ABSTRACT
Patients on dialysis (PoD) are at high risk of severe morbidity and mortality from COVID-19. Characterizing long-term vaccine immune responses in these patients will help optimize vaccine schedule for PoD. This study aimed to determine whether long-term humoral and B and T cell-responses post 3rd and 4th dose of the BNT162b2 vaccine differed between PoD and controls. Non-infected PoD and controls vaccinated with BNT162b2 were recruited in Ziv Medical Center, Israel, between 2021 and 2022. Specimens were collected 1–2 months pre 3rd dose; 1–3 months post 3rd dose; 4–5 months post 3rd dose and 3–5 months post the 4th dose. Anti-SARS-CoV-2 spike (spike) specific antibodies, spike specific memory B cells, and spike specific CD154+ T cells as well as cytokines producing CD4+/CD8+ T cells were measured using standardized assays and compared between PoD and controls at each time point using Mann Whitney and Fisher’s exact tests. We recruited 22 PoD and 20 controls. Antibody levels in PoD were lower compared to controls pre 3rd dose but not post 3rd and 4th doses. Frequencies of spike specific memory B cell populations were similar between PoD and controls overall. Frequencies of spike specific T cells, including those producing IFNγ and TNFα, were not lower in PoD. B and T cell mediated immune response in PoD following a 3rd and a 4th dose of the BNT162b2 vaccine was not inferior to controls up to 5 months post vaccination. Our results suggest that standard BNT162b2 vaccination is suitable for this group.
Introduction
Patients with end-stage kidney disease (ESKD) on dialysis (PoD) are considered a high-risk group for COVID-19.Citation1 Due to their older age and high frequency of comorbidities, such as diabetes and hypertension, PoD are more likely to develop severe COVID-19.Citation2 In addition, dialysis care usually takes place in rooms with multiple patients and frequent interactions with health care professionals, which increases the risk of infection.Citation3 Consequently, severe morbidity and mortality rates are higher in this population.Citation4–6
Patients with ESKD have altered immune responsesCitation7 caused by different factors such as uremia that may alter T cell function.Citation8 Patients with chronic kidney disease can display a lower antibody (Ab) titer after vaccination and an inability to maintain adequate Ab levels over time. They therefore require higher antigen content and different vaccination schedules for certain vaccines such against hepatitis B.Citation9
The mRNA BNT162b2 vaccine against COVID-19 that encodes the SARS-CoV-2 spike proteinCitation10 became available in Israel in December 2020.Citation11 The original vaccination schedule consisted of priming with two doses.Citation12 With accumulating evidence of waning immunity,Citation13–15 booster doses were introduced and recommended.Citation16 ESKD patients were prioritized for vaccination. However, since individuals with advanced kidney disease including PoD were largely excluded from the majority of clinical trials,Citation17 vaccine efficacy in this group was uncertain.
A systematic review showed that the humoral response following priming (1st and 2nd doses) with mRNA based vaccines were effective in PoD although somewhat less than in controls (86% vs. 100% seroconversion rate).Citation18 Neutralizing antibodies as well as vaccine-specific B and T-cell responses following priming were detected in PoD even though reduced when compared to controls.Citation19–22 Four to six months post priming, Ab levels in PoD were found to be lower than in controls,Citation23,Citation24 which may have contributed to increased breakthrough infections in PoD.Citation25 However, the first and a second booster (3rd and 4th doses) led to increased Ab titers among both PoD and healthy subjects, when measured several weeks after their application.Citation26
There are little available data on long-term vaccine responses among PoD compared to controls following a 3rd dose, and even less following a 4th dose of the vaccine In addition, most of the studies describing immune responses in PoD focused on anti-Spike (Anti-S) IgG Ab responses and did not cover cellular responses of B and T cells.Citation27,Citation28 This study aimed to characterize long-term immunity following the 3rd and 4th doses of the BNT162b2 vaccine among uninfected dialysis patients in comparison to controls in terms of circulating anti-S IgG Ab levels, and SARS-CoV-2 spike protein specific B and T cells.
Materials and methods
Participants
Participants were recruited at Ziv Medical Center (ZMC), a 300-bed government hospital in Northern Israel, between 2021 and 2022. All patients receiving maintenance dialysis care in the nephrology unit at ZMC were eligible to participate in this study. Controls with no known kidney disease and age-matched to cases within 10 year age bands were selected from a cohort of approximately 1,000 ZMC healthcare workers described elsewhere.Citation29 Inclusion criteria were (i) the ability to sign a consent form independently, (ii) having received at least three doses of the BNT162b2 vaccine, and (iii) having no documented history (clinical or laboratory) of SARS-CoV-2 infection. The infection status of PoD and controls was ascertained by checking PCR results from medical records, self report, or by evidence of clinical suspicion or a history of clinical signs and symptoms compatible with acute SARS-CoV-2 infection. The threshold of clinical suspicion for PCR testing in both groups was very low. We excluded from the study PoD and controls with unexplained rapid rises in anti-S IgG levels, as this could have represented asymptomatic, undiagnosed infection and therefore led to misclassification.
Vaccination and specimen collection
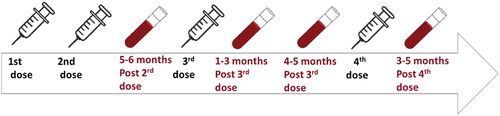
Participants were offered the second dose of the BNT162b2 vaccine about three weeks after the 1st dose. The 3rd vaccine dose was offered approximately six months after the 2nd dose and the 4th dose six months later. Vaccination status of PoD and controls was self-reported and confirmed via health care records and via occupational health records respectively. Blood specimens were collected from PoD and controls 1–2 months before the 3rd dose/5–6 months post 2nd dose (T1); 1–3 months post 3rd dose (T2); 4–5 months post 3rd dose (T3); and 3–5 months post the 4th dose (T4). outlines the vaccination and sample collection schedule.
Evaluation of SARS-CoV-2 S1/S2 IgG levels
Anti-S IgG levels were evaluated using the LIAISON SARS-CoV-2 S1/S2 IgG serology assay (DiaSorin), run according to the manufacturer‘s protocol. Serum separated from whole blood was either frozen at −80°C till measurement or immediately used.
B and T cells preparation
B and T cells were yielded from peripheral blood mononuclear cells (PBMCs); PBMCs were separated from whole blood using density gradient centrifugation with Lymphoprep (Alere Technologies ASTM) and stored in FCS supplemented with 10% CryoSure DMSO (WAk Chemie Medical) at −80°C till further use. For further details see supplementary methods.
B and T cell evaluation
B and T cells were quantified using the SARS-CoV-2 Spike B cell Analysis Kit human (Miltenyi Biotec) and the SARS-CoV-2 Prot S Complete T Cell Analysis Kit (PBMC) human (Miltenyi Biotec) respectively. For Ab panels used see Table S1 and S2 in supplementary methods. Quantification of B and T cell subpopulations was performed using flow cytometry. Details regarding B and T cell gating strategies (Figure S1 and S2), flow cytometry and B and T cell quantification are available in supplementary methods.
Statistical analysis
Data was analyzed and visualized using GraphPad prism 9. Continuous and categorical variables were evaluated using Mann Whitney test and Fisher’s exact test respectively. All tests were performed two sided with threshold of p < .05. We reported anti-S levels using geometric mean concentration (GMC) with 95% CI. Age of participants and frequencies of B and T cells of subpopulations were expressed as median with inter quartile range (IQR) or 95% CI respectively. We refrained from analyzing trends over time, since the number of participants that contributed blood specimen at T1-T4 was different. Therefore, differences between PoD and controls were calculated separately at each time point.
Ethical statement
The study was approved by ZMC ethics committee (0002–21-ZIV).
Results
Participants
The demographic and clinical characteristics of the participants are shown in . A total of 22 patients receiving hemodialysis and 20 controls fitting our inclusion/exclusion criteria consented to participate in the study. None of the participants tested positive for SARS-CoV-2 or developed symptoms suggestive of COVID-19 during the study period. However, we excluded three participants because of unexplained rises in antibody levels prior to the 3rd dose of the vaccine and suspicion of SARS-CoV-2 infection. Despite our attempt to age match, PoD were older than controls (median 71.5 years, 95% CI 61–80 vs 61 years, 95% CI 56–65, p < .005). There was no significant difference between the two groups in terms of gender (p = .35) and ethnicity (p = .1080).
Table 1. Demographic and clinical characteristics of participants.
Vaccination and specimen collection
The number of vaccinations and specimen collected at each time point are summarized in . All participants received three doses of the BNT162b2 vaccine, of those, seven PoD and ten controls received four vaccine doses. The median time of vaccination between the 2nd and the 3rd doses was 202.5 days (95% CI 193–208) in PoD vs 209 days (95% CI 202–214) in controls. The statistical significance of the difference was borderline (p = .05). Not all participants were able to contribute blood at each time point. The timing of specimen collection from PoD and controls differed: compared with controls, the median collection time in PoD was earlier at T2 and T4 (21.5 and 38 days respectively) and later at T3 (12 days). Supplementary Tables S3 and S4 provide all ranges in days for vaccination and specimen collection.
Table 2. Number of vaccinations and specimen contributions of study participants. *At T1 eleven and nine samples were collected from PoD and controls for T cell evaluation respectively and at T4 six samples were collected from PoD. PoD=Patients on dialysis.
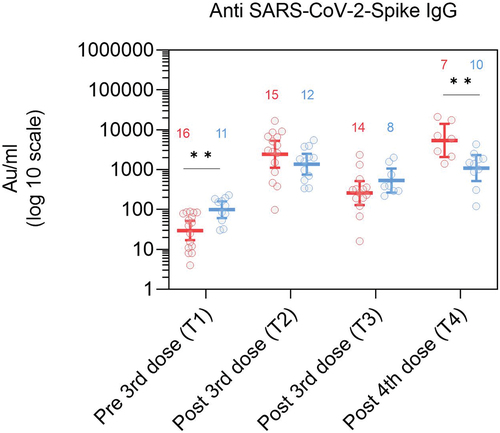
Antibody levels in PoD were lower in comparison to controls pre 3rd dose but not post 3rd and 4th doses
Anti-S IgG levels pre 3rd dose (T1) were significantly lower in PoD than in controls (29.5 vs 98.6 AU/ml, p = .004, and Table S5). Post 3rd dose, at T2 and T3, anti-S IgG levels were comparable between the two groups. At T4, post 4th dose, however, anti-Spike IgG levels were higher in PoD in comparison to controls (5393 vs 1096 AU/ml, p = .007).
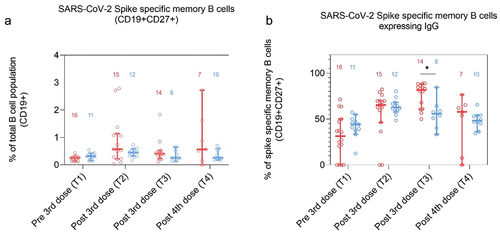
Frequencies of spike specific memory B cells were similar between PoD and controls
The proportion of spike specific memory B cells (MBCs) (CD27+CD19+) out of the whole B cell population (CD19+) was similar in both groups at all time points ( and Table S6). Similarly, proportions of spike specific MBCs expressing either IgG, IgM, or IgA were comparable ( and S3, Table S6).
Figure 3. Frequencies of SARS-CoV-2 Spike specific memory B cells in dialysis patients (red) and controls (blue) at T1 (1–2 months pre 3rd dose), T2 (1–3 months post 3rd dose), T3 (4–5 months post 3rd dose) and T4 (2–4 months post 4th dose). A: Spike specific memory B cell (CD19+CD27+) expressed as a proportion of the total B cell population (CD19+) and B: Spike specific memory B cells expressing IgG presented as a proportion of total spike specific CD19+CD27+ cells (B). Number of participants included are indicated in the graph.
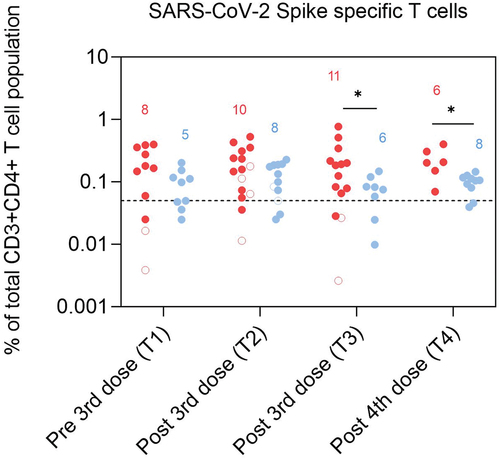
Frequencies of spike specific T cells were not lower in PoD than in controls
Cells expressing activation induced marker (AIM), CD154, were considered to be spike specific (see supplementary methods). There were no significant differences in frequencies of spike-specific T cells before the 3rd dose (T1) and 1–3 months post 3rd dose (T2). We found, however, higher levels of these cells in PoD,4–5 months post 3rd dose (at T3) and post 4th dose (at T4), (p = .05 and p = .04 respectively, and Table S7). No differences were found in the proportion of participants expressing spike specific T cells between groups at all time points (Figure S4).
Figure 4. Frequencies of SARS-CoV-2 Spike specific T cells (CD3+CD4+CD154+) in dialysis patients (red) and controls (blue) presented as a proportion of CD3+CD4+ cells at T1 (1–2 months pre 3rd dose), T2 (1–3 months post 3rd dose), T3 (4–5 months post 3rd dose) and T4 (2–4 months post 4th dose). Number of participants included in the statistical analysis are indicated in the graph.
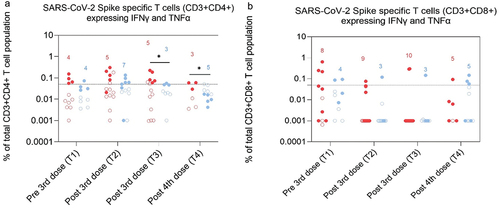
Frequencies of spike specific T cells producing IFNγ and TNFα were not lower in PoD than in controls
The Intracellular production of the cytokines IFNγ and TNFα indicates T cell functionality (see supplementary methods for more details). Differences in frequency of cytokines producing cells between PoD and controls were found only for CD3+CD4+ T cells expressing both cytokines post 3rd and 4th dose, at T3 and T4 and was higher in PoD than in controls (p = .04 at T3 and T4, and S5 and Table S8). The proportion of participants, who expressed either both cytokines, or IFNγ or TNFα only was not different between PoD and controls in both CD3+CD4+ and CD3+CD8+ T cell populations at all time points (data not shown).
Figure 5. Frequencies of SARS-CoV-2 Spike specific T cells from the CD3+CD4+ (A) and CD3+CD8+ (B) T cell population expressing both IFN∎ and TNF√ in dialysis patients (red) and controls (blue) at T1 (1–2 months pre 3rd dose), T2 (1–3 months post 3rd dose), T3 (4–5 months post 3rd dose) and T4 (2–4 months post 4th dose). Number of participants included in the statistical analysis are indicated in the graph.
Discussion
This study aimed to compare long-term immunogenicity in terms of humoral and cellular responses post 3rd and 4th doses of the BNT162b2 vaccine in PoD and controls. Overall our results suggest that up to approximately four to five months after the 3rd dose and three months after the 4th dose of the BNT162b2 vaccine, antibody titers and B and T cell responses in PoD were not inferior to patients with no kidney disease.
Older age and comorbidities such as renal disease may negatively affect the humoral response following priming with the BNT162b2 vaccine.Citation30 Despite the higher median age of hemodialysis patients in our study, we could not ascertain this finding. Data from our cohort previously published showed no difference in humoral response to the vaccine according to ethnicityCitation29 or gender,Citation31 which did not differ in their distribution between groups in our study.
In terms of Ab, our results suggested a faster waning of anti-S IgG levels in PoD six months after the 2nd dose of the BNT162b2 vaccine in agreement with existing evidence.Citation32 This suggests that although the dosage of the vaccine is suitable for boosting PoD, lower IgG levels pre 3rd dose may indicate a need to give boosters earlier to PoD to sustain Ab level. In this small cohort, mean IgG levels post 4th dose were higher in PoD than in controls, which was unexpected. This may be a result of lag time bias as the specimen collection was earlier in PoD than in controls. The pro-inflammatory state described in patients with chronic kidney diseaseCitation33 may also have contributed, although our study does not enable to identify a cause with certainty.
Our analysis of B cell expression resulted overall in similar frequencies of MBCs and MBCs expressing IgM, IgA and IgG in PoD and controls as similarly reported for MBCs post 2nd dose by Rincon-Arevalo et al.Citation22 This may indicate that the capacity of PoD to induce secondary immune response may be as effective as in healthy subjects.
In terms of T cells, we detected a comparable activation of CD154+ T cells in PoD and controls indicating a long term and effective protection against the antigen in PoD. Our T cell mediated intracellular cytokine expression data in PoD is unique for both the 3rd and the 4th doses of the BNT162b2 vaccine. Overall we found that the SARS-CoV-2 Spike specific expression of IFNγ and TNFα in PoD was at least as effective as in controls. Higher frequencies of CD4+ cells expressing both cytokines at T3 and T4 in PoD were detected. In contrast, Azzolini et al., found higher IFNγ levels in controls than in PoD pre 3rd dose in both CD4+ and CD8+ cells by using cytokines release assays.Citation24 Whether the detection of cytokines in serum in response to the antigen or the detection of antigen specific cytokine producing cells is more clinically relevant is not currently known. However, it has been discussed that aside from T cells multiple cell types such as natural killer cells may produce and release cytokines.Citation24 Our results may, therefore, reflect the true SARS-CoV-2 Spike specific cytokine production specifically by T cells. In conclusion, functionality of CD4+ and CD8+ cells was not inferior in PoD to controls.
This study has some limitations. One of them, is the limited number of participants that we were able to recruit for this study, as the number of controls and PoD that were readily infected by the virus increased and the readiness to vaccinate reduced with the progression of the pandemic. Because the patients included at each time point were not necessarily the same, we refrain from commenting on trends over time. Further, we could not fully age-match the participants due to the low numbers of healthcare workers in older age groups. As a result PoD were older. As immune responses generally decrease with age,Citation30 closer age matching would have only resulted in lower responses among controls and the age difference does not therefore challenge our findings of non-inferiority among PoD. Another limitation was the timing of specimen collection from PoD and controls. This limited our ability to interpret the differences between PoD and controls with high certainty. Whereas the difference in median time of collection at T2 was earlier in PoD by less than two weeks, at T4 the difference in median time of collection was about a month earlier in PoD compared with controls. It is possible that higher level of antibody titers, B and T cell frequencies and cytokines production at T4 in PoD were due to an earlier specimen collection. Finally, in our cytokine assay we were missing the detection of IL2, which is an additional indicator for T cell function as shown in Madelon et al.Citation34
In conclusion, the regular BNT162b2 vaccine seems adequate to achieve comparable boost of serological and B and T cellular responses in dialysis patients and in controls. A better and more granular understanding of the dynamics is needed to determine whether more frequent doses are needed among PoD. The overall non-inferior response to the 3rd and 4th doses of the mRNA vaccine contrasts with the response obtained with other vaccines against viruses, such as hepatitis B vaccines that require higher antigen content and/or differing schedules for PoD. While these vaccine are not directly comparable (protein-based HepB vaccine vs mRNA COVID-19 vaccine), the reasons behind these differences remain unclear and warrant further investigation.
Authors contribution
Y.B contributed to the study design, patient recruitment, data collection and manuscript drafting
K.A.B contributed to designing the study, controls recruitment, oversight of specimen collection and management, and manuscript editing
M.E contributed to designing the study and the analytical plan and to the drafting and editing of the manuscript.
E. E conducted flow cytometry and evaluation of B and T cell function analyzed data and contributed to writing the manuscript.
T. T. L performed all laboratory cellular work, assisted data management and analysis, and edited the manuscript.
N.T was responsible for data management, data analysis, drafting and editing of the manuscript.
C.B. Advised on the interpretation of immunological findings, reviewed data analysis and edited the manuscript.
All authors revised and gave final approval.
Supplementary material_HVI_revised.docx
Download MS Word (1.8 MB)Acknowledgments
We thank Yehudit Hakmon for assisting in participants recruitment, Victor Herrmann for assisting in sample collection and the microbiology lab in ZMC for performing the serological tests.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author, N.T, upon reasonable request.
Supplementary data
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2292376.
Additional information
Funding
References
- Geetha D, Kronbichler A, Rutter M, Bajpai D, Menez S, Weissenbacher A, Anand S, Lin E, Carlson N, Sozio S, et al. Impact of the COVID-19 pandemic on the kidney community: lessons learned and future directions. Nat Rev Nephrol. 2022;18(11):724–7. doi:10.1038/s41581-022-00618-4.
- Flythe JE, Assimon MM, Tugman MJ, Chang EH, Gupta S, Shah J, Sosa MA, Renaghan AD, Melamed ML, Wilson FP, et al. Characteristics and outcomes of individuals with pre-existing kidney disease and COVID-19 admitted to intensive care units in the United States. Am J Kidney Dis. 2021;77(2):190. doi:10.1053/J.AJKD.2020.09.003.
- Taji L, Thomas D, Oliver MJ, Ip J, Tang Y, Yeung A, Cooper R, House AA, McFarlane P, Blake PG, et al. Covid-19 in patients undergoing long-term dialysis in Ontario. CMAJ. 2021;193(8):E278–E284. doi:10.1503/CMAJ.202601/TAB-RELATED-CONTENT.
- Jager KJ, Kramer A, Chesnaye NC, Couchoud C, Sánchez-Álvarez JE, Garneata L, Collart F, Hemmelder MH, Ambühl P, Kerschbaum J, et al. Results from the ERA-EDTA registry indicate a high mortality due to COVID-19 in dialysis patients and kidney transplant recipients across Europe. Kidney Int. 2020;98(6):1540–8. doi:10.1016/j.kint.2020.09.006.
- Valeri AM, Robbins-Juarez SY, Stevens JS, Ahn W, Rao MK, Radhakrishnan J, Gharavi AG, Mohan S, Husain SA. Presentation and outcomes of patients with ESKD and COVID-19. J Am Soc Nephrol. 2020;31(7):1409–1415. doi:10.1681/ASN.2020040470/-/DCSUPPLEMENTAL.
- Cancarevic I, Nassar M, Daoud A, Ali H, Nso N, Sanchez A, Parikh A, Ul Hosna A, Devanabanda B, Ahmed N, et al. Mortality rate of COVID-19 infection in end stage kidney disease patients on maintenance hemodialysis: a systematic review and meta-analysis. World J Virol. 2022;11(5):352. doi:10.5501/WJV.V11.I5.352.
- Kato S, Chmielewski M, Honda H, Pecoits-Filho R, Matsuo S, Yuzawa Y, Tranaeus A, Stenvinkel P, Lindholm B. Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol. 2008;3(5):1526. doi:10.2215/CJN.00950208.
- Syed-Ahmed M, Narayanan M. Immune dysfunction and risk of infection in chronic kidney disease. Adv Chronic Kidney Dis. 2019;26(1):8–15. doi:10.1053/J.ACKD.2019.01.004.
- Ma BM, Yap DYH, Yip TPS, Hung IFN, Tang SCW, Chan TM. Vaccination in patients with chronic kidney disease—review of current recommendations and recent advances. Nephrology. 2021;26(1):5–11. doi:10.1111/NEP.13741.
- Lamb YN. BNT162b2 mRNA COVID-19 vaccine: first approval. Drugs. 2021;81(4):495–501. doi:10.1007/S40265-021-01480-7.
- Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA, Hernán MA, Lipsitch M, Reis B, Balicer RD, et al. BNT162b2 mRNA COVID-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384(15):1412–1423. doi:10.1056/NEJMOA2101765/SUPPL_FILE/NEJMOA2101765_DISCLOSURES.PDF.
- Haas EJ, Angulo FJ, Mclaughlin JM, Anis E, Singer SR, Khan F, Brooks N, Smaja M, Mircus G, Pan K, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397(10287):1819–29. doi:10.1016/S0140-6736(21)00947-8.
- Goldberg Y, Mandel M, Bar-On YM, Bodenheimer O, Freedman L, Haas EJ, Milo R, Alroy-Preis S, Ash N, Huppert A, et al. Waning immunity after the BNT162b2 vaccine in Israel. N Engl J Med. 2021;385(24):e85. doi:10.1056/NEJMOA2114228.
- Levin EG, Lustig Y, Cohen C, Fluss R, Indenbaum V, Amit S, Doolman R, Asraf K, Mendelson E, Ziv A, et al. Waning immune humoral response to BNT162b2 COVID-19 vaccine over 6 Months. N Engl J Med. 2021;385(24):e84. doi:10.1056/NEJMOA2114583.
- Notarte KI, Guerrero-Arguero I, Velasco JV, Ver AT, Santos de Oliveira MH, Catahay JA, Khan MSR, Pastrana A, Juszczyk G, Torrelles JB, et al. Characterization of the significant decline in humoral immune response six months post‐SARS‐CoV‐2 mRNA vaccination: a systematic review. J Med Virol. 2022;94(7):2939. doi:10.1002/JMV.27688.
- Stasi C, Meoni B, Voller F, Silvestri C. SARS-CoV-2 vaccination and the bridge between first and fourth dose: where are we? Vaccines. 2022;10(3):444. doi:10.3390/VACCINES10030444.
- Glenn D, Hegde A, Kotzen E, EWK international. Systematic review of safety and efficacy of COVID-19 vaccines in patients with kidney disease. ncbi.nlm.nih.gov. 2021 undefined [accessed 2023 Feb 22]. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7870446/.
- Falahi S, Sayyadi H, Kenarkoohi A. Immunogenicity of COVID-19 mRNA vaccines in hemodialysis patients: systematic review and meta-analysis. Heal Sci Rep. 2022;5(6):e874. doi:10.1002/HSR2.874.
- Sanders JSF, Bemelman FJ, Messchendorp AL, Baan CC, van Baarle D, van Binnendijk R, Diavatopoulos DA, Frölke SC, Geers D, GeurtsvanKessel CH, et al. The RECOVAC immune-response study: the immunogenicity, tolerability, and safety of COVID-19 vaccination in patients with chronic kidney disease, on dialysis, or living with a kidney transplant. Transplantation. 2022;106(4):821–34. doi:10.1097/TP.0000000000003983.
- Sattler A, Schrezenmeier E, Weber UA, Potekhin A, Bachmann F, Straub-Hohenbleicher H, Budde K, Storz E, Proß V, Bergmann Y, et al. Impaired humoral and cellular immunity after SARS-CoV-2 BNT162b2 (tozinameran) prime-boost vaccination in kidney transplant recipients. J Clin Invest. 2021;131(14). doi:10.1172/JCI150175.
- Strengert M, Becker M, Ramos GM, Dulovic A, Gruber J, Juengling J, Lürken K, Beigel A, Wrenger E, Lonnemann G, et al. Cellular and humoral immunogenicity of a SARS-CoV-2 mRNA vaccine in patients on haemodialysis. EBioMedicine. 2021;70:70. doi:10.1016/j.ebiom.2021.103524.
- Rincon-Arevalo H, Choi M, Stefanski AL, Halleck F, Weber U, Szelinski F, Jahrsdörfer B, Schrezenmeier H, Ludwig C, Sattler A, et al. Impaired humoral immunity to SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients and dialysis patients. Sci Immunol. 2021;6(60):1031. doi:10.1126/SCIIMMUNOL.ABJ1031/SUPPL_FILE/SCIIMMUNOL.ABJ1031_TABLE_S1.ZIP.
- Murt A, Dinc HO, Altiparmak MR, Yalin SF, Yadigar S, Parmaksiz E, Kocazeybek B, Pekpak M, Ataman MR. Waning of SARS-CoV-2 vaccine-induced immune response over 6 Months in peritoneal dialysis patients and the role of a booster dose in maintaining seropositivity. Nephron. 2022;146(6):559–563. doi:10.1159/000524658.
- Azzolini E, Pozzi C, Germagnoli L, Oresta B, Carriglio N, Calleri M, Selmi C, De Santis M, Finazzi S, Carlo-Stella C, et al. mRNA COVID-19 vaccine booster fosters B- and T-cell responses in immunocompromised patients. Life Sci Alli. 2022;5(6):e202201381. doi:10.26508/LSA.202201381.
- Anand S, Montez-Rath ME, Han J, Garcia P, Cadden L, Hunsader P, Morgan C, Kerschmann R, Beyer P, Dittrich M, et al. SARS-CoV-2 vaccine antibody response and breakthrough infection in patients receiving dialysis. Ann Intern Med. 2022;175(3):371–8. doi:10.7326/M21-4176.
- Yang X, Zhang H, Bao W, Fu S, Jin H. Immunogenicity rates after SARS-CoV-2 three-dose vaccination in patients under dialysis: a systematic review and meta-analysis. Vaccines. 2022;10(12):2070. doi:10.3390/vaccines10122070.
- Notarte KI, Catahay JA, Peligro PJ, Velasco JV, Ver AT, Guerrero JJ, Liu J, Lippi G, Benoit SW, Henry BM, et al. Humoral response in hemodialysis patients post-SARS-CoV-2 mRNA vaccination: a systematic review of literature. Vaccines. 2023;11(4):724. doi:10.3390/VACCINES11040724.
- Taheri S. Efficacy and safety of booster vaccination against SARS-CoV-2 in dialysis and renal transplant patients: systematic review and meta-analysis. Int Urol Nephrol. 2023;55(4):791–802. doi:10.1007/S11255-023-03471-X.
- Edelstein M, Wiegler Beiruti K, Ben-Amram H, Bar-Zeev N, Sussan C, Asulin H, Strauss D, Bathish Y, Zarka S, Abu Jabal K. Antibody-mediated immunogenicity against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) following priming, boosting, and hybrid immunity: insights from 11 Months of follow-up of a healthcare worker cohort in Israel, December 2020–October 2021. Clin Infect Dis. 2022;75(1):E572–E578. doi:10.1093/CID/CIAC212.
- Notarte KI, Ver AT, Velasco JV, Pastrana A, Catahay JA, Salvagno GL, Yap EPH, Martinez-Sobrido LB, Torrelles J, Lippi G, et al. Effects of age, sex, serostatus, and underlying comorbidities on humoral response post-SARS-CoV-2 Pfizer-BioNTech mRNA vaccination: a systematic review. Crit Rev Clin Lab Sci. 2022;59(6):373–390. doi:10.1080/10408363.2022.2038539.
- Jabal KA, Ben-Amram H, Beiruti K, Batheesh Y, Sussan C, Zarka S, Edelstein M. Impact of age, ethnicity, sex and prior infection status on immunogenicity following a single dose of the BNT162b2 mRNA COVID-19 vaccine: real-world evidence from healthcare workers, Israel, December 2020 to January 2021. Euro Surveill. 2021;26(6). doi:10.2807/1560-7917.ES.2021.26.6.2100096.
- Berar-Yanay N, Freiman S, Shapira M, Saffoury A, Elemy A, Hamze M, Elhaj M, Zaher M, Matanis L, Armaly ZA, et al. Waning humoral response 3 to 6 Months after vaccination with the SARS-COV-2 BNT162b2 mRNA vaccine in dialysis patients. J Clin Med. 2022;11(1):64. doi:10.3390/JCM11010064.
- Silverstein DM. Inflammation in chronic kidney disease: role in the progression of renal and cardiovascular disease. Pediatr Nephrol. 2009;24(8):1445–52. doi:10.1007/S00467-008-1046-0.
- Madelon N, Lauper K, Breville G, Sabater Royo I, Goldstein R, Andrey DO, Grifoni A, Sette A, Kaiser L, Siegrist CA, et al. Robust T cell responses in anti-CD20 treated patients following COVID-19 vaccination: a prospective cohort study. Clin Infect Dis Off Publ Infect Dis Soc Am. 2022;75(1):E1037–E1045. doi:10.1093/CID/CIAB954.