Y, Park M, Garcia JM, Alavi J, Perry N, Montgomery G, Liu B, Roybal KT. SynNotch CAR circuits enhance solid tumor recognition and promote persistent antitumor activity in mouse models. Sci Transl Med. 2021;13(591):eabd8836. doi: 10.1126/scitranslmed.abd8836. Epub 2021/04/30. PubMed PMID: 33910981; PubMed Central PMCID: PMCPMC8594452.
- Qi J, Tsuji K, Hymel D, Burke TR Jr., Hudecek M, Rader C, Peng H. Chemically programmable and switchable CAR-T therapy. Angew Chem Int Ed Engl. 2020;59(29):12178–12185. doi:10.1002/anie.202005432. Epub 2020/04/25.
- Jan M, Scarfò I, Larson RC, Walker A, Schmidts A, Guirguis AA, Gasser JA, Słabicki M, Bouffard AA, Castano AP, et al. Reversible ON- and OFF-switch chimeric antigen receptors controlled by lenalidomide. Sci Transl Med. 2021;13(575):eabb6295. doi:10.1126/scitranslmed.abb6295. Epub 2021/01/08. PubMed PMID: 33408186; PubMed Central PMCID: PMCPMC8045771.
- Huang Z, Wu Y, Allen ME, Pan Y, Kyriakakis P, Lu S, Chang Y-J, Wang X, Chien S, Wang Y. Engineering light-controllable CAR T cells for cancer immunotherapy. Sci Adv. 2020;6(8):eaay9209. doi:10.1126/sciadv.aay9209. Epub 2020/03/05.
- Wu Y, Liu Y, Huang Z, Wang X, Jin Z, Li J, Limsakul P, Zhu L, Allen M, Pan Y, et al. Control of the activity of CAR-T cells within tumours via focused ultrasound. Nat Biomed Eng. 2021;5(11):1336–1347. doi:10.1038/s41551-021-00779-w. Epub 2021/08/14. PubMed PMID: 34385696.
- Guercio M, Manni S, Boffa I, Caruso S, Di Cecca S, Sinibaldi M, Abbaszadeh Z, Camera A, Ciccone R, Polito VA, et al. Inclusion of the inducible caspase 9 suicide gene in CAR construct increases safety of CAR.CD19 T cell therapy in B-Cell malignancies. Front Immunol. 2021;12:755639. doi:10.3389/fimmu.2021.755639. Epub 2021/11/06. PubMed PMID: 34737753; PubMed Central PMCID: PMCPMC8560965.
- Ying Z, Huang XF, Xiang X, Liu Y, Kang X, Song Y, Guo X, Liu H, Ding N, Zhang T, et al. A safe and potent anti-CD19 CAR T cell therapy. Nat Med. 2019;25(6):947–53. doi:10.1038/s41591-019-0421-7. Epub 2019/04/24. PubMed PMID: 31011207; PubMed Central PMCID: PMCPMC7518381.
- Singh N, Frey NV, Engels B, Barrett DM, Shestova O, Ravikumar P, Cummins KD, Lee YG, Pajarillo R, Chun I, et al. Antigen-independent activation enhances the efficacy of 4-1BB-costimulated CD22 CAR T cells. Nat Med. 2021;27(5):842–50. doi:10.1038/s41591-021-01326-5. Epub 2021/04/24. PubMed PMID: 33888899; PubMed Central PMCID: PMCPMC8451032.
- Schmidts A, Wehrli M, Maus MV. Toward better understanding and management of CAR-T cell–associated toxicity. Annu Rev Med. 2021;72(1):365–382. doi:10.1146/annurev-med-061119-015600. Epub 2020/08/11.
- Neelapu SS, Tummala S, Kebriaei P, Wierda W, Gutierrez C, Locke FL, Komanduri KV, Lin Y, Jain N, Daver N, et al. Chimeric antigen receptor T-cell therapy — assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15(1):47–62. doi:10.1038/nrclinonc.2017.148. Epub 2017/09/20. PubMed PMID: 28925994; PubMed Central PMCID: PMCPMC6733403.
- Morris EC, Neelapu SS, Giavridis T, Sadelain M. Cytokine release syndrome and associated neurotoxicity in cancer immunotherapy. Nat Rev Immunol. 2022;22(2):85–96. doi: 10.1038/s41577-021-00547-6. Epub 2021/05/19. PubMed PMID: 34002066.
- Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, Maus MV, Park JH, Mead E, Pavletic S, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25(4):625–38. doi:10.1016/j.bbmt.2018.12.758. Epub 2018/12/29. PubMed PMID: 30592986.
- Shimabukuro-Vornhagen A, Gödel P, Subklewe M, Stemmler HJ, Schlößer HA, Schlaak M, Kochanek M, Böll B, von Bergwelt-Baildon MS. Cytokine release syndrome. J Immunother Cancer. 2018;6(1):56. doi:10.1186/s40425-018-0343-9. Epub 2018/06/17.
- Hong R, Hu Y, Huang H. Biomarkers for chimeric antigen receptor T cell therapy in acute lymphoblastic leukemia: prospects for personalized management and prognostic prediction. Front Immunol. 2021;12:627764. doi:10.3389/fimmu.2021.627764. Epub 2021/03/16.
- Tedesco V, Mohan C. Biomarkers for predicting cytokine release syndrome following CD19-targeted CAR T cell therapy. The Journal Of Immunology. 2021;206(7):1561–1568. doi:10.4049/jimmunol.2001249. Epub 2021/03/12.
- Santomasso BD, Nastoupil LJ, Adkins S, Lacchetti C, Schneider BJ, Anadkat M, Atkins MB, Brassil KJ, Caterino JM, Chau I, et al. Management of immune-related adverse events in patients treated with chimeric antigen receptor T-cell therapy: ASCO guideline. J Clin Oncol. 2021;39(35):3978–92. doi:10.1200/jco.21.01992. Epub 2021/11/02. PubMed PMID: 34724386.
- Liu Y, Fang Y, Chen X, Wang Z, Liang X, Zhang T, Liu M, Zhou N, Lv J, Tang K, et al. Gasdermin E–mediated target cell pyroptosis by CAR T cells triggers cytokine release syndrome. Sci Immunol. 2020;5(43):eaax7969. doi:10.1126/sciimmunol.aax7969. Epub 2020/01/19. PubMed PMID: 31953257.
- Freyer CW, Porter DL. Cytokine release syndrome and neurotoxicity following CAR T-cell therapy for hematologic malignancies. J Allergy Clin Immunol. 2020;146(5):940–8. doi:10.1016/j.jaci.2020.07.025. Epub 2020/08/11. PubMed PMID: 32771558.
- Schuster SJ, Maziarz RT, Rusch ES, Li J, Signorovitch JE, VV R, Locke FL, Maloney DG. Grading and management of cytokine release syndrome in patients treated with tisagenlecleucel in the JULIET trial. Blood Adv. 2020;4(7):1432–1439. doi:10.1182/bloodadvances.2019001304. Epub 2020/04/10.
- Miao L, Zhang J, Zhang Z, Wang S, Tang F, Teng M, Li Y. A bibliometric and knowledge-map analysis of CAR-T cells from 2009 to 2021. Front Immunol. 2022;13:840956. doi:10.3389/fimmu.2022.840956. PubMed PMID: 35371087; PubMed Central PMCID: PMC8971369.
- Cooper ID. Bibliometrics basics. J Med Libr Assoc. 2015;103(4):217–228. doi:10.3163/1536-5050.103.4.013. Epub 2015/10/30. PubMed PMID: 26512226; PubMed Central PMCID: PMCPMC4613387.
- Chen C, Song M, Glanzel W. Visualizing a field of research: a methodology of systematic scientometric reviews. PloS one. 2019;14(10):e0223994. doi:10.1371/journal.pone.0223994. Epub 2019/11/02.
- Chen C. Searching for intellectual turning points: progressive knowledge domain visualization. Proc Natl Acad Sci U S A. 2004;101(Suppl 1):5303–5310. doi:10.1073/pnas.0307513100. Epub 2004/01/16. PubMed PMID: 14724295; PubMed Central PMCID: PMCPMC387312.
- van Eck NJ, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. 2010;84(2):523–538. doi:10.1007/s11192-009-0146-3. Epub 2010/06/30.
- Chen C, Leydesdorff L. Patterns of connections and movements in dual-map overlays: a new method of publication portfolio analysis. J Assoc Inf Sci Technol. 2013;65(2):334–351. doi:10.1002/asi.22968.
- Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, Braunschweig I, Oluwole OO, Siddiqi T, Lin Y, et al. Axicabtagene Ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531–44. doi:10.1056/NEJMoa1707447. Epub 2017/12/12. PubMed PMID: 29226797; PubMed Central PMCID: PMCPMC5882485.
- Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, Bader P, Verneris MR, Stefanski HE, Myers GD, et al. Tisagenlecleucel in children and young adults with B-Cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439–48. doi:10.1056/NEJMoa1709866. Epub 2018/02/01. PubMed PMID: 29385370; PubMed Central PMCID: PMCPMC5996391.
- Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, Jäger U, Jaglowski S, Andreadis C, Westin JR, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-Cell lymphoma. N Engl J Med. 2019;380(1):45–56. doi:10.1056/NEJMoa1804980. Epub 2018/12/07. PubMed PMID: 30501490.
- Park JH, Rivière I, Gonen M, Wang X, Sénéchal B, Curran KJ, Sauter C, Wang Y, Santomasso B, Mead E, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med. 2018;378(5):449–59. doi:10.1056/NEJMoa1709919. Epub 2018/02/01. PubMed PMID: 29385376; PubMed Central PMCID: PMCPMC6637939.
- Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, Oluwole OO, Lin Y, Braunschweig I, Hill BT, Timmerman JM, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. The Lancet Oncology. 2019;20(1):31–42. doi:10.1016/s1470-2045(18)30864-7. Epub 2018/12/07. PubMed PMID: 30518502; PubMed Central PMCID: PMCPMC6733402.
- Hay KA, Hanafi LA, Li D, Gust J, Liles WC, Wurfel MM, López JA, Chen J, Chung D, Harju-Baker S, et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor–modified T-cell therapy. Blood. 2017;130(21):2295–2306. doi:10.1182/blood-2017-06-793141. Epub 2017/09/20. PubMed PMID: 28924019; PubMed Central PMCID: PMCPMC5701525.
- Gust J, Hay KA, Hanafi LA, Li D, Myerson D, Gonzalez-Cuyar LF, Yeung C, Liles WC, Wurfel M, Lopez JA, et al. Endothelial activation and Blood–brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discovery. 2017;7(12):1404–1419. doi:10.1158/2159-8290.Cd-17-0698. Epub 2017/10/14. PubMed PMID: 29025771; PubMed Central PMCID: PMCPMC5718945.
- Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, Fry TJ, Orentas R, Sabatino M, Shah NN, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385(9967):517–528. doi:10.1016/s0140-6736(14)61403-3. Epub 2014/10/17. PubMed PMID: 25319501; PubMed Central PMCID: PMCPMC7065359.
- Norelli M, Camisa B, Barbiera G, Falcone L, Purevdorj A, Genua M, Sanvito F, Ponzoni M, Doglioni C, Cristofori P, et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med. 2018;24(6):739–48. doi:10.1038/s41591-018-0036-4. Epub 2018/05/29. PubMed PMID: 29808007.
- Turtle CJ, Hanafi LA, Berger C, Gooley TA, Cherian S, Hudecek M, Sommermeyer D, Melville K, Pender B, Budiarto TM, et al. CD19 CAR–T cells of defined CD4+: CD8+ composition in adult B cell all patients. Journal Of Clinical Investigation. 2016;126(6):2123–2138. doi:10.1172/jci85309. Epub 2016/04/26. PubMed PMID: 27111235; PubMed Central PMCID: PMCPMC4887159.
- Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–17. doi:10.1056/NEJMoa1407222. Epub 2014/10/16. PubMed PMID: 25317870; PubMed Central PMCID: PMCPMC4267531.
- Ma D, Yang B, Guan B, Song L, Liu Q, Fan Y, Zhao L, Wang T, Zhang Z, Gao Z, et al. A bibliometric analysis of pyroptosis from 2001 to 2021. Front Immunol. 2021;12:731933. doi:10.3389/fimmu.2021.731933. Epub 2021/09/07. PubMed PMID: 34484243; PubMed Central PMCID: PMCPMC8416445.
- Maalej KM, Merhi M, Inchakalody VP, Mestiri S, Alam M, Maccalli C, Cherif H, Uddin S, Steinhoff M, Marincola FM, et al. CAR-cell therapy in the era of solid tumor treatment: current challenges and emerging therapeutic advances. Mol Cancer. 2023;22(1):20. doi:10.1186/s12943-023-01723-z. Epub 2023/02/01. PubMed PMID: 36717905; PubMed Central PMCID: PMCPMC9885707.
- Jain MD, Smith M, Shah NN. How I treat refractory CRS and ICANS after CAR T-cell therapy. Blood. 2023;141(20):2430–42. doi:10.1182/blood.2022017414. Epub 2023/03/30. PubMed PMID: 36989488; PubMed Central PMCID: PMCPMC10329191.
- Tan Y, Shan L, Zhao L, Deng B, Ling Z, Zhang Y, Peng S, Xu J, Duan J, Wang Z, et al. Long-term follow-up of donor-derived CD7 CAR T-cell therapy in patients with T-cell acute lymphoblastic leukemia. J Hematol Oncol. 2023;16(1):34. doi:10.1186/s13045-023-01427-3. Epub 2023/04/06. PubMed PMID: 37020231; PubMed Central PMCID: PMCPMC10074659.
- Aldoss I, Khaled SK, Wang X, Palmer J, Wang Y, Wagner JR, Clark MC, Simpson J, Paul J, Vyas V, et al. Favorable activity and safety profile of memory-enriched CD19-targeted chimeric antigen receptor T-cell therapy in adults with high-risk relapsed/refractory all. Clin Cancer Res. 2023;29(4):742–53. doi:10.1158/1078-0432.Ccr-22-2038. Epub 2022/10/19. PubMed PMID: 36255386.
- Raghunandan S, Pauly M, Blum WG, Qayed M, Dhodapkar MV, Elkhalifa M, Watkins B, Schoettler M, Horwitz E, Parikh S, et al. BCMA CAR-T induces complete and durable remission in refractory plasmablastic lymphoma. J Immunother Cancer. 2023;11(5):e006684. doi:10.1136/jitc-2023-006684. Epub 2023/05/04. PubMed PMID: 37137553; PubMed Central PMCID: PMCPMC10163502.
- Gazeau N, Liang EC, Wu QV, Voutsinas JM, Barba P, Iacoboni G, Kwon M, Ortega JLR, López-Corral L, Hernani R, et al. Anakinra for refractory cytokine release syndrome or immune effector cell-associated neurotoxicity syndrome after chimeric antigen receptor T cell therapy. Transplant Cell Ther. 2023;29(7):430–437. doi:10.1016/j.jtct.2023.04.001. Epub 2023/04/10. PubMed PMID: 37031746; PubMed Central PMCID: PMCPMC10330552.
- Park JH, Nath K, Devlin SM, Sauter CS, Palomba ML, Shah G, Dahi P, Lin RJ, Scordo M, Perales M-A, et al. CD19 CAR T-cell therapy and prophylactic anakinra in relapsed or refractory lymphoma: phase 2 trial interim results. Nat Med. 2023;29(7):1710–17. doi:10.1038/s41591-023-02404-6. Epub 2023/07/04. PubMed PMID: 37400640.
- Pensato U, Amore G, Muccioli L, Sammali S, Rondelli F, Rinaldi R, D’Angelo R, Nicodemo M, Mondini S, Sambati L, et al. CAR t-cell therapy in BOlogNa–NEUrotoxicity TReatment and assessment in lymphoma (CARBON–NEUTRAL): proposed protocol and results from an Italian study. J Neurol. 2023;270(5):2659–2673. doi:10.1007/s00415-023-11595-4. Epub 2023/03/05. PubMed PMID: 36869888.
- Leclercq-Cohen G, Bacac M, Klein C. Rationale for combining tyrosine kinase inhibitors and T cell redirecting antibodies to mitigate cytokine release syndrome (CRS). Expert Opin Biol Ther. 2023;23(3):223–225. Epub 2023/01/12 PubMed PMID: 36629122. doi: 10.1080/14712598.2023.2166786.
- Harris K, LaBelle JL, Bishop MR. Current status of CAR T cell therapy for leukemias. Curr Treat Options Oncol. 2021;22(7):62. doi:10.1007/s11864-021-00859-8. Epub 2021/06/08. PubMed PMID: 34097135.
- Denlinger N, Bond D, Jaglowski S. CAR T-cell therapy for B-cell lymphoma. Curr Probl Cancer. 2021;46(1):100826. doi:10.1016/j.currproblcancer.2021.100826. Epub 2022/01/12. PubMed PMID: 35012754.
- Haslauer T, Greil R, Zaborsky N, Geisberger R. CAR T-cell therapy in hematological malignancies. Int J Mol Sci. 2021;22(16):8996. doi:10.3390/ijms22168996. Epub 2021/08/28.
- Viardot A, Sala E. Investigational immunotherapy targeting CD19 for the treatment of acute lymphoblastic leukemia. Expert Opin Investig Drugs. 2021;30(7):773–784. doi:10.1080/13543784.2021.1928074. Epub 2021/05/18.
- Sheth VS, Gauthier J. Taming the beast: CRS and ICANS after CAR T-cell therapy for all. Bone Marrow Transplant. 2021;56(3):552–566. Epub 2020/11/25. PubMed PMID: 33230186; PubMed Central PMCID: PMCPMC8592274.
- Cosenza M, Sacchi S, Pozzi S. Cytokine release syndrome associated with T-Cell-based therapies for hematological malignancies: pathophysiology, clinical presentation, and treatment. Int J Mol Sci. 2021;22(14):7652. doi:10.3390/ijms22147652. Epub 2021/07/25.
- Xiao X, Huang S, Chen S, Wang Y, Sun Q, Xu X, Li Y. Mechanisms of cytokine release syndrome and neurotoxicity of CAR T-cell therapy and associated prevention and management strategies. J Exp Clin Cancer Res. 2021;40(1):367. doi:10.1186/s13046-021-02148-6. Epub 2021/11/20.
- Akhoundi M, Mohammadi M, Sahraei SS, Sheykhhasan M, Fayazi N. CAR T cell therapy as a promising approach in cancer immunotherapy: challenges and opportunities. Cellular Oncology (Dordrecht). 2021;44(3):495–523. doi:10.1007/s13402-021-00593-1. Epub 2021/03/25. PubMed PMID: 33759063.
- Shah NN, Lee DW, Yates B, Yuan CM, Shalabi H, Martin S, Wolters PL, Steinberg SM, Baker EH, Delbrook CP, et al. Long-term follow-up of CD19-CAR T-cell therapy in children and young adults with B-all. J Clin Oncol. 2021;39(15):1650–9. doi:10.1200/jco.20.02262. Epub 2021/03/26. PubMed PMID: 33764809; PubMed Central PMCID: PMCPMC8274806.
- Westin JR, Kersten MJ, Salles G, Abramson JS, Schuster SJ, Locke FL, Andreadis C. Efficacy and safety of CD19-directed CAR-T cell therapies in patients with relapsed/refractory aggressive B-cell lymphomas: observations from the JULIET, ZUMA-1, and TRANSCEND trials. Am J Hematol. 2021;96(10):1295–1312. doi:10.1002/ajh.26301. Epub 2021 Aug 13. PMID: 34310745; PMCID: PMC9290945.
- Samra B, Jabbour E, Ravandi F, Kantarjian H, Short NJ. Evolving therapy of adult acute lymphoblastic leukemia: state-of-the-art treatment and future directions. J Hematol Oncol. 2020;13(1):70. doi:10.1186/s13045-020-00905-2. Epub 2020/06/07.
- Zhao J, Song Y, Liu D. Recent advances on blinatumomab for acute lymphoblastic leukemia. Exp Hematol Oncol. 2019;8(1):28. doi:10.1186/s40164-019-0152-y. Epub 2019/11/12.
- Stein A, Franklin JL, Chia VM, Arrindell D, Kormany W, Wright J, Parson M, Amouzadeh HR, Choudhry J, Joseph G. Benefit–risk assessment of blinatumomab in the treatment of relapsed/refractory B-cell precursor acute lymphoblastic leukemia. Drug Safety. 2019;42(5):587–601. doi:10.1007/s40264-018-0760-1. Epub 2018/12/20.
- Jain T, Litzow MR. Management of toxicities associated with novel immunotherapy agents in acute lymphoblastic leukemia. Ther Adv Hematol. 2020;11:2040620719899897. doi:10.1177/2040620719899897. Epub 2020/02/06.
- Chen C. Science mapping: a systematic review of the literature. J Data Inf Sci. 2017;2(2):1–40. doi:10.1515/jdis-2017-0006.
- Gardner RA, Ceppi F, Rivers J, Annesley C, Summers C, Taraseviciute A, Gust J, Leger KJ, Tarlock K, Cooper TM, et al. Preemptive mitigation of CD19 CAR T-cell cytokine release syndrome without attenuation of antileukemic efficacy. Blood. 2019;134(24):2149–58. doi:10.1182/blood.2019001463. Epub 2019/11/08. PubMed PMID: 31697826; PubMed Central PMCID: PMCPMC6908832.
- Nastoupil LJ, Jain MD, Feng L, Spiegel JY, Ghobadi A, Lin Y, Dahiya S, Lunning M, Lekakis L, Reagan P, et al. Standard-of-care axicabtagene ciloleucel for relapsed or refractory large B-cell lymphoma: results from the US lymphoma CAR T consortium. J Clin Oncol. 2020;38(27):3119–28. doi:10.1200/jco.19.02104. Epub 2020/05/14. PubMed PMID: 32401634; PubMed Central PMCID: PMCPMC7499611.
- Neelapu SS. Managing the toxicities of CAR T-cell therapy. Hematol Oncol. 2019;37(Suppl 1):48–52. doi:10.1002/hon.2595. Epub 2019/06/13. PubMed PMID: 31187535.
- Xu J, Chen LJ, Yang SS, Sun Y, Wu W, Liu YF, Xu J, Zhuang Y, Zhang W, Weng X-Q, et al. Exploratory trial of a biepitopic CAR T-targeting B cell maturation antigen in relapsed/refractory multiple myeloma. Proc Natl Acad Sci U S A. 2019;116(19):9543–51. doi:10.1073/pnas.1819745116. Epub 2019/04/17. PubMed PMID: 30988175; PubMed Central PMCID: PMCPMC6510991.
- Pasquini MC, Hu ZH, Curran K, Laetsch T, Locke F, Rouce R, Pulsipher MA, Phillips CL, Keating A, Frigault MJ, et al. Real-world evidence of tisagenlecleucel for pediatric acute lymphoblastic leukemia and non-Hodgkin lymphoma. Blood Adv. 2020;4(21):5414–24. doi:10.1182/bloodadvances.2020003092. Epub 2020/11/05. PubMed PMID: 33147337; PubMed Central PMCID: PMCPMC7656920.
- Curran KJ, Margossian SP, Kernan NA, Silverman LB, Williams DA, Shukla N, Kobos R, Forlenza CJ, Steinherz P, Prockop S, et al. Toxicity and response after CD19-specific CAR T-cell therapy in pediatric/young adult relapsed/refractory B-all. Blood. 2019;134(26):2361–8. doi:10.1182/blood.2019001641. Epub 2019/10/28. PubMed PMID: 31650176; PubMed Central PMCID: PMCPMC6933289.
- Jacobson CA, Hunter BD, Redd R, Rodig SJ, Chen PH, Wright K, Lipschitz M, Ritz J, Kamihara Y, Armand P, et al. Axicabtagene Ciloleucel in the non-trial setting: outcomes and correlates of response, resistance, and toxicity. J Clin Oncol. 2020;38(27):3095–106. doi:10.1200/jco.19.02103. Epub 2020/07/16. PubMed PMID: 32667831; PubMed Central PMCID: PMCPMC7499617.
- Cohen AD, Garfall AL, Stadtmauer EA, Melenhorst JJ, Lacey SF, Lancaster E, Vogl DT, Weiss BM, Dengel K, Nelson A, et al. B cell maturation antigen–specific CAR T cells are clinically active in multiple myeloma. Journal Of Clinical Investigation. 2019;129(6):2210–2221. doi:10.1172/jci126397. Epub 2019/03/22. PubMed PMID: 30896447; PubMed Central PMCID: PMCPMC6546468.
- Gong N, Han X, Xue L, El-Mayta R, Metzloff AE, Billingsley MM, Hamilton AG, Mitchell MJ. In situ PEGylation of CAR T cells alleviates cytokine release syndrome and neurotoxicity. Nat Mater. 2023. doi:10.1038/s41563-023-01646-6. Epub ahead of print. PMID: 37696939.
- Li X, Gong N, Tian F, Zhang S, Zhang Y, Wang Y, Qing G, Wang Y, Li F, Xu Y, et al. Suppression of cytokine release syndrome during CAR-T-cell therapy via a subcutaneously injected interleukin-6-adsorbing hydrogel. Nat Biomed Eng. 2023;7(9):1129–1141. doi:10.1038/s41551-023-01084-4. Epub 2023/09/12. PubMed PMID: 37696984.
- Butt OH, Zhou AY, Ances BM, DiPersio JF, Ghobadi A. A systematic framework for predictive biomarkers in immune effector cell-associated neurotoxicity syndrome. Front Neurol. 2023;14:1110647. doi:10.3389/fneur.2023.1110647. Epub 2023/03/03. PubMed PMID: 36860569; PubMed Central PMCID: PMCPMC9969296.
- De Matteis S, Dicataldo M, Casadei B, Storci G, Laprovitera N, Arpinati M, Maffini E, Cortelli P, Guarino M, Vaglio F, et al. Peripheral blood cellular profile at pre-lymphodepletion is associated with CD19-targeted CAR-T cell-associated neurotoxicity. Front Immunol. 2022;13:1058126. doi:10.3389/fimmu.2022.1058126. Epub 2023/02/03. PubMed PMID: 36726971; PubMed Central PMCID: PMCPMC9886226.
- Rodriguez-Otero P, Ailawadhi S, Arnulf B, Patel K, Cavo M, Nooka AK, Manier S, Callander N, Costa LJ, Vij R, et al. Ide-cel or standard regimens in relapsed and refractory multiple myeloma. N Engl J Med. 2023;388(11):1002–14. doi:10.1056/NEJMoa2213614. Epub 2023/02/11. PubMed PMID: 36762851.
- Mailankody S, Matous JV, Chhabra S, Liedtke M, Sidana S, Oluwole OO, Malik S, Nath R, Anwer F, Cruz JC, et al. Allogeneic BCMA-targeting CAR T cells in relapsed/refractory multiple myeloma: phase 1 UNIVERSAL trial interim results. Nat Med. 2023;29(2):422–9. doi:10.1038/s41591-022-02182-7. Epub 2023/01/24. PubMed PMID: 36690811.
- Li W, Zhang B, Cao W, Zhang W, Li T, Liu L, Xu L, Gao F, Wang Y, Wang F, et al. Identification of potential resistance mechanisms and therapeutic targets for the relapse of BCMA CAR-T therapy in relapsed/refractory multiple myeloma through single-cell sequencing. Exp Hematol Oncol. 2023;12(1):44. doi:10.1186/s40164-023-00402-5. Epub 2023/05/09. PubMed PMID: 37158921; PubMed Central PMCID: PMCPMC10165782.
- Cowan AJ, Pont MJ, Sather BD, Turtle CJ, Till BG, Libby EN 3rd, Coffey DG, Tuazon SA, Wood B, Gooley T, et al. γ-Secretase inhibitor in combination with BCMA chimeric antigen receptor T-cell immunotherapy for individuals with relapsed or refractory multiple myeloma: a phase 1, first-in-human trial. Lancet Oncol. 2023;24(7):811–22. doi:10.1016/s1470-2045(23)00246-2. Epub 2023/07/07. PubMed PMID: 37414012.
- Miao L, Zhang J, Huang B, Zhang Z, Wang S, Tang F, Teng M, Li Y. Special Chimeric Antigen Receptor (CAR) modifications of T cells: a review. Front Oncol. 2022;12:832765. doi:10.3389/fonc.2022.832765. Epub 2022/04/09. PubMed PMID: 35392217; PubMed Central PMCID: PMCPMC8981721.