ABSTRACT
Booster vaccinations against SARS-CoV-2 are recommended 6–12 months after the last dose or infection in elderly and high-risk groups. The present analysis aims to evaluate whether an interval shorter than 12 months is required in multiple sclerosis patients receiving ofatumumab. Neutralizing antibody status over 1 year in patients receiving booster vaccination in the non-interventional, multicenter KYRIOS study under continued ofatumumab treatment was analyzed. Fifteen patients were included. At the time of the first booster vaccination, ten patients were seropositive for neutralizing antibodies, four patients were seronegative, and for one patient, no baseline levels were available. All patients who were seropositive at baseline showed >2-fold increase in neutralizing antibody titers after the first booster and two patients (20%) showed a >10-fold increase. Among seronegative patients, three (75%) had a >10-fold increase in neutralizing antibody titers. Seropositivity was maintained in almost all patients until month 12. One initially seronegative patient had less than 2-fold increase in neutralizing antibody titers after the booster vaccination and can be considered a non-responder. Most patients with continued ofatumumab treatment are able to maintain permanent seropositivity and therefore presumably constant protection against severe courses of COVID-19 if repeated booster vaccinations are applied.
Introduction
mRNA vaccinations against SARS-CoV-2 are established, and booster vaccinations are routinely applied. The Robert Koch-Institute (RKI) issued recommendations for regular booster vaccinations 12 months after the last vaccination or infection in elderly and high-risk groups including immunocompromised patients. However, the RKI points out that it may be necessary to shorten the interval of 12 months for further booster vaccinations in people with a compromised immune response.Citation1 The World Health Organization (WHO) recommends receiving first and additional booster doses 6 or 12 months after the last dose, depending on factors such as age and immunocompromising conditions.Citation2
Whether an interval shorter than 12 months is required in patients receiving disease-modifying therapy (DMT) remains unclear. For ofatumumab, an anti-CD20 antibody used to treat relapsing forms of multiple sclerosis (MS), data are available showing that initial SARS-CoV-2 mRNA vaccination elicits both cellular and humoral immune responses in ofatumumab-treated MS patients. Ofatumumab treatment interruption is not necessary for the purpose of SARS-CoV-2 mRNA vaccination.Citation3,Citation4
However, data are still lacking on the sustainability of the immune response and the necessary frequency of booster vaccinations in MS patients treated with ofatumumab. It is important that booster vaccinations are able to maintain adequate immune response (i.e., seropositivity) under continued ofatumumab therapy, as treatment interruption is associated with an increased risk of relapses and disease progression.Citation5,Citation6
The KYRIOS study investigated the immune response toward SARS-CoV-2 mRNA vaccination in patients receiving ofatumumab. Results on the immune responses 1 month after initial vaccination and 1 month after booster vaccination during continued ofatumumab treatment have already been published.Citation3,Citation4 New data are now available from the KYRIOS study on the maintenance of the immune response over 12 months after booster vaccination.
Materials and methods
KYRIOS (NCT04869358; registered 2021-04-29) was a non-interventional, multicenter, two-cohort study on the immune response toward SARS-CoV-2 mRNA vaccination before (cohort 1) or during (cohort 2) ofatumumab treatment. Details on the design of the KYRIOS study and patient characteristics have been published previously.Citation3,Citation4 Here, we report neutralizing antibody status over 1 year in patients who received only their booster vaccination in the KYRIOS study under continued ofatumumab treatment. These patients are a subgroup of cohort 2 and had been initially vaccinated outside of the KYRIOS study either during treatment with other DMT than ofatumumab or without receiving DMT. Further booster vaccinations were optional. Assessment of immune responses was planned at month 0 (before first booster), month 1 (results have already been publishedCitation3), month 6, and month 12 after first booster. Optional assessments could be performed 1 month after second (optional) booster vaccination. The study complied with principles of the Declaration of Helsinki, it was approved by an ethics committee, and written informed consent was obtained from all patients included.
Results
In total, 15 patients received only their booster vaccination during the KYRIOS study under ofatumumab and were included in the present analysis. Of these, 13 patients received the SARS-CoV-2 mRNA vaccine by BioNTech/Pfizer and 2 patients received the SARS-CoV-2 mRNA vaccine by MODERNA as booster. During their initial vaccination cycle, seven of these patients had been treated with DMT and eight patients were not treated with any DMT (). After their initial vaccination cycle but before the first booster, the patients started treatment with ofatumumab. The mean time since initiation of ofatumumab to first booster was 1.87 months (standard deviation [SD] 0.85).
Table 1. DMT during initial vaccination.
shows the titers of neutralizing antibodies as well as timing of covid infections and further booster vaccinations (if applicable). For one patient no baseline neutralizing antibody titers were available. For another patient month 12 neutralizing antibody titers were not available.
Figure 1. Quantification of SARS-CoV-2-specific neutralizing antibody titer in U/ml after booster vaccination during ofatumumab treatment. All patients with available data were included in the analysis, and individual values are represented by dots. Dotted line indicates assay-specific cutoff for seropositivity. The color scheme indicates initial neutralizing antibody titers (yellow: seronegative patients at the time of the first booster; blue: seropositive patients with moderate level of neutralizing antibody titers at the time of the first booster; black: seropositive patients with high level of neutralizing antibody titers at the time of the first booster; gray: no baseline level of neutralizing antibodies available). Time of COVID-19 infections and timing of additional boosters are indicated in the individual courses. An integrated table gives an overview of key parameters per case (sorted by the level of neutralizing antibody titers at month 12): seropositivity at the time of vaccination (+), additional booster vaccination received during the study (+), COVID-19 infection reported during the study (+), strength of increase in neutralizing antibody titers after first booster compared to baseline (<2-fold; >2-fold, >4-fold, and >10-fold); DMT received during initial vaccination (yes = continued DMT/no = DMT interrupted/naïve = no DMT prior and during vaccination). *no neutralizing antibody titer available at month 12 due to technical issues; **no baseline value; ***rounded. DMT: disease-modifying therapy; n.a.: not available; nAB: neutralizing antibody.
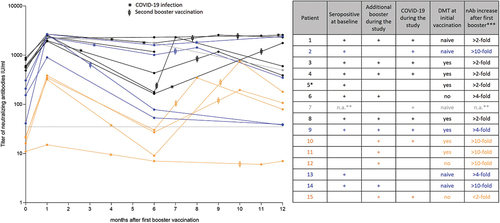
At the time of the first booster vaccination, ten patients were seropositive for neutralizing antibodies and four patients were seronegative. Among seropositive patients, all (100%) showed a >2-fold increase of neutralizing antibody titers after the first booster, three (33%) showed a >4-fold increase and two (20%) showed a >10-fold increase. Among seronegative patients, three (75%) had a >10-fold increase in neutralizing antibody titers. After 12 months, nine (90%) of the initially seropositive patients remained seropositive (for one patient information on neutralizing antibody titers at month 12 was missing). Among the initially seronegative patients, three (75%) were seropositive at month 12 and one (25%) remained seronegative. The latter patient had less than 2-fold increase in neutralizing antibody titers after the booster vaccination ().
During the study, 11 patients received an additional booster vaccination (second booster). The timing of additional booster vaccinations is indicated in . Of the patients with additional booster, ten (91%) were seropositive at month 12. In comparison, three of four patients without additional booster (75%) were seropositive at month 12 (for one patient information on neutralizing antibody titers at month 12 was missing) ().
Among patients with and without DMT during the initial vaccination, 5 (71%) vs. 5 (63%) were seropositive at the time of the first booster, 7 (100%) vs. 6 (75%) showed a >2-fold increase in neutralizing antibody titers after the first booster, 3 (43%) vs. 5 (63%) showed a >4-fold increase, and 2 (29%) vs. 3 (38%) showed a >10-fold increase (for one patient without DMT during initial vaccination, increase in neutralizing antibody titers could not be estimated due to missing baseline value). At month 12, 7 (100%) vs. 7 (88%) were seropositive ().
The number and time of occurrence of COVID-19 infections are indicated in . Overall, nine COVID-19 infections were reported. These were mild in three patients (20%) and moderate in six patients (40%). The duration of infection was 7–24 days, and all patients fully recovered.
Discussion
According to the present analysis, almost all patients had an increase in neutralizing antibody titers after the first booster vaccination irrespective of the serostatus at the time of the first booster. Only one patient showed an increase less than 2-fold. This patient had also been seronegative at the time of the booster and thus can be considered a non-responder. Seropositivity was maintained in almost all patients until month 12. Whether or not continued DMT during the initial vaccination impacted the booster response cannot be answered based on the present booster cohort.
The present analysis focused on neutralizing antibodies as these are thought to be a stronger correlate with protection than total SARS-CoV-2-specific antibody titers.Citation7 This has to be kept in mind when comparing the present results to previous reports on immune responses, as some studies assessed total immunoglobulin G (IgG) levels only.
Similar courses of neutralizing antibodies over time were also found in vaccinated healthy individuals. Arunachalam et al. have investigated the durability of neutralizing antibodies in healthy, immunocompetent volunteers who received their first booster 6–10 months after the initial vaccination and a second booster 6–8 months after the first booster. According to their results on antibodies with neutralizing capacity against wildtype as well as omicron variants, individuals with persistent response, rapid decline, and low response can be identified. In addition, the results show a category of non-responders who remained below the cutoff for seropositivity for neutralizing antibodies against omicron variants after a first or second booster.Citation8
Neutralizing antibody titers observed under continued ofatumumab in the present analysis on average were as high as previously reported for patients receiving their booster before ofatumumabCitation3 or during treatment with other DMT.Citation9 Compared to healthy individuals,Citation8 neutralizing antibody titers reached after booster vaccination under continued ofatumumab treatment seem slightly lower. However, a threshold for a protective effect has not been quantified and possibly will never be, as humoral response constitutes only one pillar of the immune response against SARS-CoV-2. Therefore, the clinical relevance of this difference in neutralizing antibody titers remains unclear. The level of T-cell activity after SARS-CoV-2 mRNA vaccination in patients treated with ofatumumab has been previously published. Accordingly, an early T-cell response 1 week after completed initial vaccination was induced in all patients treated with ofatumumab.Citation4
In patients treated with ocrelizumab, booster vaccinations are also able to induce seroconversion for neutralizing antibodies in some patients, but an additional booster is necessary to achieve a near similar seroconversion rate as in healthy controls. In a recently published study, seven out of ten healthy controls had neutralizing antibodies against omicron variant BA.2 after the first booster compared to three (after the first booster) and six (after the second booster) out of ten patients treated with ocrelizumab.Citation10 Total anti-spike IgG titers over time in ocrelizumab-treated patients remained below those in an untreated control group.Citation11 Nevertheless, compared to total anti-spike IgG titers after the initial vaccination, booster vaccinations induced an increase in ocrelizumab-treated patients.Citation11,Citation12 This increase was shown to sustain until month 3.Citation11
A booster vaccination or infection during ofatumumab treatment increases neutralizing antibody titers to reach sustained seropositivity in most patients as in a healthy population. This suggests that humoral immune memory responses can in principle be generated under ofatumumab after initial vaccinationCitation4 and immune memory can also be maintained. Recent data support that memory B cells can be detected under ofatumumab treatment.Citation13
According to the present results, neutralizing antibody titers generally decline over time like in healthy individuals and most patients treated with ofatumumab need repeated boosters to remain seropositive. Having a booster at regular intervals increases the chance of long-term seropositivity and therefore protection against severe courses of COVID-19. Although a threshold for protective antibody titers has not yet been established, the present results suggest that an interval of 12 months might be too long to ensure constant protective antibody titers in most patients. However, the present results need to be interpreted with caution due to the small sample size and the uncontrolled study setting, which does not allow statistical testing of the observed effects. Furthermore, the sample size is not sufficient for a conclusive clinical evaluation. The present results should be considered hypothesis-generating rather than hypothesis-testing. Overall, nine COVID-19 cases were reported in the KYRIOS booster cohort. In the ALITHIOS study, an open-label extension of the pivotal study on ofatumumab, seven COVID-19 cases have been reported in 476 ofatumumab-treated patients (1.5%) after completed initial vaccination.Citation14 Until the cutoff of the ALITHIOS study, no COVID-19 cases have been reported in 27 patients, who already had received a booster vaccination.Citation14 As the booster cohort from the ALITHIOS study is also small and the setting was uncontrolled, a clinical evaluation of the optimal booster timing is pending and the present assumptions have to be confirmed in a controlled study setting in a larger patient population.
In conclusion, the majority of patients with continued ofatumumab treatment are able to maintain permanent seropositivity and therefore presumably constant protection against severe courses of COVID-19 if repeated booster vaccinations are applied.
Author contributions
TZ: conzeptualization, investigation, and writing (review and editing); MG: investigation, project administration, and writing (review and editing); BE: conzeptualization, project administration, investigation, and writing (review and editing); TB: conzeptualization, investigation, and writing (review and editing).
Acknowledgments
Medical writing support (preparation of the manuscript draft) was provided by Karin Eichele (mediwiz).
Disclosure statement
Tjalf Ziemssen has received personal compensation for participating on advisory boards, trial steering committees, and data and safety monitoring committees, as well as for scientific talks and project support from: Almirall, Bayer, BAT, Biogen, Celgene, Sanofi Genzyme, Merck, Novartis, Roche, Vitaccess, and Teva. Marie Groth and Benjamin Ettle are employees of Novartis Pharma GmbH, Nuremberg, Germany. Tobias Bopp has received consulting fee and honoraria for lectures from Biogen, Celgene, Merck, Novartis, Pathios Therapeutics, Roche, Sanofi Genzyme, and Teva.
Data availability statement
Data will be provided upon reasonable request.
Additional information
Funding
References
- Robert Koch-Institut. Stellungnahme der STIKO anlässlich der Zulassung von XBB.1.5-Varianten-adaptierten COVID-19-Impfstoffen für die Auffrischimpfung von Personen mit erhöhtem Risiko für einen schweren Krankheitsverlauf; 2023. https://www.rki.de/DE/Content/Kommissionen/STIKO/Empfehlungen/Stellungnahme-COVID-19-Varianten-adaptierte-Impfstoffe.html.
- World Health Organization. COVID-19 advice for the public: getting vaccinated; 2023. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines/advice.
- Ziemssen T, Schlegel E, Groth M, Ettle B, Bopp T. Results on SARS-CoV-2 mRNA vaccine booster from an open-label multicenter study in ofatumumab-treated participants with relapsing multiple sclerosis. Vaccines (Basel). 2023;11(5):11. doi:10.3390/vaccines11050978.
- Ziemssen T, Groth M, Ettle B, Bopp T. Immune response to SARS-CoV-2 mRNA vaccines in an open-label multicenter study in participants with relapsing multiple sclerosis treated with Ofatumumab. Vaccines (Basel). 2022;10(12):2167. doi:10.3390/vaccines10122167.
- He A, Merkel B, Brown JWL, Zhovits Ryerson L, Kister I, Malpas CB, Sharmin S, Horakova D, Kubala Havrdova E, Spelman T, et al. Timing of high-efficacy therapy for multiple sclerosis: a retrospective observational cohort study. Lancet Neurol. 2020;19(4):307–4. doi:10.1016/S1474-4422(20)30067-3.
- Koeditz D, Frensch J, Bierbaum M, Ness N-H, Ettle B, Vudumula U, Gudala K, Adlard N, Tiwari S, Ziemssen T. Comparing the long-term clinical and economic impact of ofatumumab versus dimethyl fumarate and glatiramer acetate in patients with relapsing multiple sclerosis: a cost-consequence analysis from a societal perspective in Germany. Mult Scler J Exp Transl Clin. 2022;8(1):20552173221085741. doi:10.1177/20552173221085741.
- Carrillo J, Izquierdo-Useros N, Avila-Nieto C, Pradenas E, Clotet B, Blanco J. Humoral immune responses and neutralizing antibodies against SARS-CoV-2; implications in pathogenesis and protective immunity. Biochem Biophys Res Commun. 2021;538:187–91. doi:10.1016/j.bbrc.2020.10.108.
- Arunachalam PS, Lai L, Samaha H, Feng Y, Hu M, Hui HSY, Wali B, Ellis M, Davis-Gardner ME, Huerta C, et al. Durability of immune responses to mRNA booster vaccination against COVID-19. J Clin Invest. 2023;133(10):133. doi:10.1172/jci167955.
- Ziemssen T, Groth M, Winkelmann VE, Bopp T. Immune response to initial and booster SARS-CoV-2 mRNA vaccination in patients treated with siponimod—final analysis of a nonrandomized controlled clinical trial (AMA-VACC). Vaccines (Basel). 2023;11(8):11. doi:10.3390/vaccines11081374.
- Otto C, Schwarz T, Jeworowski LM, Schmidt ML, Walper F, Pache F, Schindler P, Niederschweiberer M, Krumbholz A, Rose R, et al. Humoral immune responses remain quantitatively impaired but improve qualitatively in anti-CD20-treated patients with multiple sclerosis after three or four COVID-19 vaccinations. Mult Scler. 2023;29(7):884–888. doi:10.1177/13524585231161253.
- Woopen C, Dunsche M, Al Rahbani GK, Dillenseger A, Atta Y, Haase R, Raposo C, Pedotti R, Ziemssen T, Akgün K, et al. Long-term immune response profiles to SARS-CoV-2 vaccination and infection in people with multiple sclerosis on anti-CD20 therapy. Vaccines (Basel). 2023;11(9):11. doi:10.3390/vaccines11091464.
- Schiavetti I, Inglese M, Frau J, Signoriello E, Caleri F, Stromillo ML, Ferrò MT, Rilla MT, Gandoglia I, Gazzola P, et al. Antibody response elicited by the SARS-CoV-2 vaccine booster in patients with multiple sclerosis: who gains from it? Eur J Neurol. 2023;30(8):2357–2364. doi:10.1111/ene.15830.
- Haase S, Krickl A-L, Freudenstein D, Burner F, Angstwurm K, Lee DH, Linker R. Subcutaneous anti-CD20 targeted B cell depletion preserves memory B cells and IgG serum levels in patients with RRMS (P280/442). Mult Scler J. 2023;29. doi:10.1177/13524585231196192.
- Cross AH, Delgado S, Habek M, Davydovskaya M, Ward BJ, Cree BAC, Totolyan N, Pingili R, Mancione L, Hu X, et al. COVID-19 outcomes and vaccination in people with relapsing multiple sclerosis treated with ofatumumab. Neurol Ther. 2022;11(2):741–758. doi:10.1007/s40120-022-00341-z.
