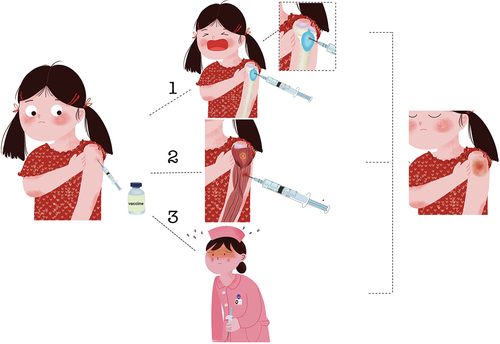
ABSTRACT
To prevent COVID-19, the COVID-19 vaccine has been widely administered worldwide, but various complications accompany this vaccine. The aim of this study was to investigate the demographic patterns, clinical features, diagnostic findings, and treatment outcomes associated with shoulder injury related to vaccine administration (SIRVA). This study examined 22 patients with SIRVA following COVID-19 vaccination from the Web of Science (WOS) and PubMed databases. The patients were categorized based on sex, age, type of COVID-19 vaccine received, dose administered, latency of symptom onset, and the presence of specific clinical manifestations. Patients, evenly distributed by sex (12 females, 10 males), and aged 21 to 84 years (mean age 46.6), were analyzed. SIRVA cases were reported across all age groups. The Pfizer – BioNTech COVID-19 vaccine had the highest incidence (n = 8), followed by the Oxford/AstraZeneca COVID-19 vaccine (n = 4). Symptoms, primarily shoulder pain (n = 22) and shoulder mobility disorders (n = 18), occurred within three days post-vaccination. Some patients also reported shoulder swelling (n = 5) and fever (n = 2). Imaging revealed nonspecific X-ray findings, supraspinatus tendon calcification (n = 2), and shoulder edema and inflammation on MRI (n = 12). This study provides insights into the clinical aspects of SIRVA related to COVID-19 vaccination. Recognition and appropriate management of these complications are crucial for optimal patient outcomes.
Introduction
Since the COVID-19 pandemic began, countries around the world have gradually completed vaccination against COVID-19, though this way followed by side effects, including fever and local pain. As the number of people vaccinated gradually increased, more SIRVA patients were reported.Citation1–3
SIRVA refers to shoulder pain and rigidity within hours after vaccine injection, due to incorrect injection of the vaccine into the shoulder joint bursa instead of the deltoid muscle. Moreover, because of improper muscle injection, unsuitable syringe selection or incorrect selection of the injection site, all the reasons above will cause accidental injection of the vaccine into the joint capsule below the deltoid muscle and its surroundings. Finally, inflammation is induced, even injuring the skeletal muscle system of the shoulder.Citation4
Currently, there is no systematic diagnosis or treatment plan for SIRVA, and treatment relies mostly on nonsteroidal anti-inflammatory agent (NSAID) drugs and glucocorticoids; moreover, some patients with combined tendon and bone injuries may require further surgical treatment. Therefore, how to effectively prevent SIRVA is particularly important.Citation5
In this study, we focused on analyzing reported SIRVA cases and contributing to the standardization of COVID-19 vaccine injection.
Method
Strategies
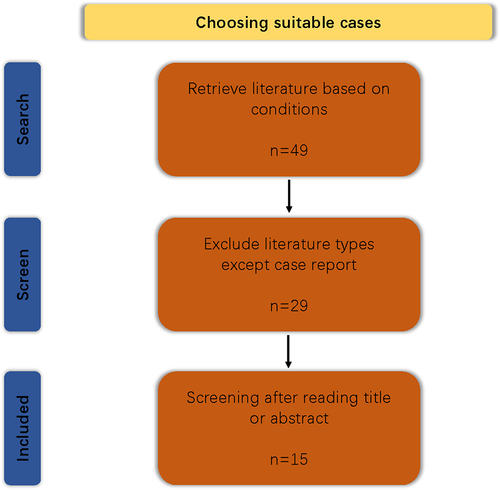
We used the Web of Science (WOS) database to obtain SIRVA patient information that appeared after COVID-19 vaccine injection without any restrictions. The search strategies used were as follows: TS = (“Covid-19 vaccine” OR “Coronavirus vaccine” OR “COVID-19 vaccination” OR “Coronavirus vaccination” OR “Covid-19 vaccinate” OR “Coronavirus vaccinate”) AND TS = (“Shoulder Injury” OR “Shoulder Pain” OR “SIRVA”). Moreover, the paper type was limited to “case reports.” The same strategies were applied in PubMed. Finally, duplicate and studies were excluded, as some patients with myocarditis after injection of the COVID-19 vaccine had clinical manifestations of shoulder pain, causing errors; the procedure is shown in . Inclusion criteria: 1. SIRVA related to COVID-19 vaccine. 2. Paper type is “case reports.” 3. Language limits to English. Exclusion criteria: Shoulder pain caused by other complications after injection of COVID-19 vaccine, without primary focus on the shoulder.
Study choices
After deleting duplicate and unrelated literature, we obtained a total of 16 articles and 19 cases. The following information was collected from these patients: 1. patient age; 2. sex; 3. vaccine type; 4. vaccine dose; 5. when symptoms appeared; 6. laboratory examination; 7. imaging examination; 8. treatment, and 9. outcomes. The data are shown in .
Table 1. Information of selected cases. Here, we summarized the basic information, vaccine information, physical examination, laboratory examination, imaging examination, treatment, and outcome of each case.
Table 2. Detailed information of subgroups. The frequency and proportion of different genders, age groups, vaccine types, injections, and causes of different diseases.
Results
Basic information
In this study, 19 patients were included in the analysis of SRIVA after receiving the COVID-19 vaccine. As shown in , there was no significant difference in the proportion of males to females (12; 10). Compared to those by sex, patients who underwent SIRVA after receiving the COVID-19 vaccine were distributed into all age groups; the oldest patient was 84 years old, the youngest was 21 years old, and the mean age was 45.8 years (n = 22).
Clinical features
Most of the SIRVAs were initiates after the patients received the Pfizer – BioNTech COVID-19 vaccination (n = 8; p = 36.4%), the second was the Oxford/AstraZeneca COVID-19 vaccine (n = 4; p = 18.2%), and the relationship between dose and the morbidity of SIRVA was inconspicuous (first dose: 7; second dose: 7; not recorded: 8).The latency of SIRVA was concentrated within three days, while the longest latency was 15 days (average 4 days). However, the etiology of SIRVA patients after COVID-19 vaccine has been unclear. However, false selection of the injection site (n = 6; p = 27.3%) was the top priority, given the record of the cause of SIRVA. Additionally, unprofessional practitioner (n = 2; p = 9.1%) and inappropriate syringe selection (n = 3; p = 13.6%) were the significant causes of SIRVA according to the records.
Examinations
In terms of SRIVA symptoms, all the patients suffered from shoulder pain (n = 22 p = 100%: severe: 12 p = 54.5%; mild: 10 p = 45.5%), and the majority of patients had shoulder mobility disorders (n = 18; p = 81.8%) regardless of whether they were positive or negative. Moreover, a minority of the SIRVA patients suffered from shoulder swelling (n = 5; p = 22.7%), and fever (n = 2; p = 9.1%). In addition, some of the Neer, Hawkins, Yocum, and O’Brien test results (n = 3; p = 13.6%) were positive, indicating rotator cuff injury and synovial lesions. There was only one patient with a positive Hornblower sign (n = 1; p = 4.5%), which may indicate a greater risk of recurrent rupture of the rotator cuff ligament group in the future. As shown in , only a few patients had laboratory test records. Based on the recorded results, SIRVA patients generally exhibited an increase in inflammatory indicators such as white blood cells C-reactive protein. Few patients underwent shoulder joint puncture fluid extraction (n = 2; p = 9.1%), and result showed shoulder joint injury and suppuration. On imaging, most patients who underwent SIRVA underwent X-ray, MRI and shoulder ultrasound only when shoulder swelling was severe. The X-ray imaging findings of most SIRVA patients were nonspecific, and some patients with a longer disease course may have had calcification of the supraspinatus tendon (n = 2; p = 9.1%). MRI often revealed edema and inflammation of the shoulder (n = 12; p = 54.5%). On ultrasound, inflammatory reactions in the shoulder could often be detected (n = 4; p = 18.2%).
Treatment and outcomes
In terms of SIRVA treatment, most patients only took oral painkillers in combination, while a small number of patients underwent intra-articular injection (n = 2). Surgical indications were available for only three patients, but only two patients underwent surgical treatment; only one specific surgical procedure, shoulder joint perfusion dilation surgery, was recorded. After standardized treatment, the vast majority of patients recover. However, two patients still experienced shoulder pain and movement disorders after treatment.
Discussion
With the large-scale use of COVID-19 vaccines, cervical cancer vaccines, influenza vaccines and other vaccines, the complication rate of vaccination in the future will increase daily. The incidence rate of SIRVA increased from 2.5% in 2011 to 41.9% in 2016 according to the National Vaccine Injury Compensation Program (VICP). Therefore, it is necessary to have a solid theoretical foundation and strong technical skills to reduce the fear of vaccination recipients and decrease the incidence rate of SIRVA.
Injury mechanisms
According to our collected information, patients were mechanically injured for various reasons. Mechanistically, SIRVA involves the injection of vaccines into the synovial sac of the shoulder joint or damage to the skeletal muscles, ligaments, or even bone tissue at the injection site.Citation6 The lack of professional practice by the practitioner, the incorrect choice of injection site and the improper selection of needles that we concluded ultimately led to injury of the shoulder musculoskeletal system are consistent with the results of previous studies. Additionally, consistent with our research, clinical trials in the U.S. showed that the COVID-19 mRNA vaccine, Pfizer-BioNTech vaccine and Moderna vaccine could easily cause an immune response; in other words, the immunogenicity was strong. This may be related to the protein shell and mRNA of the virus in the vaccine. Adjuvants are used routinely in vaccines, leading to enhanced immunogenicity of vaccines.Citation2 This could effectively explain why the highest incidence of SIRVA occurred after Pfizer-BioNTech vaccine injection.
Injection techniques
Immunogenicity can be achieved by providing vaccines through abundant blood vessels near the deltoid muscle and suitable adipose tissue which can be quickly identified by the immune system. Currently, five locations are used for deltoid muscle injection: A: transverse finger width 1–3 below the midpoint of the shoulder peak.Citation21 B: Middle one-third of the deltoid muscle.Citation22 C: The injection site is a triangular area, where a horizontal line is drawn 2.5–5 cm below the acromial process. The bottom of the triangle is the middle half of the horizontal line, and its vertex is inverted at the midpoint of the “arm side aligned with the armpit.”Citation23 D: This site is located ‘by drawing an imaginary horizontal line two to three finger breadths 2.5–5.0 cm below the lower edge of the acromion process.’ The acromion process was identified as the upper marker, the deltoid tuberosity (in line with the axilla) as the lower marker, and an imaginary triangle was drawn pointing downward from the acromion. The injection site is in the center of the triangle or the point halfway between the markers (it will be from one- to four-finger widths from the acromion, depending on the size of the arm).Citation24 E: The intersection between the anteroposterior axillary line (the line between the upper end of the anterior axillary line and the upper end of the posterior axillary line) and the line perpendicular to the mid-acromion.Citation25 A site is easy to distinguish, and is used frequently in the clinic. B site is an improvement over the A site and is more accurate than A site. C and D sites are the areas with the densest muscle tissue in the deltoid muscle, however, these sites are safer than A and B sites are, but the disadvantage is that they are difficult to identify. However, all four injection sites have been reported to cause vascular and/or nerve damage after injection.Citation26 E site was first proposed in 2017, and it was best to avoid the axillary nerve, anterior humeral artery, subacromial and deltoid bursa at this location. Research has shown that when receiving vaccination, patients should extend their arms by approximately 60 degrees and support their palms on the iliac bone, which can effectively reduce pain during injection and help medical staff grasp the injection location.Citation25 We have summarized the detailed information, advantages and disadvantages, as well as usage frequency of each injection site in . We documented that SIRVA was caused by “the incorrect injection site,” and most patients recalled “too high injection site.” This means that individuals with thinner deltoid coverage are more likely to be damaged.
Table 3. Information on commonly used injection sites for the deltoid muscle. Including its injection site, advantages and disadvantages, and frequency of use.
The selection of syringes should be judged based on the patient’s body shape. Long needles can lead to injection into the synovial sac and even damage the periosteum, while short needles can cause subcutaneous emphysema and ineffective vaccination. Researchers recommend the use of 16 mm needles for patients weighing 60 kilograms or less, 25 mm needles for women weighing 60–90 kilograms and for men weighing 60–118 kilograms. Patients weighing more than these weights were advised to use a 38 mm needle. In addition, some scholars believe that the selection of needles can be optimized by squeezing the skin to determine the thickness of subcutaneous tissue.Citation27 A total of three patients were categorized as inappropriate syringe selection according to our results. A 52-year-old female weighted 58 kg received injection of the vaccine via a 1-inch length (25 mm) syringe. A standard weight 52-year-old male was injected with the vaccine via a 1.5-inches syringe (38 mm). Another 55-year-old female suffered from SIRVA caused by 1 (25 mm) inch syringe selection. The incorrect needle selection in these patients was consistent with the results discussed above.Vaccination should be accomplished by medical technicians. However, nearly one-third of influenza vaccines were injected by pharmacists who were not qualified for vaccination in America. During the COVID-19 pandemic, the United States even passed bills to allow pharmacy staff and some interns to inject vaccines. The influx of large-scale nonprofessional practitioners was also a reason for the increasing incidence of vaccine related complications.Citation8
Diagnosis, treatment and prognosis
The most common side effect of deltoid muscle vaccination is local redness, swelling, and pain, which can be relieved within a few days However, when the pain persists or gradually intensifies, SIRVA such as adhesive cystitis, tendonitis, or subacromial deltoid synovitis, should be considered. Imaging examination and joint cavity puncture play important roles in the diagnosis of SIRVA. At present, there is no unified diagnosis or treatment method for SIRVA internationally, but most patients can recover through oral anti-inflammatory and analgesic drugs and physical therapy. Early identification markers for SIRVA have been proposed: 1. Shoulder pain occurred <48 h after injection. 2. The patient had a duration of at least 7 days. 3. Restricted range of motion. 4. No symptoms before vaccination. Patients for whom oral medication was ineffective could choose intra-articular injection. Surgical treatment may be considered for patients with ineffective conservative treatment or secondary suppurative arthritis. Most patients have a good prognosis, but a small number of patients may experience residual shoulder pain and mild mobility disorders.Citation28
Limitations
This study has several limitations. First, all the objects were derived from case reports through December 11, 2023. Some reviews extensively reported on this case but lacked detailed information and some academic research lags, resulting in this study not including all cases. Although this study summarized the case characteristics of COVID-19-related SIRVA, analyzed the etiology of SIRVA, and tried to avoid SIRVA as much as possible, we did not propose effective new solutions. Moreover, due to the different focuses of each case series and the lack of data, this study was unable to analyze the collected results in depth.
Conclusion
SIRVA is a common complication of COVID-19 vaccine injection even any other vaccine, and has various causes. Although most patients have a good prognosis after receiving formal treatment, a small number of severe patients also require shoulder joint puncture, fluid extraction, or even surgical treatment. Therefore, it is necessary to provide training for practitioners and choose different injection points and needles for different patients. Finally, a timely diagnosis of SIRVA and an optimal treatment plan should be established. In this way, the incidence rate of SIRVA could be reduced, and the prognosis of SIRVA patients could be guaranteed.
Author contributions
LZX was the authors of the paper’s main body producers. CSJ and ZMY were the leading designer of this study and provided us with research ideas and directions.
abstract graphica.jpg
Download JPEG Image (896.2 KB)Acknowledgments
The authors thank everyone who has contributed to this study, especially Dan Li who provided a schematic diagram for this article.
Supplemental data
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2024.2321672
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors without undue reservation.
Additional information
Funding
References
- Eisenberg MT, Tingey C, Fulton O, Owen J, Snyder T. Quadrilateral space region inflammation and other incidental findings on shoulder MRI following recent COVID-19 vaccination: three case reports. Radiol Case Rep. 2021 Oct;16(10):3024–16. doi:10.1016/j.radcr.2021.07.028.
- Honarmand AR, Mackey J, Hayeri R. Shoulder injury related to vaccine administration (SIRVA) following mRNA COVID-19 vaccination: report of 2 cases of subacromial-subdeltoid bursitis. Radiol Case Rep. 2021 Dec;16(12):3631–4.
- Klabklay P, Chuaychoosakoon C. Septic arthritis of shoulder joint following a COVID-19 vaccination: a case report. Int J Surg Case Rep. 2022 Oct;99:107686. doi:10.1016/j.ijscr.2022.107686.
- Atanasoff S, Ryan T, Lightfoot R, Johann-Liang R. Shoulder injury related to vaccine administration (SIRVA). Vaccine. 2010 Nov 29;28(51):8049–52.
- Macomb CV, Evans MO, Dockstader JE, Montgomery JR, Beakes DE. Treating SIRVA early with corticosteroid injections: a case series. Mil Med. 2020 Feb 12;185(1–2):298–300. doi:10.1093/milmed/usz269.
- Yuen WLP, Loh SYJ, Wang DB. SIRVA (shoulder injury related to vaccine administration) following mRNA COVID-19 vaccination: case discussion and literature review. Vaccine. 2022 Apr 20;40(18):2546–50.
- Mayer EN, Gajewski CR, Bernthal NM, Jensen AR. Arthroscopic debridement for acute hemorrhagic subacromial bursitis following COVID-19 vaccine administration: a case report. Shoulder Elbow. 2023 Oct;15(5):527–533. doi:10.1177/17585732221090821.
- Flores C, Choate WS, Tupler R. Shoulder injury related to vaccine administration. Ochsner J. 2022;22(3):261–264. doi:10.31486/toj.21.0114.
- Kashkosh A, Peake CM, Narvani AA, Imam MA. Spontaneous avascular necrosis of the humeral head following COVID-19 vaccination. Arch Bone Jt Surg. 2023;11(2):140–143. doi:10.22038/ABJS.2022.67994.3243.
- Aldosary AH. Prolonged shoulder dysfunction after coronavirus disease vaccination: a case of shoulder injury related to vaccine administration. SAGE Open Med Case Rep. 2022;10:2050313X221089494. doi:10.1177/2050313X221089494.
- Boonsri P, Chuaychoosakoon C. Combined subacromial-subdeltoid bursitis and supraspinatus tear following a COVID-19 vaccination: a case report. Ann Med Surg (Lond). 2021 Sep;69:102819. doi:10.1016/j.amsu.2021.102819.
- Nakajima K, Miyata A, Kato S, Oshima Y, Tanaka S. Calcific tendinitis of the shoulder induced by an mRNA vaccine for COVID-19: a case report. Mod Rheumatol Case Rep. 2023 Jan 3;7(1):211–4. doi:10.1093/mrcr/rxac006.
- Cantarelli Rodrigues T, Hidalgo PF, Skaf AY, Serfaty A. Subacromial-subdeltoid bursitis following COVID-19 vaccination: a case of shoulder injury related to vaccine administration (SIRVA). Skeletal Radiol. 2021 Nov;50(11):2293–7.
- Miyano M, Tsukuda Y, Hiratsuka S, Hamasaki M, Iwasaki N. Chronic shoulder injury related to vaccine administration following coronavirus disease 2019 vaccination: a case report. J Med Case Rep. 2023 Oct 17;17(1):456. doi:10.1186/s13256-023-04198-0.
- Chuaychoosakoon C, Parinyakhup W, Tanutit P, Maliwankul K, Klabklay P. Shoulder injury related to Sinovac COVID-19 vaccine: a case report. Ann Med Surg (Lond). 2021 Aug;68:102622. doi:10.1016/j.amsu.2021.102622.
- Sukhija S, Singh S, Saxena S, Ambwani S, Khera PS. Shoulder injury related to vaccine administration (SIRVA) with COVID-19 vaccination - a case report. J Family Med Prim Care. 2022 Dec;11(12):7937–40.
- Sahu D. Shoulder pain and injury after COVID-19 vaccination. Yale J Biol Med. 2022 Jun;95(2):217–220. doi:10.3390/vaccines9050502.
- jpts-35-831.pdf.
- Wharton BR, Doan KC, Wolcott ML. Shoulder injury related to COVID-19 vaccine administration: a case report. JSES Rev Rep Tech. 2022;2(2):178–181. doi:10.1016/j.xrrt.2021.10.005.
- Moya D, Gómez D, Patiño P, Altamirano NN, Balzarini M, Freitag K. Shoulder injury related to vaccine administration following misplaced SARS-CoV-2 vaccination: a case report and review of literature. J Orthop Case Rep. 2022;12(3):100–3. doi:10.13107/jocr.2022.v12.i03.2736.
- Beyea SC, Nicoll LH. Administration of medications via the intramuscular route: an integrative review of the literature and research-based protocol for the procedure,” (in eng. Appl Nurs Res. 1995 Feb;8(1):23–33. doi:10.1016/S0897-1897(95)80279-7.
- Gray R, Spilling R, Burgess D, Newey T. Antipsychotic long-acting injections in clinical practice: medication management and patient choice,” (in eng), Br J Psychiatry Suppl. 2009 Nov;52:S51–6. doi:10.1192/bjp.195.52.s51.
- Cook IF. Best vaccination practice and medically attended injection site events following deltoid intramuscular injection. Hum Vaccin Immunother. 2015;11(5):1184–91. doi:10.1080/21645515.2015.1017694.
- Cocoman A, Murray J. Intramuscular injections: a review of best practice for mental health nurses. J Psychiatr Ment Health Nurs. 2008 Jun;15(5):424–34. doi:10.1111/j.1365-2850.2007.01236.x.
- Nakajima Y, Mukai, K., Takaoka, K., Hirose, T., Morishita, K., Yamamoto, T., Yoshida, Y., Urai, T., Nakatani, T. Establishing a new appropriate intramuscular injection site in the deltoid muscle. Hum Vaccin Immunother. 2017;13(9):2123–2129. doi:10.1080/21645515.2017.1334747.
- Cook IF. An evidence based protocol for the prevention of upper arm injury related to vaccine administration (UAIRVA). Hum Vaccin. 2011 Aug;7(8):845–8.
- Nakajima Y, Mukai K, Takaoka K, Hirose T, Morishita K, Yamamoto T, Yoshida Y, Urai T, Nakatani T. Establishing a new appropriate intramuscular injection site in the deltoid muscle. Hum Vaccin Immunother. 2017 Sep 2;13(9):2123–9. doi:10.1080/21645515.2017.1334747.
- Veera S, Chin J, Kleyn L, Spinelli S, Tafler L. Use of osteopathic manipulation for treatment of chronic shoulder injury related to vaccine administration. Cureus. 2020 Jul 12;12(7):e9156. doi:10.7759/cureus.9156.