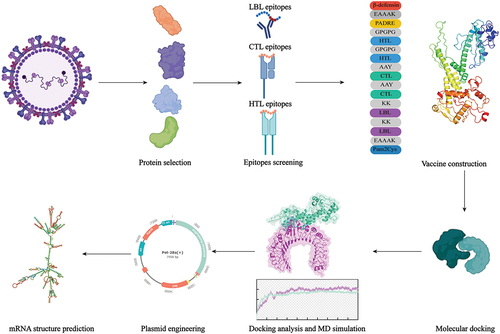
ABSTRACT
Human metapneumovirus (HMPV) is one of the main pathogens causing severe respiratory infections in children, as a common cause of immunodeficiency-related deaths in children and elderly individuals, the prevalence of HMPV has been showing an increasing trend during the last years. However, no vaccines or effective treatment plans are available currently. In this present, based on candidate proteins highly associated with viral virulence and has promising protective potential, we screened for immunodominant cytotoxic T cells, helper T cells, and Linear B-cell epitopes from the most promising candidate Fusion protein, together with G, SH, M, and M2. All epitopes were predicted to have strong antigenicity by Vaxijen and pose no potential toxicity, allergenicity, or hormonology to human proteins by Toxinpred, Allerpred, and Blast analysis, meanwhile, high conservancy is demanded to cover different subtypes. adjuvants β-defensin II and Pam2Cys was attached with EAAAK linkers to improve vaccine’s efficiency. Then, calculation of physicochemical properties proved the protein vaccine as a product can stably exist in the human body. Besides, we assessed the docking between the vaccine and immune receptors to evaluate its ability to stimulate immune responses, and the dynamic simulation further confirmed that the vaccine can tightly bind with immune receptors, which approved that the construction has the potential to induce strong humoral and cellular immune response. Finally, the vaccine was constructed into a multi-epitope mRNA vaccine, the immune simulations suggest that this is a vaccine candidate for controlling HMPV infection.
Introduction
Human metapneumovirus (HMPV), first identified in 2001 from 28 young children from the Netherlands, was recognized as a leading cause of Acute respiratory tract infections (RTIs) in children, the elderly and other population with immunodeficiency.Citation1 Usually, as a seasonal epidemic, HMPV threaten is controllable, its circulation lasts from winter to next year’s spring along with other respiratory viruses and generates a limited number of cases, the average detection rate of individuals under the age of 18 between 2016 and 2021 was approximately 4%.Citation2 However, the outbreak of HMPV exceeded previous years in 2023, the number of HMPV antigen-positive patients reported by hospitals and clinical laboratories from various states across the United States reached 19.6% in March (average 3.6% from 2008 to 2014 from same organization), resulting in overcrowding in pediatric and intensive care units.Citation3,Citation4
HMPV belongs to the Pneumoviridae Metapneumovirus family. With a disease spectrum similar to respiratory syncytial virus (RSV), HMPV is responsible for about 15% acute respiratory infections among young children and about 5% in adults.Citation5,Citation6 According to the World Health Organization’s report, HMPV is responsible for 10% of deaths in children under the age of 5 in 2017. While in 2018, it is estimated that the number of children under the age of 5 infected with HMPV worldwide reaches 14 million, among which 640,000 require hospitalization and 8,000 result in death.Citation7 Typically, HMPV infection results in mild symptoms including fever, cough, nasal congestion and so on, but individuals with compromised immune function, such as premature infants, when infected, may develop bronchiolitis, pneumonia, and even severe pneumonia requiring admission to the ICU. In general, HMPV causes a significant medical burden every year, especially in middle- and low-income countries. Thus, it is highly necessary to have a targeted intervention strategy to protect infants and young children. Unfortunately, there is no solid treatment or vaccine for HMPV so far, the lack of vaccines is a significant factor leading to a large number of hospitalizations during the epidemic season, while the scarcity of treatment options has resulted in cases of death among hospitalized patients.
Supportive treatments such as immunoglobulins, glucocorticoids, and other symptomatic treatment methods are considered the main method against HMPV infection. In clinical practice, ribavirin and polyclonal intravenous immune globulin (IVIG) are utilized for severe infections in cases, though there is currently no study exploring their solid effectiveness in patients and further RCT studies are needed, these therapies are still indicated based on experiences.Citation8 There are also some methods that have demonstrated antivirus effects in mice, including monoclonal antibodies, RNAi, NMSO3, and heparin, etc.Citation9 However, none of these methods have been approved for licensed clinical use.
Developing a vaccine seems to be a feasible way to alleviate the pressure of viral infection in the absence of effective treatments. Human metapneumovirus (HMPV) has evolved into two genetic lineages with antigenic distinctiveness, A and B. Each lineage contains two subtypes, A1 and A2, B1 and B2, A2 can be further divided to A2a and A2b.Citation10 Different subtypes have distinct genetic sequences, but still demonstrate considerable homology, as evidenced by protein sequence similarities between different subtypes exceeding 80%,by selecting the epitopes that are conserved in both lineages, subsequent vaccine candidates can provide protection against various strains of HMPV.Citation11 The HMPV virus carries a non-segmented negative-sense RNA as its genome, which has a total length of 13kb. There are eight genes on this RNA, collectively encoding nine proteins, among which nucleoprotein (N), phosphoprotein (P), and large protein (L) participates in synthetization of RNA. And matrix protein (M) is involved in viral assembly and budding, while M2 is involved in RNA transcription. The rest three proteins are anchored to the viral lipid envelope, F protein mediates membrane fusion while G protein mediates attachment. Less is known about small hydrophobic (SH) protein, studies suggest SH protein may be a viroporin forming ion channels, but it is believed to regulate host immune response.Citation12,Citation13 After many attenuated vaccines failed due to insufficient attenuation or poor protective effects, subunit vaccine became the main focus of research. The F protein plays a crucial role in the fusion of the virus envelope and cell membrane, which means that targeting the F protein can terminate the invasion and subsequent replication of the virus. Moreover, the F protein is highly conserved (more than 90%) among different strains of HMPV, which make F protein the most important target in vaccine development.Citation14–16 Meanwhile, though other proteins are not considered as protective vaccine candidates before, they are also believed to be involved in immune progress, their epitopes have been identified to participate in immune regulation, thus improving immune responses against HMPV infection, the G and SH protein are also considered weaker protective vaccine provides neutralizing antibodies.Citation17,Citation18 Considering the importance of F protein in immune response of HMPV, we set F protein as a main target for further epitope vaccine design, at the same time, given that G, SH, M, and M2 proteins are also highly related to viral replication, some immunodominant epitopes could be screened from their sequences to enhance the vaccine construction.
In total, there has been little progress made in HMPV vaccine development, a multi-epitope mRNA vaccine is a proven strategy against bacterial and viral infection, an mRNA vaccine uses mRNA as a carrier to encode exogenous antibodies and introduce them into body cells. By utilizing the expression system to synthesize antigens, they induce cellular and humoral immune responses. Multi-epitope mRNA vaccines select immunodominant epitopes from different proteins as exogenous antibodies, thereby further enhancing the specificity and protective ability of the vaccine. With immunoinformatic methods, we designed a multi-epitope mRNA vaccine based on the F protein as well as some epitopes from G, M1, M2, and SH proteins to address the potential threat of an HMPV epidemic,
Method and materials
Reference sequence acquisition
The NCBI virus database stores the gene sequence and protein sequence of each virus, and the reference protein sequences of human metapneumovirus are obtained from this database. The G protein (YP_009513272.1), F protein (YP_009513268.1), M protein (YP_009513267.1), M2–1 protein (YP_009513269.1), M2–2 protein (YP_009513270.1), and SH protein (YP_009513271.1) were selected for further analysis.
T cell epitope prediction
The NetCTL1.2 is a server,comprehensively predicts CTL epitopes by means of peptide-MHCI binding, C-terminal cleavage of the proteasome, and TAP transport efficiency.Citation19 Twelve supertypes, including A1, A2, A3, A24, A26, B7, B8, B27, B39, B44, B58, and B62 are selected, and the default threshold is 0.75. We used the MHC-II Binding Predictions tool from IEDB to predict the HTL epitope via the IEDB recommendation algorithm (NetMHCIIpan 4.1 EL).Citation20 Vaxijen 2.0 server was employed to predict the antigenicity of the epitopes according to their physical and chemical properties of amino acids.Citation21 Epitopes with antigenicity scores >0.4 were used for further screening. Moreover, the AllerTOP v2.0 was utilized to predict the allergenicity of epitopes, and the ToxinPred servers were utilized to predict the toxicity of epitopes.Citation22,Citation23
For CTL epitopes, their affinities to MHC I molecules were further predicted by the MHC-I Binding Predictions tool based on the NetMHCpan EL 4.1 algorithm. In general, CTL epitopes with rank percentiles <0.5% were considered to have strong binding to the MHC I molecules.
For HTL epitopes, epitopes with rank percentiles <2% were considered to have strong binding to the MHC II molecules. Cytokines induced by HTL epitopes are important in adaptive immunity. IFNepitope, IL4Pred and IL10Pred servers were employed to analyze the ability of HTL epitopes to induce IFN-γ, IL-4, and IL10, respectively.Citation24,Citation25
Prediction of linear B cell epitopes
The ABCpred server predicted linear B cell epitopes with a threshold of 0.51 and an accuracy of 65.93%.Citation26 Then, the antigenicity, allergenicity, and toxicity of the LBL epitopes were predicted through the VaxiJen 2.0 server, the AllerTOP v2.0 server, and the ToxinPred server. LBL epitopes with good antigenicity, non-toxicity, and non-allergenicity were used for further analysis.
Conservative analysis of epitopes and homology analysis with human protome
The complete genomes of 392 human metapneumovirus strains isolated from human were obtained from the NCBI virus database (https://www.ncbi.nlm.nih.gov/labs/virus/vssi/#/), and the human metapneumovirus genome database was established via BLAST v2.120+. The tBLASTn was used to query the conservatism of epitopes, and more than 80% of the epitopes are used for vaccine construction. PeptideMatch server was employed to identify homology between epitopes and human proteins, thus minimizing the risk of self-antigens immune reactions.Citation27
Vaccine construct and calculation of physicochemical properties
The final vaccine consists of HTL epitopes, CTL epitopes, LBL epitopes, and adjuvants. AAY linkers, GPGPG linkers, and KK linkers were used to connect the CTL epitopes, HTL epitopes, and LBL epitopes, respectively. In order to enhance the immunogenicity of the construction, TLR4 agonist and TLR2 agonist were attached to the N-terminal and C-terminal of the vaccine, respectively. Further, A pan HLA DR-binding epitope (PADRE) that can bind to multiple MHC II molecules was incorporated into the construction to enhance the adaptive immunity induced by the vaccine. To figure out the physicochemical characteristics of the vaccine, the ProtParam tool was employed. The ProtParam tool can calculate the number of amino acids, theoretical PI, instability index, aliphatic index, half-life, and hydrophilicity values of the vaccine. Moreover, the VaxiJen 2.0 server and the ANTIGENpro server were utilized to evaluate the antigenicity of the vaccine. The AllerTOP v2.0 server and the ToxinPred 2.0 server were employed to evaluate the allergenicity and toxicity of the vaccine. To understand the probability of solubility of the vaccine when overexpressed in E. coli, the SOLpro server was used (http://scratch.proteomics.ics.uci.edu/).
Secondary structure, tertiary structure prediction, and refinement
The vaccine’s secondary structure was predicted with GOR4. The tertiary structure model of the vaccine was constructed by the Robetta server (http://robetta.bakerlab.org/). GalaxyWEB (http://sysbio.rnet.missouri.edu/3Drefine/) was then employed to refine the tertiary model. Three main indicators, namely GDT-HA, RMSD, and MolProbity were utilized to refine the tertiary model, which can reveal the model’s quality.Citation28 In addition, the quality of the model can be evaluated with Procheck, ERRAT, and Pro-sa Web servers. A model is considered to be of high quality if it has an ERRAT score above 85. The overall quality of the protein’s tertiary structure can be assessed using the Z-score, with a positive value indicating structural errors.Citation29,Citation30
Molecular docking
Toll-like receptors (TLRs) play a crucial role in the recognition of different pathogen-related molecular patterns thereby contributing significantly to the innate immune response. The Crystal structures of TLR2 (PDBID:2Z81) and TLR4 (PDBID:2Z63) were obtained from the RCSB PDB Database. The ClusPro 2.0 server performed molecular docking between the vaccine and the TLRs, which used the PIPER16 program based on the on the Fast Fourier Transform (FFT) correlation approach to perform rigid-body docking. Further, the top rank result was submitted to the HADDOCK 2.4 server for refinement using default parameters. Meanwhile, we used the PDBsum server to analyze the interaction between vaccine and TLRs.Citation31
Molecular dynamics simulation
Molecular dynamics (MD) simulation evaluated the stability of vaccine-receptor complexes. Then, a 100 ns molecular dynamics simulation was conducted by using Gromacs v2022.1 software.Citation32 The Amber14SB_ armbsc1 force field and TIP3P water model were added to the whole system. Then, the anion (Cl-) was added to balance the charges of the whole system. Subsequently, energy minimization was performed based on steep algorithm until the maximum force ≤1000.0 kJ/mol/nm. NVT and NPT were performed 200ps and 1 ns, respectively. The modified Berendsen thermostat was employed to regulate the system’s temperature to 310k, while the Parinello-Rahman barostat was used to equilibrate the pressure to 1 atm. The hydrogen atom bonds were constrained by the LINCS algorithm and long-range electrostatic interactions were calculated by the Particle-Mesh Ewald summation scheme.
MM-GBSA calculation
The gmx_MMPBSA v1.56 tool calculated the binding energy between the vaccine and the receptors. In this study, we used the Molecular Mechanics Generalized Born Surface Area (MM-GBSA) methods to calculate the binding energy between the vaccine and the receptors via the last 10 ns trajectory.
Immune simulation and population coverage
Immune simulation can be used to evaluate the immune response of the human body after three injections of vaccine. C-IMMSIM server was employed to perform a progress of immune simulation, which identified epitopes via the PSSM methods.Citation33 Vaccination was injected on days 1, 28, and 56, respectively, and the simulation was performed for a duration of 350 d. The host HLA selection was set to HLA-A * 02:01, HLA-B * 07:02, and HLA-DRB1 * 01:01. The coverage of the vaccine in the world was computed with the Tepitool according to MHC corresponding of epitopes.Citation34
Construction of multi-epitope mRNA vaccine and secondary structure prediction
Jcat server (http://www.jcat.de/Start.jsp.) was utilized for reverse translation and optimization of the vaccine. To construct the conclusive multi-epitope mRNA vaccine, the DNA sequence of the vaccine is further modified, a Kozak sequence is attached to the 5’ end to improve RNA stability and translation efficiency,Citation35 signal peptide Tissue Plasminogen Activator (tPA) follows the Kozak sequence to facilitate the extracellular secretion of proteins and improve antigen presentation.Citation36 Meanwhile, an MHC I-targeting domain (MITD) sequence is attached to the 3’ end of the vaccine DNA sequence to guide the presentation of CTL epitopes,Citation37 then a TAA condon ends the translation. Besides, to stabilize the mRNA partial sequence of cytomegalovirus immediate-early gene Untranslated Region (UTR) was added as 5’ untranslated region (UTR), and partial sequence of the human growth hormone was attached as 3’ UTR.Citation38,Citation39 The full DNA sequence was translated into mRNA using the Transcription Tool. The RNAfold server (http://rna.tbi.univie.ac.at/cgi-bin/RNAWebSuite/RNAfold.cgi) and mFold server (http://www.bioinfo.rpi.edu/applications/mfold) predicted the mRNA’s secondary structure.
Construction of vaccine vectors
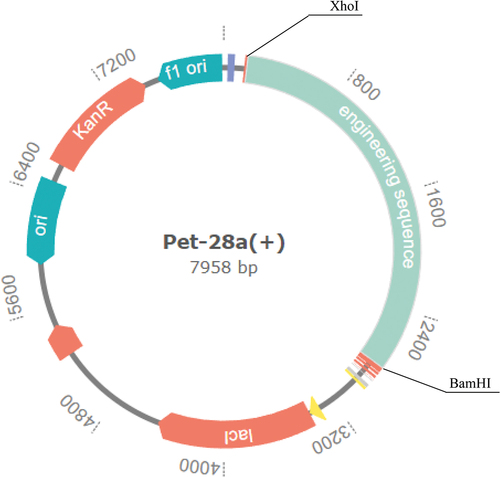
XhoI and BamHI were selected from E. coli K12 for reverse translation and optimization of the vaccine sequence, gensmart tool was used for expression vector construction. XhoI and BamHI inserted the vaccine gene sequence into the pET28a (+) plasmid to construct expression vectorCitation40().
Results
Screening of epitopes
After being obtained from NCBI database, sequences of F, G, SH, M, M2–1, and M2–2 were put into the process of screening, Overall, all epitopes share some common screening principles, these epitopes are supposed to possess strong antigenicity (antigenicity >0.4), and proper immunogenicity, no toxicity, allergenicity, or homology to human proteins is allowed, besides, to provide protection against all subtypes, selected epitopes should be able to cover the majority of strains in 392 strains obtained from NCBI database. Then, epitopes that are deemed suitable and have high overall scores and rankings in the prediction are selected for further computation. Finally, 15 CTL epitopes (), 4 HTL epitopes () and 9 LBL epitopes () were selected. Most epitopes are from F and M proteins, none were from G proteins. In addition, the ability of inducing cytokines containing IFN-γ, Il-4, and Il-10 was considered when selecting HTL epitopes.
Table 1. Screen of CTL epitopes.
Table 2. Screen of HTL epitopes.
Table 3. Screen of LBL epitopes.
Vaccine construction and physicochemical properties calculate
To improve the effectiveness of the vaccine construct, β-defensin II and Pam2Cys were introduced to the vaccine sequence as adjuvants. Additionally, the vaccine also contains a sequence of PADRE to supplement the helper T cell (HTL) epitopes. The final vaccine consists of 2 adjuvants (Pam2Cys and β-defensin II), 15 CTL, 5 HTL (including PADRE), and 9 LBL epitopes. The vaccine’s final antigenicity is 0.6487, nontoxic and non-allergenic. The construction consists of 507 aas and has a 55.2 kDa molecular weight, its theoretical pI is predicted as 9.41. The half-life of the vaccine is estimated to be 30 hours in mammalian reticulocytes, in vitro, more than 20 hours in yeast, and more than 10 hours in E. coli. The protein’s instability index (II) is computed to be 22.50 which suggests the protein is stable. Furthermore, it’s 81.56 aliphatic index indicates that the vaccine has good thermal stability. The grand average of hydropathicity (GRAVY) for the protein was −0.051, indicating that it is hydrophilic as it is less than 0. The solubilization probability of the vaccine when overexpressed in E. coli was predicted to be 0.7936 according to SOLpro.
Secondary and tertiary structures prediction
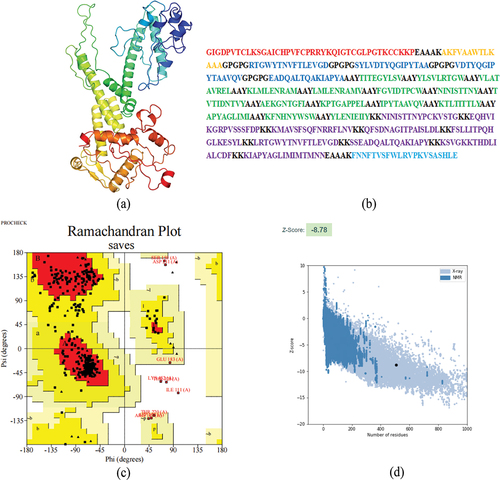
The GOR4 server is utilized to predict the construction’s secondary structure, the sequence consists of 38.86% alpha helix, 18.15% extended strand, and 43% random coil (). The vaccine’s tertiary structure was predicted using the RoseTTAfold, and Galaxyweb was then utilized to refine the structure (). The best model achieved a Molprobity score of 2.071, GDT-HA of 0.9763, and RMSD of 0.343. Ramachandran plots reveal that 375 residues (85.4%) lie in the most favored regions, with only 55 residues (12.5%) in the additional allowed regions, 4 residues (0.9%) in the generously allowed regions and 5 residues (1.1%) in the disallowed regions (). The Pro-sa web server analyzed the structure of the model, which achieved a Z-score of −7.6 (<0), indicating good quality ().
Figure 2. Prediction of secondary structure of vaccine.
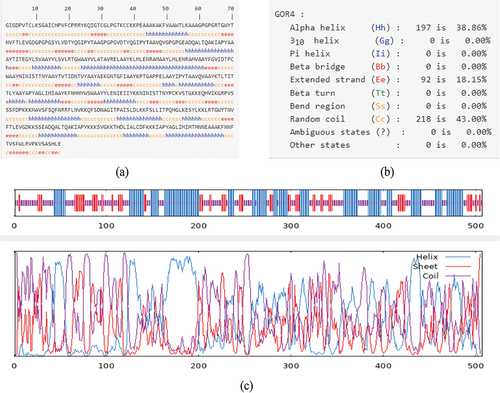
Figure 3. Construction of multi-epitope vaccine.
Molecular docking
The ClusPro 2.0 server was utilized to perform molecular docking between the vaccine and TLRs, while the preliminary molecular docking screening was run on HADDOCK. The vaccine-TLR2 complex has a center energy of −947.3 and a lowest energy of −1056.8, while the vaccine-TLR4 complex has a center energy of −819.5 and a lowest energy of −968.3. The HADDOCK refinement scored the vaccine-TLR2 complex to be −212.1 ± 2.3 and the vaccine-TLR4 complex to be −258.8 ± 2.1. The PRODIGY calculated the ΔG of vaccine-TLR2 to be −11.3 and Kd (M) at 25°C to be 5.4 × 10−9, which data are −16.9 and 4.1 × 10−13 separately for vaccine-TLR4 complex (). The interactions on the docking surface of the vaccine-TLRs complex are evaluated by PDBsum, results suggested plural salt bridges, hydrogen bonds as well as disulfide bonds are formed between proteins ().
Figure 4. Docking analysis of vaccine-TLR2 complex, the docking result is on the left, and the analysis of the interactions inside the complex are on the right.
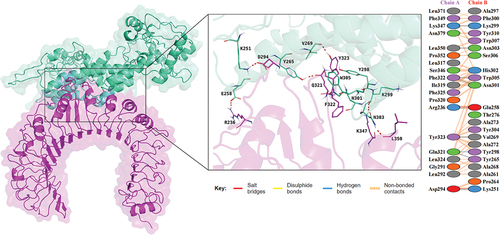
Figure 5. Docking analysis of vaccine-TLR4 complex, the docking result is on the left, and the analysis of the interactions inside the complex are on the right.
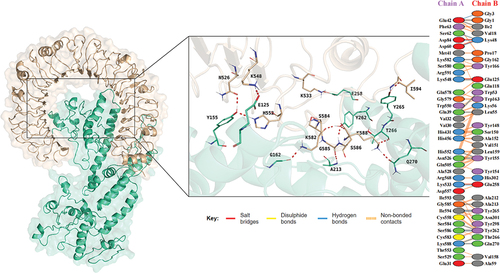
Table 4. Docking analysis of vaccine–TLR combination.
Molecular dynamics simulations
Gromacs was employed to possess molecular dynamics simulations to understand the binding affinity of the vaccine-TLRs complexes. The RMSD (root mean square deviation) served as an indicator of the structural fluctuations of the vaccine-TLRs complexes, The curves revealed both complexes tend to maintain a stable binding, and the RMSD of vaccine-TLR2 and vaccine-TLR4 remains stable after 10 ns in the 100 ns simulation, the average RMSD of vaccine-TLR2 is 1.284 while the average RMSD of vaccine-TLR4 is 1.382 (). The radius of gyration (Rg) was used to assess the degree of loosening in the complexes the curves of both vaccine-TLRs maintained relative equilibrium, the average Rg of vaccine-TLR2 is 3.801 while the average Rg of vaccine-TLR4 is 3.527, indicating both complexes gradually tighten in the 100 ns progress (). RMSF (root mean square fluctuation) analysis provided insights into the flexibility of the residues of vaccine and TLR proteins in complexes, the results demonstrated that residues around 400–500 of the vaccine-TLR2 exhibited relatively high flexibility while other residues kept a low level, the residues of vaccine-TLR4 complex always stay in a low level (). Meanwhile, the H-bonds curves showed both complexes tend to form more H-bonds during the interaction (). The gmx_MMPBSA v1.56 tool calculated the average ΔTOTAL binding energy of vaccine−TLR2 to be −91.73 and −126.59 kcal mol−1 for vaccine-TLR4 complex ( and full results can be found in Table S1 and S2).
Figure 6. The results of molecular dynamics simulation.
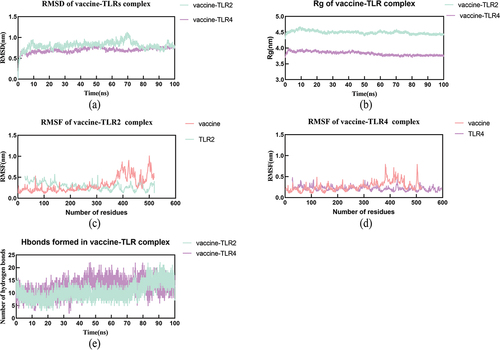
Table 5. MMPBSA of vaccine-TLR2/TLR4 delta (complex - receptor - ligand).
Immunization simulation
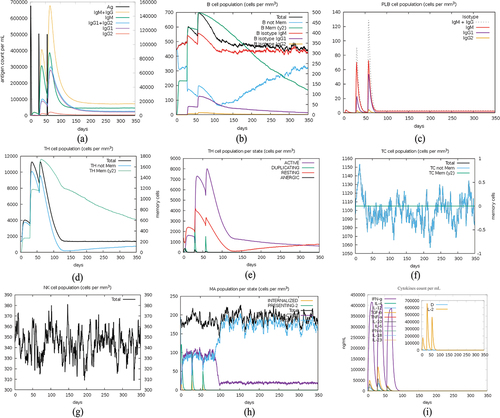
The C-IMMSIM tool accurately predicted immune responses in humans after three doses of vaccination, administered at 4-week intervals, the predicted results of the immune simulations generally align with the actual results of the vaccine injections. The second and third vaccinations induced significantly increased immune responses compared to the response after the first vaccination. The immune response exhibited elevated levels of IgM and combined IgG1+IgG2 antibodies, the peak titer of the antibody increases with the number of injections and gradually decreases after each injection, eventually reaching a stable presence in the body. (). The population of B cells and T cells also increased after each vaccination, and high levels of memory B cells and TH cells were generated simultaneously (). Meanwhile, the vaccination promotes the activation of the innate immune system, as evidenced by the activation and increase of macrophages (). Due to the introduction of HTL epitopes that have a strong stimulating effect on cytokines expression, the immunomodulatory factor, mainly IFN-g and IL-2, significantly increases after the vaccination (). These results indicate that the vaccine can elicit durable immune responses in both B cells and T cells, thereby demonstrating its effectiveness as a prophylactic measure.
Figure 7. Immune simulation after 3 doses of vaccination.
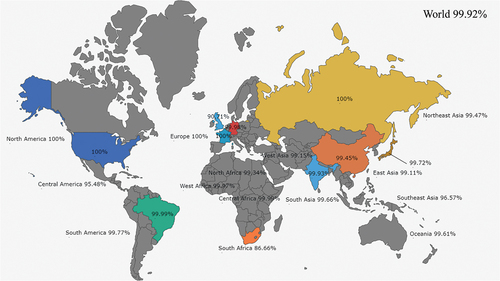
Population coverage
The distribution of MHC varies among different ethnic groups, and the population coverage of the constructed vaccine is calculated based on MHC alleles corresponding with epitopes in the sequence. The constructed vaccine has the capacity to provide coverage to 99.92% of the world’s population, and more than 99% in most regions (). The graphical result is drawn based on pixelmap(https://pixelmap.amcharts.com/).
Construction of multi-epitope mRNA vaccine and vaccine vector
Reverse translation and optimization were possessed on Jcat server, after improvement, a 2573 bp length cDNA was captured, which including full-length epitope vaccine as well as tPA and MITD sequence, its CAI value is 1.0 and its GC content is 49.98, both lies in an ideal range, suggesting good density and thermos-stability. After connecting with UTR sequence, the full-length DNA has 2624 bps. Then, RNAfold predicted its free energy of the thermodynamic ensemble (free energy of the secondary structure of vaccine mRNA) to be −796.96 kcal/mol, and the plasmid structure’s minimum free energy is −507.49 kcal/mol, graphical output can be found in supplement materials. The results predicted revealed the stability of the multi-epitope mRNA vaccine (Figures S1 and S2).
Finally, the DNA sequence of mRNA vaccine was inserted into Pet28a (+) plasmid between BamHI and XhoI with gensmart server to construct the expression vector ().
Discussion
HMPV has grown into a novel threat to public health these days, in the period from birth to 5 y old, almost 100% of the population experience infection once or more, after adulthood, people still face the threat of reinfection, especially those with poor immune response. Vaccination is considered the most effective method to combat epidemics, the final aim is to reduce RTIs in the target population including infants and the elderly. In earlier presents, Cseke et al. immunized cotton rats with soluble F protein, F DNA plasmid, or F protein after F DNA plasmid, all three groups were protected from HMPV infection, with the protection provided by the F protein being the strongest.Citation41 F protein vaccines vectored on alphavirus replicon or other respiratory viruses have also shown varying degrees of protection in immunized animals.Citation42 VLP-based vaccine is also a hopeful candidate, Cox et al. designed a VLP vaccine based on F and M proteins, similarly, Levy et al. developed a VLP vaccine based on F and G proteins, both vaccines provided protection for HMPV infected animal models.Citation43,Citation44 Shaw’s team developed an mRNA vaccine coding F protein of HMPV and PIV3, which has entered Phase I clinical trials.Citation45 In the past decades, many attenuated live vaccines have been developed, most of which performed well in preclinical trials. rHMPV-Pa entered clinical trial but did not achieve the expected effects because of over attenuation.Citation46 Meanwhile, the knockout of G, SH, and M2–1, M2–2 proteins can reduce viral virulence, further confirming the role of proteins other than F in the immune process of HMPV infection.Citation47,Citation48 The short discovery time and lack of sufficient attention have led to the absence of specific vaccines against HMPV to date, successful experiences in developing the Respiratory syncytial virus (RSV) vaccine indicated the important role of the F protein, however, led to the neglection of other proteins. Recently, doctor August’s team developed a mRNA vaccine mRNA-1653 based on F protein of both HMPV and Parainfluenza Virus Type 3. The safety and ability to induce antibodies of this vaccine have been verified in humans, further suggesting that mRNA is a safe and reliable platform, as well as the effectiveness of F protein as a target.Citation49 Thus, at this present, we managed to design a novel multi-epitope mRNA vaccine with immunoinformatic methods to face the threat of HMPV.
Advancements in immune informatics technologies, represented by epitope prediction, have brought new insights to vaccine development. Screening CTL, HTL, and B epitopes from target proteins and combining them with selected adjuvant sequences for design can enhance the safety and efficacy of peptide vaccines, methods such as dynamic simulations and immune simulations provide validation to vaccine design. Further animal experiments validate the protective effect of in silico designed vaccines on Streptococcus,Citation50 Shigella flexneri ,Citation51 and other pathogens. To conclude results have proved the reliability of immunoinformatic methods.
Epitope selection is of paramount importance in the design of multi-epitope vaccines. In this report, our team predicted epitopes from mainly F proteins, as well as epitopes from G, SH, M1, M2–1, and M2–2. F protein is the most favorable target for vaccine development. F protein participates a vital role in recognition and fusion with host cells, and it has the strongest immunogenicity and proved protective response.Citation52,Citation53 Meanwhile, most identified epitopes are located on F protein, the use of the F protein alone can induce the production of protective monoclonal antibodies, while currently no other proteins have been found to generate neutralizing antibodies.Citation54,Citation55 However, proteins other than F are also involved in immune response, SH can inhibit the secretion of inflammatory cytokines, while G protein has the ability to decrease innate immune responses. Although viruses lacking G and SH proteins can still replicate, their replication efficiency will be greatly reduced.Citation56–58 M2–1 is a protein regulating RNA synthesis, it is highly associated with viral replication efficiency. And M2–2 helps HMPV escape from the immune system by inhibiting MAVS-dependent cellular response, knockdown of M2 proteins influences the immunogenicity of the virus.Citation47,Citation48,Citation59,Citation60 Therefore, we mainly focus on the F protein epitope, and selected some predicted epitopes with better results from other proteins, and finally 9 LBL epitopes, 15 CTL epitopes, and 4 HTL epitopes were selected. Stimulating humoral immunity to generate a sufficient titer of antibodies is the focused point in vaccine design. However, simultaneously activating B cells and T cells can generate a stronger immune response, cellular immune response also responds to viral infection, and the ability to promote cytokine production of HTL epitopes mobilizes innate immunity to participate in viral defense. To provide protection for different serum types of HMPV, the epitopes are supposed to be conservative among 392 strains from 5 subtypes, due to high variability among different strains, there was no ideal epitopes found in G protein. The candidates were selected from prediction results by their composite score, antigenicity, and immunogenicity, also they are supposed to be not toxic or homologous with humanoid protein in case of potential harm. Besides, a PADRE sequence severs as a supplement to HTL epitopes, which is able to correspond with 15 MHC II alleles.
Appropriate adjuvants and linkers can enhance the efficacy of vaccines, GPGPG, AAY, and KK linkers are utilized to connect epitopes, these linkers are applied for better cleavage and enhanced immune response.Citation61,Citation62 Two adjuvants are applied in the design, Pam2Cys is a short sequence agonizing TLR2/6 pathway and mediating a series of pro-inflammatory cytokine (including IL-1β, TNF-α, IL-12 and IL-17) releases.Citation63 Pam2Cys can enhance the humoral and cellular immune responses stimulated by mRNA vaccines without safety concerns, making it suitable as an adjuvant for mRNA vaccines.Citation64 While β-defensin II is a commonly used antiviral adjuvant that can enhance innate immunity and immune induction against viruses.Citation65 Thus, a sequence of Pam2Cys is attached to the C-terminal of the vaccine, meanwhile, β-defensin II is attached to the N-terminal both with EAAAK linkers.Citation66 Finally, the vaccine sequence contains 507 residues, its molecular weight is 55.2 kDa and its PI is predicted as 9.41, both are ideal for a vaccine construct.Citation67 With an instability index of 22.50 and an aliphatic of 81.56, the vaccine protein is considered stable and possesses high thermal stability, usually an instability index below 40 is considered stable, lower index perform better results, while higher aliphatic indicates higher thermal stability. High-level stability and thermal stability suggest that in-body synthesized vaccine proteins can stably exist, providing more opportunities for immune responses. With a hydrophilicity index of −0.051, the vaccine demonstrates its hydrophilic properties.Citation68 Meanwhile, the inspection of the constructed candidate revealed it has high antigenicity, and also immunogenicity without toxicity or allergenicity.
To ensure the vaccine can interact with immune receptors to induce immune response, molecular docking and dynamics simulation between the vaccine and TLRs are processed. Toll-like receptors (TLRs) are important immune receptors in viral inflammatory, the respiratory syncytial virus (RSV) virus, which is closely related to HMPV, can induce immune responses through TLR4 activation, TLR4-deficient mice are unable to clear RSV infection.Citation69–71 Additionally, as a molecule that recognizes a wide range of ligands, TLR2 also participates in the signal recognition of RSV, activating pro-inflammatory responses.Citation72,Citation73 Thus, we simulated the docking between vaccine and TLR2/TLR4 to valid it has the ability to induce immune responses. Firstly, we predicted the vaccine’s tertiary structure and refined it, the model quality is acceptable through Ramachandran plotting and ERRAT quality analysis. In molecular docking, we observed that the vaccine binds tightly with TLR2 and TLR4, and plural bridges are formed in the interaction interface, these bridges include salt bridges and hydrogen bonds, disulfide bridges, etc., the more interactions formed, the more closely the molecular interaction is. Further MD simulation estimated the complex, during the 100 ns simulation, the vaccine and receptors tend to form stable interactions in simulated environments, RMSD (Root Mean Square Deviation) measures the difference between the simulated molecular structure and the experimental structure. The smaller the RMSD, the closer the simulation result is to the experimental result. both RMSD curves run flat at a low level, the average RMSD of vaccine-TLR2 is 1.284 while the average RMSD is 1.382 for vaccine-TLR4 complex. Rg (Radius of Gyration) describes the size and shape of the molecule. Smaller Rg values indicate a more compact molecular shape, while larger Rg values indicate a looser molecular shape. The Rg curves decreased smoothly, indicating the complexes tightened gradually until it reached a relative equilibrium state where it stabilized. Meanwhile, RMSF (Root Mean Square Fluctuation) measures the degree of variation in the positions of atoms within the molecule. Smaller RMSF values indicate more stable movements during the simulation process, and in the RMSF analysis of two complexes, only the vaccine-TLR2 complex has a slightly higher level of 400–500 residues of the vaccine, while the other residues maintain a lower level of RMSF, indicating that most of the residues are relatively stable in the simulation. Docking and analyzing of vaccine with TLRs indicates the candidate has an ideal affinity with receptors and the potential to stimulate strong immune responses, which is also validated by immune simulation. In immune simulation, the vaccine can elicit high titer antibody response, the total IgG and IgM levels exceed 65,000/ml after the third infection, which suggests that vaccination can generate a strong antibody response to neutralize the virus. Meanwhile, an increase in the population of B cells was observed after immunization, with multiple isotypes maintained at a higher level for an extended period of time following the final immunization. Similarly, vaccination leads to an increase in TH and TC cells, as well as elevated levels of cell factors such as IFN-g. Results align with the epitope selection and also suggests that the vaccine can induce strong humoral and cellular immune responses. Lastly, the protein sequence is converted into DNA and engineered to perform multi-epitope mRNA vaccine, and the full-length sequence was transferred to the pET28(a) plasmid as a precursor for synthesis in E. coli expression system.
There are still certain limitations in this study. Though many vaccines designed through epitope selection have achieved ideal protective effects equal to in silico predictions in animal experiments, still we cannot determine this candidate vaccine’s efficacy only in silico. Meanwhile, the experimentally validated epitopes can usually be obtained from epitope prediction tools, and they rank high in the overall evaluation. However, not all high-scoring peptides can be experimentally validated. Therefore, we have to appropriately increase the number of epitopes in the vaccine sequence, which will result in an increased total length, possibly affecting the stability of the final product and increasing the difficulty of acquisition.
Conclusion
In this present, we developed a novel multi-epitope mRNA vaccine candidate against HMPV with the assistant of immunoinformatic methods. Compared with previous researches, we choose multi-epitope mRNA vaccine as the formation, through incorporating immunodominant epitopes, we aim to achieve stronger targeting while minimizing undesirable effects. Meanwhile, a design with conserved epitopes can provide a broader range of protection among different subtypes, and a vaccine containing T cell and B cell epitopes is supposed to elicit both humoral and cellular immune responses. Besides, this design introduced epitopes from SH, M1, and M2 proteins to support F epitopes, by targeting other proteins with immune-modulatory functions, we can enhance the functionality of the vaccine. It should be pointed out that the final efficacy of the vaccine still needs to be determined through in vitro and in vivo experiments. But in summary, the design of this vaccine is a promising vaccine candidate that performs well in simulations. Given the limited research on HMPV vaccines, it can provide an effective strategy for the prevention of HMPV.
Author contributions
Conceptualization, F.Z, and S.M.; methodology, F.Z.; validation, S.M. and Y.X., and M.R.; formal analysis, P.Z, W.P, H.Y and Y.C; writing – original draft preparation, S.M, Y.X and C.T; writing – review and editing:J.C; supervision, P.P and J.C.; project administration, P.P and J.C; funding acquisition, P.P
Data available statement
The data that support the findings of this study are available from the corresponding author, Pinhua Pan, upon reasonable request.
Declaration of generative AI in scientific writing
No AI technology is used in scientific writing.
Submission declaration and verification
This article has not been published previously, and it is not under consideration for publication elsewhere, its publication is approved by all authors and tacitly or explicitly by the responsible authorities where the work was carried out.
supplement materials.docx
Download MS Word (290 KB)Acknowledgments
Thanks to the support from HY.W, C.Z, and L.Z.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2293300.
Additional information
Funding
References
- van den Hoogen BG, de Jong JC, Groen J, Kuiken T, de Groot R, Fouchier RA, Osterhaus ADME. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7(6):719–16. doi:10.1038/89098.
- Perez A, Lively JY, Curns A, Weinberg GA, Halasa NB, Staat MA, Szilagyi PG, Stewart LS, McNeal MM, Clopper B, et al. Respiratory virus surveillance among children with acute respiratory illnesses — new vaccine surveillance network, United States, 2016–2021. MMWR Morb Mortal Wkly Rep. 2022;71(40):1253–9. doi:10.15585/mmwr.mm7140a1.
- CDC amecira. Human metapneumovirus (HMPV). National Trends. https://www.cdc.gov/surveillance/nrevss/hmpv/natl-trend.html.
- Haynes AK, Fowlkes AL, Schneider E, Mutuc JD, Armstrong GL, Gerber SI. Human metapneumovirus circulation in the United States, 2008 to 2014. Pediatrics. 2016;137(5). doi:10.1542/peds.2015-2927.
- Williams JV, Harris PA, Tollefson SJ, Halburnt-Rush LL, Pingsterhaus JM, Edwards KM, Wright PF, Crowe JE. Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N Engl J Med. 2004;350(5):443–50. doi:10.1056/NEJMoa025472.
- Walsh EE, Peterson DR, Falsey AR. Human metapneumovirus infections in adults: another piece of the puzzle. Arch Intern Med. 2008;168(22):2489–96. doi:10.1001/archinte.168.22.2489.
- Wang X, Li Y, Deloria-Knoll M, Madhi SA, Cohen C, Ali A, Basnet S, Bassat Q, Brooks WA, Chittaganpitch M, et al. Global burden of acute lower respiratory infection associated with human metapneumovirus in children under 5 years in 2018: a systematic review and modelling study. Lancet Glob Health. 2021;9(1):e33–e43. doi:10.1016/S2214-109X(20)30393-4.
- Shahda S, Carlos WG, Kiel PJ, Khan BA, Hage CA. The human metapneumovirus: a case series and review of the literature. Transpl Infect Dis. 2011;13(3):324–8. doi:10.1111/j.1399-3062.2010.00575.x.
- Haas LE, Thijsen SF, van Elden L, Heemstra KA. Human metapneumovirus in adults. Viruses. 2013;5(1):87–110. doi:10.3390/v5010087.
- Gaunt ER, Jansen RR, Poovorawan Y, Templeton KE, Toms GL, Simmonds P, Fouchier R. Molecular epidemiology and evolution of human respiratory syncytial virus and human metapneumovirus. PLoS One. 2011;6(3):e17427. doi:10.1371/journal.pone.0017427.
- Biacchesi S, Skiadopoulos MH, Boivin G, Hanson CT, Murphy BR, Collins PL, Buchholz UJ. Genetic diversity between human metapneumovirus subgroups. Virology. 2003;315(1):1–9. doi:10.1016/S0042-6822(03)00528-2.
- Shafagati N, Williams J. Human metapneumovirus - what we know now. F1000Res. 2018;7:135. doi:10.12688/f1000research.12625.1.
- Wilson RL, Fuentes SM, Wang P, Taddeo EC, Klatt A, Henderson AJ, He B. Function of small hydrophobic proteins of paramyxovirus. J Virol. 2006;80(4):1700–9. doi:10.1128/JVI.80.4.1700-1709.2006.
- Herfst S, Fouchier RA. Vaccination approaches to combat human metapneumovirus lower respiratory tract infections. J Clin Virol. 2008;41(1):49–52. doi:10.1016/j.jcv.2007.10.022.
- Herfst S, Schrauwen EJ, de Graaf M, van Amerongen G, van den Hoogen BG, de Swart RL, Osterhaus ADME, Fouchier RAM. Immunogenicity and efficacy of two candidate human metapneumovirus vaccines in cynomolgus macaques. Vaccine. 2008;26(33):4224–30. doi:10.1016/j.vaccine.2008.05.052.
- Olmedillas E, Cano O, Martinez I, Luque D, Terron MC, McLellan JS, Melero JA, Más V. Chimeric Pneumoviridae fusion proteins as immunogens to induce cross-neutralizing antibody responses. EMBO Mol Med. 2018;10(2):175–87. doi:10.15252/emmm.201708078.
- Herd KA, Mahalingam S, Mackay IM, Nissen M, Sloots TP, Tindle RW. Cytotoxic T-lymphocyte epitope vaccination protects against human metapneumovirus infection and disease in mice. J Virol. 2006;80(4):2034–44. doi:10.1128/JVI.80.4.2034-2044.2006.
- Melendi GA, Zavala F, Buchholz UJ, Boivin G, Collins PL, Kleeberger SR, Polack FP. Mapping and characterization of the primary and anamnestic H-2 d -restricted cytotoxic T-lymphocyte response in mice against human metapneumovirus. J Virol. 2007;81(20):11461–7. doi:10.1128/JVI.02423-06.
- Larsen MV, Lundegaard C, Lamberth K, Buus S, Lund O, Nielsen M. Large-scale validation of methods for cytotoxic T-lymphocyte epitope prediction. BMC Bioinform. 2007;8(1):424. doi:10.1186/1471-2105-8-424.
- Reynisson B, Barra C, Kaabinejadian S, Hildebrand WH, Peters B, Nielsen M. Improved prediction of MHC II antigen presentation through integration and motif deconvolution of mass spectrometry MHC eluted ligand data. J Proteome Res. 2020;19(6):2304–15. doi:10.1021/acs.jproteome.9b00874.
- Doytchinova IA, Flower DR. VaxiJen: a server for prediction of protective antigens, tumour antigens and subunit vaccines. BMC Bioinform. 2007;8(1):4. doi:10.1186/1471-2105-8-4.
- Dimitrov I, Bangov I, Flower DR, Doytchinova I. AllerTOP v.2—a server for in silico prediction of allergens. J Mol Model. 2014;20(6):2278. doi:10.1007/s00894-014-2278-5.
- Gupta S, Kapoor P, Chaudhary K, Gautam A, Kumar R, Raghava GPS, Open Source Drug Discovery C. In silico approach for predicting toxicity of peptides and proteins. PLoS One. 2013;8(9):e73957. doi:10.1371/journal.pone.0073957.
- Dhanda SK, Gupta S, Vir P, Raghava GP. Prediction of IL4 inducing peptides. Clin Dev Immunol. 2013;2013:1–9. doi:10.1155/2013/263952.
- Dhanda SK, Vir P, Raghava GP. Designing of interferon-gamma inducing MHC class-II binders. Biol Direct. 2013;8(1):30. doi:10.1186/1745-6150-8-30.
- Saha S, Raghava GP. Prediction of continuous B-cell epitopes in an antigen using recurrent neural network. Proteins. 2006;65(1):40–8. doi:10.1002/prot.21078.
- Chen C, Li Z, Huang H, Suzek BE, Wu CH, UniProt C. A fast peptide match service for UniProt knowledgebase. Bioinformatics. 2013;29(21):2808–9. doi:10.1093/bioinformatics/btt484.
- Baek M, DiMaio F, Anishchenko I, Dauparas J, Ovchinnikov S, Lee GR, Wang J, Cong Q, Kinch LN, Schaeffer RD, et al. Accurate prediction of protein structures and interactions using a three-track neural network. Science. 2021;373(6557):871–6. doi:10.1126/science.abj8754.
- Narang PK, Dey J, Mahapatra SR, Ghosh M, Misra N, Suar M, Kumar V, Raina V. Functional annotation and sequence-structure characterization of a hypothetical protein putatively involved in carotenoid biosynthesis in microalgae. S Afr J Bot. 2021;141:219–26. doi:10.1016/j.sajb.2021.04.014.
- Wiederstein M, Sippl MJ. ProSA-web: interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res. 2007;35(Web Server):W407–10. doi:10.1093/nar/gkm290.
- van Zundert GCP, Rodrigues J, Trellet M, Schmitz C, Kastritis PL, Karaca E, Melquiond ASJ, van Dijk M, de Vries SJ, Bonvin AMJJ. The HADDOCK2.2 web server: user-friendly integrative modeling of biomolecular complexes. J Mol Biol. 2016;428(4):720–5. doi:10.1016/j.jmb.2015.09.014.
- Kutzner C, Kniep C, Cherian A, Nordstrom L, Grubmuller H, de Groot BL, Gapsys V. GROMACS in the cloud: a global supercomputer to speed up alchemical drug design. J Chem Inf Model. 2022;62(7):1691–711. doi:10.1021/acs.jcim.2c00044.
- Rapin N, Lund O, Castiglione F. Immune system simulation online. Bioinformatics. 2011;27(14):2013–4. doi:10.1093/bioinformatics/btr335.
- Rapin N, Lund O, Bernaschi M, Castiglione F, Brusic V. Computational immunology meets bioinformatics: the use of prediction tools for molecular binding in the simulation of the immune system. PLoS One. 2010;5(4):e9862. doi:10.1371/journal.pone.0009862.
- Kim SC, Sekhon SS, Shin WR, Ahn G, Cho BK, Ahn JY, Kim Y-H. Modifications of mRNA vaccine structural elements for improving mRNA stability and translation efficiency. Mol Cell Toxicol. 2022;18(1):1–8. doi:10.1007/s13273-021-00171-4.
- Kou Y, Xu Y, Zhao Z, Liu J, Wu Y, You Q, Wang L, Gao F, Cai L, Jiang C, et al. Tissue plasminogen activator (tPA) signal sequence enhances immunogenicity of MVA-based vaccine against tuberculosis. Immunol Lett. 2017;190:51–7. doi:10.1016/j.imlet.2017.07.007.
- Kreiter S, Selmi A, Diken M, Sebastian M, Osterloh P, Schild H, Huber C, Türeci O, Sahin U. Increased antigen presentation efficiency by coupling antigens to MHC class I trafficking signals. J Immunol. 2008;180(1):309–18. doi:10.4049/jimmunol.180.1.309.
- Rcheulishvili N, Mao J, Papukashvili D, Liu C, Wang Z, Zhao J, Xie F, Pan X, Ji Y, He Y, et al. Designing multi-epitope mRNA construct as a universal influenza vaccine candidate for future epidemic/pandemic preparedness. Int J Biol Macromol. 2023;226:885–99. doi:10.1016/j.ijbiomac.2022.12.066.
- Rybakova Y, Kowalski PS, Huang Y, Gonzalez JT, Heartlein MW, DeRosa F, Delcassian D, Anderson DG. mRNA delivery for therapeutic anti-HER2 antibody expression in vivo. Mol Ther. 2019;27(8):1415–23. doi:10.1016/j.ymthe.2019.05.012.
- Grote A, Hiller K, Scheer M, Munch R, Nortemann B, Hempel DC, Jahn D. JCat: a novel tool to adapt codon usage of a target gene to its potential expression host. Nucleic Acids Res. 2005;33:W526–31. doi:10.1093/nar/gki376.
- Cseke G, Wright DW, Tollefson SJ, Johnson JE, Crowe JE Jr., Williams JV. Human metapneumovirus fusion protein vaccines that are immunogenic and protective in cotton rats. J Virol. 2007;81(2):698–707. doi:10.1128/JVI.00844-06.
- Ogonczyk Makowska D, Hamelin ME, Boivin G. Engineering of live chimeric vaccines against human metapneumovirus. Pathogens. 2020;9(2):135. doi:10.3390/pathogens9020135.
- Cox RG, Erickson JJ, Hastings AK, Becker JC, Johnson M, Craven RE, Tollefson SJ, Boyd KL, Williams JV, et al. Human metapneumovirus virus-like particles induce protective B and T cell responses in a mouse model. J Virol. 2014;88(11):6368–79. doi:10.1128/JVI.00332-14.
- Levy C, Aerts L, Hamelin ME, Granier C, Szecsi J, Lavillette D, Boivin G, Cosset F-L. Virus-like particle vaccine induces cross-protection against human metapneumovirus infections in mice. Vaccine. 2013;31(25):2778–85. doi:10.1016/j.vaccine.2013.03.051.
- Shaw CL, Smolenov S, Panther T, Kalidindi I, Zaks L, Smolenov I, Panther L. 2754. Phase 1 trial of an mRNA-based combination vaccine against hMPV and PIV3. Open Forum Infect. 2019;6(Supplement_2):S970. doi:10.1093/ofid/ofz360.2431.
- Karron RA, San Mateo J, Wanionek K, Collins PL, Buchholz UJ. Evaluation of a live attenuated human metapneumovirus vaccine in adults and children. J Pediatric Infect Dis Soc. 2018;7(1):86–9. doi:10.1093/jpids/pix006.
- Biacchesi S, Pham QN, Skiadopoulos MH, Murphy BR, Collins PL, Buchholz UJ. Infection of nonhuman primates with recombinant human metapneumovirus lacking the SH, G, or M2-2 protein categorizes each as a nonessential accessory protein and identifies vaccine candidates. J Virol. 2005;79(19):12608–13. doi:10.1128/JVI.79.19.12608-12613.2005.
- Buchholz UJ, Biacchesi S, Pham QN, Tran KC, Yang L, Luongo CL, Skiadopoulos MH, Murphy BR, Collins PL. Deletion of M2 gene open reading frames 1 and 2 of human metapneumovirus: effects on RNA synthesis, attenuation, and immunogenicity. J Virol. 2005;79(11):6588–97. doi:10.1128/JVI.79.11.6588-6597.2005.
- August A, Shaw CA, Lee H, Knightly C, Kalidindia S, Chu L, Essink BJ, Seger W, Zaks T, Smolenov I, et al. Safety and immunogenicity of an mRNA-based human metapneumovirus and parainfluenza virus type 3 combined vaccine in healthy adults. Open Forum Infect Dis. 2022;9(7):ofac206. doi:10.1093/ofid/ofac206.
- Zhang Y, Liang S, Zhang S, Zhang S, Yu Y, Yao H, Liu Y, Zhang W, Liu G. Development and evaluation of a multi-epitope subunit vaccine against group B Streptococcus infection. Emerg Microbes Infect. 2022;11(1):2371–82. doi:10.1080/22221751.2022.2122585.
- Leon Y, Zapata L, Molina RE, Okanovic G, Gomez LA, Daza-Castro C, Flores-Concha M, Reyes JL, Oñate AA. Intranasal immunization of mice with multiepitope chimeric vaccine candidate based on conserved autotransporters SigA, Pic and Sap, confers protection against shigella flexneri. Vaccines (Basel). 2020;8(4):563. doi:10.3390/vaccines8040563.
- Van Den Bergh A, Bailly B, Guillon P, von Itzstein M, Dirr L. Antiviral strategies against human metapneumovirus: targeting the fusion protein. Antiviral Res. 2022;207:105405. doi:10.1016/j.antiviral.2022.105405.
- Guo L, Li L, Liu L, Zhang T, Sun M. Neutralising antibodies against human metapneumovirus. Lancet Microbe. 2023;4(9):e732–e44. doi:10.1016/S2666-5247(23)00134-9.
- Stepanova E, Matyushenko V, Rudenko L, Isakova-Sivak I. Prospects of and barriers to the development of epitope-based vaccines against human metapneumovirus. Pathogens. 2020;9(6):481. doi:10.3390/pathogens9060481.
- Ryder AB, Tollefson SJ, Podsiad AB, Johnson JE, Williams JV. Soluble recombinant human metapneumovirus G protein is immunogenic but not protective. Vaccine. 2010;28(25):4145–52. doi:10.1016/j.vaccine.2010.04.007.
- Bao X, Kolli D, Liu T, Shan Y, Garofalo RP, Casola A. Human metapneumovirus small hydrophobic protein inhibits NF-κB transcriptional activity. J Virol. 2008;82(16):8224–9. doi:10.1128/JVI.02584-07.
- Bao X, Liu T, Shan Y, Li K, Garofalo RP, Casola A, Baric RS. Human metapneumovirus glycoprotein G inhibits innate immune responses. PLoS Pathog. 2008;4(5):e1000077. doi:10.1371/journal.ppat.1000077.
- Skiadopoulos MH, Biacchesi S, Buchholz UJ, Amaro-Carambot E, Surman SR, Collins PL, Murphy BR. Individual contributions of the human metapneumovirus F, G, and SH surface glycoproteins to the induction of neutralizing antibodies and protective immunity. Virology. 2006;345(2):492–501. doi:10.1016/j.virol.2005.10.016.
- Ren J, Wang Q, Kolli D, Prusak DJ, Tseng CT, Chen ZJ, Li K, Wood TG, Bao X. Human metapneumovirus M2-2 protein inhibits innate cellular signaling by targeting MAVS. J Virol. 2012;86(23):13049–61. doi:10.1128/JVI.01248-12.
- Schowalter RM, Smith SE, Dutch RE. Characterization of human metapneumovirus F protein-promoted membrane fusion: critical roles for proteolytic processing and low pH. J Virol. 2006;80(22):10931–41. doi:10.1128/JVI.01287-06.
- Dey J, Mahapatra SR, Singh P, Patro S, Kushwaha GS, Misra N, Suar M. B and T cell epitope-based peptides predicted from clumping factor protein of staphylococcus aureus as vaccine targets. Microb Pathog. 2021;160:105171. doi:10.1016/j.micpath.2021.105171.
- Li G, Huang Z, Zhang C, Dong BJ, Guo RH, Yue HW, Yan L-T, Xing X-H. Construction of a linker library with widely controllable flexibility for fusion protein design. Appl Microbiol Biotechnol. 2016;100(1):215–25. doi:10.1007/s00253-015-6985-3.
- Ashhurst AS, Johansen MD, Maxwell JWC, Stockdale S, Ashley CL, Aggarwal A, Siddiquee R, Miemczyk S, Nguyen DH, Mackay JP, et al. Mucosal TLR2-activating protein-based vaccination induces potent pulmonary immunity and protection against SARS-CoV-2 in mice. Nat Commun. 2022;13(1):6972. doi:10.1038/s41467-022-34297-3.
- Gu Y, Yang J, He C, Zhao T, Lu R, Liu J, Mo X, Wen F, Shi H. Incorporation of a Toll-like receptor 2/6 agonist potentiates mRNA vaccines against cancer and infectious diseases. Sig Transduct Target Ther. 2023;8(1):273. doi:10.1038/s41392-023-01479-4.
- Kim J, Yang YL, Jang SH, Jang YS. Human β-defensin 2 plays a regulatory role in innate antiviral immunity and is capable of potentiating the induction of antigen-specific immunity. Virol J. 2018;15(1):124. doi:10.1186/s12985-018-1035-2.
- Mei HF, Jin XB, Zhu JY, Zeng AH, Wu Q, Lu XM, Li X-B, Shen J. β-defensin 2 as an adjuvant promotes anti-melanoma immune responses and inhibits the growth of implanted murine melanoma in vivo. PLoS One. 2012;7(2):e31328. doi:10.1371/journal.pone.0031328.
- Dey J, Mahapatra SR, Lata S, Patro S, Misra N, Suar M. Exploring Klebsiella pneumoniae capsule polysaccharide proteins to design multiepitope subunit vaccine to fight against pneumonia. Expert Rev Vaccines. 2022;21(4):569–87. doi:10.1080/14760584.2022.2021882.
- Wilkins MR, Gasteiger E, Bairoch A, Sanchez JC, Williams KL, Appel RD. Protein identification and analysis tools in the ExPASy server. Methods Mol Biol. 1999;112:531–52.
- Funchal GA, Jaeger N, Czepielewski RS, Machado MS, Muraro SP, Stein RT, Bonorino CBC, Porto BN. Respiratory syncytial virus fusion protein promotes TLR-4–dependent neutrophil extracellular trap formation by human neutrophils. PLoS One. 2015;10(4):e0124082. doi:10.1371/journal.pone.0124082.
- Kurt-Jones EA, Popova L, Kwinn L, Haynes LM, Jones LP, Tripp RA, Walsh EE, Freeman MW, Golenbock DT, Anderson LJ, et al. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat Immunol. 2000;1(5):398–401. doi:10.1038/80833.
- Rassa JC, Meyers JL, Zhang Y, Kudaravalli R, Ross SR. Murine retroviruses activate B cells via interaction with toll-like receptor 4. Proc Natl Acad Sci USA. 2002;99(4):2281–6. doi:10.1073/pnas.042355399.
- Murawski MR, Bowen GN, Cerny AM, Anderson LJ, Haynes LM, Tripp RA, Kurt-Jones EA, Finberg RW. Respiratory syncytial virus activates innate immunity through Toll-like receptor 2. J Virol. 2009;83(3):1492–500. doi:10.1128/JVI.00671-08.
- Alshaghdali K, Saeed M, Kamal MA, Saeed A. Interaction of ectodomain of respiratory syncytial virus G protein with TLR2/TLR6 heterodimer: an in vitro and in silico approach to decipher the role of RSV G protein in pro-inflammatory response against the virus. Curr Pharm Des. 2021;27(44):4464–76. doi:10.2174/1381612827666210716160030.