ABSTRACT
Rotavirus (RV) vaccines were first introduced in 2011 and adopted for universal vaccination in 2020 in Japan. However, the effectiveness of RV vaccines after being adopted for universal vaccination in 2020 has not been reported. Because of the easy accessibility of clinics in Japan, many children are not usually hospitalized for RV gastroenteritis (RVGE). Therefore, in order to evaluate the impact of the RV vaccine since 2008, we investigated the incidence of hospitalization for RVGE as well as the frequency of children aged < 5 years who received medical treatment for severe RVGE at clinics in Shibata City, Japan. The RV vaccine coverage rate was 94.0% (1,046/1,113) in Shibata City after universal vaccination in 2020; this was a significant increase from previous rates. The incidence per 1000 person – years for RVGE hospitalization and severe RVGE at clinics were significantly higher among children aged < 3 years than in previous time periods. The incidence in children with all acute gastroenteritis (AGE) decreased significantly after universal vaccination during the COVID-19 pandemic. The proportion of severe RVGE among all AGE cases also decreased significantly after universal vaccination among children aged < 3 years (0.0%) and those aged 3–4 years (0.6%). There were significant differences in the distribution of RV genotypes isolated from the feces of children with RVGE between different eras divided by RV vaccination rates, especially G1P[8], which was the major genotype before it recently almost disappeared. Further studies are warranted to assess the impact of the COVID-19 pandemic.
Introduction
Rotavirus (RV) is one of the pathogens responsible for acute severe gastroenteritis (GE), especially in children younger than 5 years of age. RV causes many deaths among children globally.Citation1 There are fewer fatalities among children with RV in Japan than in fewer countries; however, almost all children under 5 years of age have had RV infection causing GE (RVGE) in Japan, similar to the situation in other countries.Citation1,Citation2
To prevent RVGE, four RV vaccines, RotarixⓇ (RV1; GlaxoSmithKline; Rixensart, Belgium), RotaTeqⓇ (RV5; Merck & Co., Inc., Rahway, NJ, USA), ROTAVACⓇ (Bharat Biotech, Hyderabad, India), and Rotasiil (Serum Institute of India, Pune, India) have been approved in more than 100 countries.Citation3 In Japan, these vaccines were first introduced in 2011 and 2012, and several reports have indicated the effectiveness of the RV vaccine in lowering the incidence of RVGE hospitalizations among children <5 years old in Japan.Citation4–7
However, there are some differences in access to medical centers between Japan and other countries. In Japan, many children with RVGE receive outpatient fluid transfusion at clinics, which helps prevent hospitalization. We analyzed data from children with RVGE who were admitted to hospitals since 2008 and those treated with outpatient fluid transfusion at clinics since 2011.Citation8
In our previous report, we studied Japanese children aged <3 years, which was considered the target age in reports on the effectiveness of RV vaccination.Citation9–11 However, many children aged <5 years, including those aged 3 years and 4 years, develop RVGE. The RV vaccine was adopted as part of the universal vaccination schedule in October 2020 in Japan, after our last report.Citation12 No similar studies have been conducted in children under 5 years of age. Therefore, we investigated the impact of the RV vaccine in children <5 years of age after universal vaccination in Japan.
Materials and methods
Study participants
This prospective, observational study was conducted in Shibata City, which has a population of approximately 100,000, and approximately 600 births annually. We collaborated with pediatricians at four primary health care pediatric clinics and one hospital in Shibata City. We enrolled children <5 years of age with acute AGE who (1) visited the four pediatric clinics most visited by children in Shibata City from February 2012 to May 2022 or (2) were admitted to Niigata Prefectural Shibata Hospital from 2008 to 2022. Niigata Prefectural Shibata Hospital is the only hospital with a pediatric ward in Shibata city, and all children in the city are usually admitted there.
Since these outpatient clinics cater to almost the entire population of Shibata City, we made the assumption that all cases of AGE among children were covered during the study duration.
Study items
When enrolling children <5 years old in the study, information on dates of visits, date of birth, sex, residence location, symptoms such as diarrhea (loose, watery stools three or more times a day) and vomiting, and intravenous administration of rehydration fluids were collected.
We defined AGE based on the occurrence of symptoms such as diarrhea and vomiting lasting no more than 14 days. Severe AGE was defined when the patient required intravenous rehydration. Furthermore, we took fecal samples for RV antigen testing using the ImmunoCardTM ST Rotavirus (catalog number: 750030; Fujirebio Inc., Tokyo, Japan) and RapidTestaⓇ ROTA-ADENO (Sekisui Medical Inc., Tokyo, Japan), for which the cut off was 1.1 × 10−6 mg/mL from all children with suspected severe AGE.
We defined severe RVGE as positive RV antigens in fecal samples and the need for intravenous rehydration. To ensure objective introduction of intravenous rehydration, we calculated scores on the Vesikari scale for children, which indicated the severity of dehydration, by collecting information from enrolled children.Citation13 Oral informed consent was obtained from the parents/guardians of the pediatric patients before participation in the study. Relevant information was extracted from medical charts and analyzed only for children who fulfilled the inclusion criteria.
The numbers of hospitalized patients with AGE and RVGE at the Niigata Prefectural Shibata Hospital were calculated retrospectively. We evaluated RV vaccine coverage rates using vaccination records from the four pediatric clinics, Shibata Hospital, and Tomita gynecologic clinic, which are the main administrators of vaccinations for children in the area. We counted the full RV vaccination as one single vaccination. The researchers performing the laboratory examinations were blinded to participant vaccination statuses, which were collected after the examinations. The research period was divided into four eras based on vaccination rates as follows. First was the pre-vaccination era when RV vaccines had not been introduced yet in 2008–2011. Second was the transitional era when the vaccine coverage rates were increasing yearly in 2012–2014. Third was the popularization era when vaccine coverage rates almost plateaued in 2015–2020. Fourth was the universal era after the RV vaccine was adopted as a universal vaccination in Japan in 2021–2022. However, we started this study in 2012 in outpatients after the introduction of the RV vaccine in Japan; for these outpatients, we divided the time into three periods, the transitional era, popularization era, and universal era, and we did not include the pre-vaccination era. For inpatients, we divided the observation period into the above four eras in 2008–2022.
Fecal samples were taken at the first visit after symptom onset. RV genotyping was performed using fecal samples from patients with RVGE. Reverse transcription-polymerase chain reaction was performed as previously described for the samples at the Niigata Prefectural Institute of Public Health and Environmental Sciences.Citation14
Statistical analyses
Incidences were calculated from the number of patients who visited the four clinics during each observation period (by May 31) and the number of children who lived in Shibata City. Fisher’s exact or Chi-square tests were performed to examine the statistically significant differences in incidence rate and in the ratio of severe RVGE in all AGE among different eras. We used a Poisson model to compare the hospitalization rate among eras and age groups. The normality of the 20-point Vesikari scale data was checked using the Shapiro‒Wilk test, and t-tests were performed to compare the scores between eras.
For all analyses, two-tailed p-values <.05 were considered statistically significant. All statistical analyses were performed using STATA (v14; StataCorp, College Station, TX, USA) and Microsoft Excel (Microsoft Corp., Redmond, WA, USA).
Ethical adherence
This study complied with the Ethical Guidelines for Epidemiological Research (Partial revision: December 1, 2008, The Ministry of Education, Culture, Sports, Science, and Technology; Ministry of Health, Labor, and Welfare), which is based on the guiding principles of the Declaration of Helsinki – Ethical for Medical Research Involving Human Participants, and the Ethics Guidelines for Medical Research for Humans (December 22, 2014, The Ministry of Education, Culture, Sports, Science, and Technology; Ministry of Health, Labor, and Welfare). Before conducting the study, the Ethical Review Committee of the Niigata Prefectural Shibata Hospital reviewed the ethical and scientific aspects of the study protocol (e.g., study design, population, and time period) and approved the proposed study (approval number: 65).
Results
RV vaccine coverage rates (RV1 and/or RV5) in Shibata City
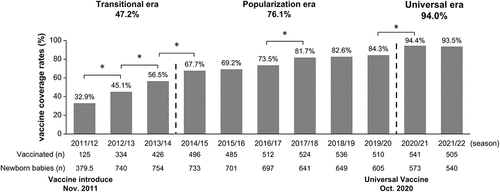
As shown in , the annual RV vaccine coverage rate in Shibata City increased significantly from 2011/2012, when the RV vaccine was first introduced, to 2014/2015. The rate gradually increased until 2019/2020, and it again increased significantly in 2020/2021, when it was adopted as a universal vaccine (p < .05). The RV vaccine coverage rates in each of the three periods were 47.2%, 76.1%, and 94.0%, respectively.
Figure 1. Vaccine coverage rates for RV1 and RV5 in Shibata City by season.
Incidences of severe RVGE, severe AGE, and all AGE years
The incidences of severe RVGE and severe AGE, as well as all cases of AGE, per year among children aged <3 years () and those aged 3–4 years were calculated (). The average incidences of severe RVGE and severe AGE in each era among children aged <3 years and those aged 3–4 years significantly decreased over time (p < .05) (). The incidence of all AGE among children aged 3–4 years also significantly decreased with time (p < .05) (). However, the incidence of all AGE among children aged <3 years at the four clinics significantly increased between the transitional and popularization eras (p < .05) ().
Figure 2. Incidence rates of severe RVGE (A), severe AGE (B), and AGE (C) among children younger than 3 years of age. Incidence rates of severe RVGE (D), severe AGE(E), and AGE (F), among children from 3 to 4 years of age. Values in graphs represent averages.
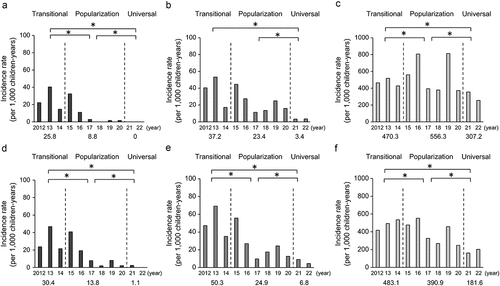
In comparisons of the incidences of severe RVGE, severe AGE, and all AGE between children aged <3 years and those aged 3–4 years, there were no significant differences other than in the incidence of AGE between the two age groups in both the popularization and universal eras.
Comparison of the proportion of severe RVGE to all AGE and Vesikari scale scores of patients with severe RVGE
presents the proportion of severe RVGE in all AGE and 20-point Vesikari scale scores among children aged <3 years and those aged 3–4 years. The proportion of severe RVGE in all AGE was significantly reduced among children aged <3 years. The ratio among children aged 3–4 years was not significantly reduced; however, it significantly differed between the transitional and universal eras (p < .05). There was no difference in the 20-point Vesikari scale scores between the transitional and popularization eras among children aged 3–4 years.
Table 1. Proportion of severe RVGE to all AGE per 1000 person-years and mean scores of Vesikari scales for patients with severe RVGE according to the era.
Hospitalization for RVGE and GE
The hospitalization rates per era in Shibata City (/1,000 person – years) and the annual number of hospitalizations at Shibata Hospital due to AGE and RVGE among children aged <3 years and those aged 3–4 years are shown in .
Figure 3. Hospitalization rates and annual number of hospitalizations for RVGE and AGE in Shibata City (/1,000 person-years) among children younger than 3 years of age (a) and those aged 3 to 4 years (b).
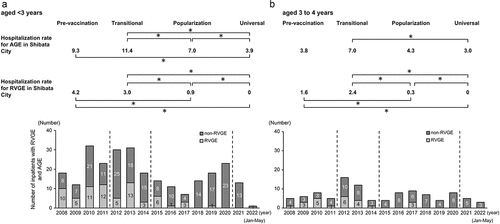
Among children aged <3 years, the hospitalization rate for AGE significantly decreased with the eras except for between the pre-vaccination and transitional eras and between pre-vaccination and popularization eras. Among children aged 3–4 years, the hospitalization rate for AGE decreased significantly only between the transitional and universal eras. For both age groups, the hospitalization rate for RVGE was significantly reduced between eras except for between the pre-vaccination and transitional eras and between the pre-vaccination and popularization eras.
Comparing the hospitalization rates for RVGE and AGE between children aged <3 years and those aged 3–4 years, there were significant differences in the incidence of RVGE in the pre-vaccination era and in the incidence of AGE in the pre-vaccination, transitional, and popularization eras between the two age groups.
Comparison of the proportion of hospitalization for RVGE to the hospitalization for all AGE by eras
presents the comparison of the proportion of RVGE in all AGE hospitalizations among children aged <3 years and those aged 3–4 years by era. In children <3 years of age, there were significant differences in the proportion of RVGE in all AGE hospitalizations between any two eras except between the popularization and universal eras. There were significant differences between the pre-vaccination and popularization eras, pre-vaccination and universal eras, and transitional and popularization eras in those aged 3–4 years.
Table 2. Proportion of hospitalization for RVGE to all AGE according to the era.
Comparison of the distribution of RV genotypes detected among three eras
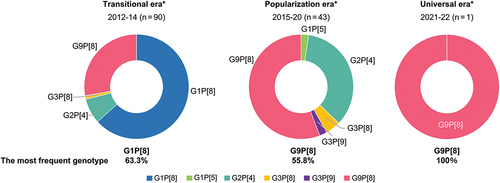
Comparisons of the distribution of RV genotypes in samples isolated from participants are indicated in . The predominant RV genotype combination in the transitional era was G1P[8], while G9P[8] dominated in the popularization and universal eras. There were significant differences in the distribution of RV genotypes between eras.
Figure 4. Distributions of detected RV genotypes in three eras. There were significant differences in the distribution of RV genotypes isolated from the feces of children with RVGE between different eras divided by RV vaccination rates.
Discussion
Since RV vaccines were adopted as a universal vaccination in Japan in October 2020, there have been few reports on the effectiveness of RV vaccines in Japan. The RV vaccine was introduced in 118 countries as of the end of 2021.Citation15 Among the 70 countries that adopted national immunization programs for the RV vaccine, the average RV coverage rate was 82% in 2016.Citation16 Therefore, the 94% vaccine coverage rate in the universal era in our study was very high compared with other countries. The global coverage RV vaccination is 49%,Citation15 which is similar to the average coverage rate in the transitional era in Japan.
The incidence of severe RVGE significantly decreased over time not only among children <3 years of age but also among children aged 3–4 years, including those who were unable to receive vaccinations and those for whom vaccine effectiveness may have waned. Thus, the RV vaccine seems to have a strong impact on vaccinated as well as unvaccinated children,Citation17 and its popularization has reduced the incidence of severe RVGE among vaccinated and unvaccinated children in Japan.
Strict preventive measures for infections due to the COVID-19 pandemic reduced the incidence of AGE, including severe RVGE.Citation18–20 In our research, the incidence of severe RVGE in the popularization era was significantly lower than that in other eras in both age groups (). Furthermore, the proportion of severe RVGE in all AGE also significantly decreased in both age groups except for between the transitional and popularization eras in children aged 3–4 years (). This suggests that RV vaccinations were more effective in the popularization era than in the previous era. The incidence of all AGE among children aged <3 years increased significantly during the popularization era (); this can be attributed to the prevalence of GE types other than RVGE in this age group. Hospitalization rates for RVGE were significantly higher in the pre-vaccination and transitional eras than in the other eras.
Some studies have analyzed hospitalization rates after the introduction of RV vaccines in Japan, and the number of hospitalizations for RVGE has decreased since 2014 in these studies.Citation4–7 RV vaccine coverage rates ranged from 37.9% to 67.2% at the time. The coverage rate in the present study was 56.5%, which is within this range, as shown in . The hospitalization rates for RVGE in the popularization era (after 2014) were significantly lower than those in the previous years. Therefore, the trends in hospitalization for RVGE are similar in Japan. Between the two age groups, there was a significant difference in the hospitalization rate for RVGE in the pre-vaccination era; however, there was no difference in other eras. It is suggested that there was more hospitalization for RVGE among children aged <3 years and those aged 3–4 years in the pre-vaccination era; however, we believe the reason for this is the rapid decrease in the number of hospitalizations for children aged <3 years and the gradual decrease in the number of hospitalizations for children aged 3–4 years after the introduction of RV vaccinations. Therefore, the difference in hospitalization rates for RVGE between the two age groups became smaller in each era, exhibiting no significance.
Although there was a significant reduction of the incidence of severe RVGE between the popularization and universal eras, there were no significant differences in hospitalization rates for RVGE and for all AGE (). This is possibly because the total number of hospitalizations for all AGE during the COVID-19 pandemic was very small compared with the total number of visitors at clinics.
The distribution of RV genotypes was also significantly different among the eras. It was described in the report on the distributions of RV genotypes in Sapporo, Japan that the dominant GP genotype has changed after the introduction of RV. Specifically, the five major GP genotypes, including G1P[8], G2P[4], G3P[8], G4P[8], and G9P[8] decreased after 2012, and new GP genotypes have emerged.Citation21 However, there are some reports on the reemergence of G9P[8], including reassortants.Citation22–24 Therefore, we should continue to observe genotype transitions in the future.
Our study had some limitations. First, there were no data form the pre-vaccination era at clinics. We obtained data from children <3 years of age from only one year, 2011. The incidence of severe RVGE was 77.1 (per 1,000 children – years) at clinics.Citation12 As shown in , there were some differences in the number of hospitalizations for RVGE between the pre-vaccination eras. Therefore, it is difficult to assert whether there was a difference in the incidence rate of severe RVGE at clinics between the pre-vaccination and transient eras using only the 2011 data. Second, as previously mentioned, our study might have been affected by the COVID-19 pandemic. However, the ratio of severe RVGE cases to all AGE cases also decreased significantly between before and after the COVID-19 pandemic (). This suggests that the real incidence rate of severe RVGE also decreased significantly in the universal era compared with that in the popularization era. Third, children with RVGE who were treated with outpatient fluid transfusion at clinics were considered as having severe RVGE. There could be some differences among such children because of subjective evaluations by the clinician. However, we also checked the Vesikari scales for each patient with severe RVGE, and there was no significant difference among the eras (). Therefore, the evaluation for severe RVGE at clinics is thought to be reasonable. Whereas there were no scales for severe AGE other than RVGE. Therefore, it was not able to deny that there might be some bias for the diagnosis of severe AGE among clinicians. However, we make a guess that there might be not so much difference among them from the points of view that there was no significant difference in severe RVGE. Fourth, we also investigated RV serotypes, but not enough stool was collected from patients with severe RVGE for genotyping, and we could not take samples from all patients with severe RVGE. Therefore, we could not check the severity of RVGE for children from whom samples for RV serotypes were collected. Therefore, the correlation between serotypes and severity is unclear. Furthermore, the number of samples was too small, especially in the universal vaccination era because of rapid decreasing the children with RVGE. Therefore, we need to take more samples to clarify if there are any differences or not in RV serotypes between before and after universal vaccinations in the future. In addition to the conventional binary classification system, an extended RV genotyping system was established in 2008 based on the sequences of all 11 genome segments to clarify RV epidemiology and evolution.Citation25 Based on this classification, an unusual strain, DS-1-like G1P[8], was detected in Japan.Citation26,Citation27 This strain is thought to be a double-reassortant strain derived from the recombination of the human Wa-like G1P[8] and human DS-1-like backbone strains.Citation27 Furthermore, the other RV vaccine genotype including the equine-like G3 and Unusual G9P[8] were reported even after RV vaccine introduction in Japan.Citation28,Citation29 Therefore, we will investigate the serotypes including the sequences of all 11 genome segments in the future. Lastly, intussusception (IS) is a major side effect of the RV vaccine, but we did not choose to investigate the frequency of IS in our study. Based on the report on IS in Japan, the incidence rates of patients with IS aged <1 year (susceptible age) were 102.8 per 100,000 person-years in the pre-vaccination era (2007‒2011) and 94.0 in the post-vaccination era (2012‒2014), respectively.Citation30 That is, the incidence of IS did not increase after the introduction of RV vaccine. The number of births was 570 in Shibata City in 2022. Therefore, if we adopt the incidence rates of patients aged <1 year with IS in Japan,Citation30 those in Shibata City are expected to be almost zero through the pre- and post-vaccination eras. Therefore, the effect of IS was not investigated in this study.
We investigated the changes in vaccine coverage and AGE incidence, including severe RVGE, over different periods among Japanese children <5 years of age until the establishment of universal RV vaccination in Japan. The RV vaccine coverage rate was > 90% after universal vaccination. The incidences for hospitalization for RVGE and severe RVGE at clinics, and that of all AGE, were significantly reduced after universal vaccination during the COVID-19 pandemic. The ratio of cases of severe RVGE to all AGE at clinics significantly decreased after universal vaccination among children <3 years of age and those aged 3–4 years. Further follow-up studies are needed after the COVID-19 pandemic.
Author contributions
Tomohiro Oishi conceptualized the study and drafted the original manuscript. Satoshi Hasegawa, Tokushi Nakano, Shoji Sudo, Hiroaki Kuwajima, and Shuko Tokuriki took samples and supervised the study. Tsutomu Tamura contributed to data analysis and interpretation. All the authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work.
Acknowledgments
We would like to thank Professor Takashi Nakano at the Department of Pediatrics, Kawasaki Medical School for his helpful advice; Dr. Masatoshi Tomita at Tomita Obstetrics and Gynecology Clinic for providing medical records of rotavirus vaccination; Dr. Tetsuo Taguchi, Dr. Shinya Tsukano, and Dr. Masamichi Matsunaga at Niigata Prefectural Shibata Hospital for supervising the progress the study; and Seiko Naka at MC&P Co., Ltd. for her assistance with statistical analysis and study reports.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author, T.O., upon reasonable request.
Additional information
Funding
References
- Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD. WHO-coordinated global rotavirus surveillance network. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:136–9. doi:10.1016/S1473-3099(11)70253-5.
- Cortese MM, Parashar UD. Prevention of rotavirus gastroenteritis among infants and children: recommendations of the advisory committee on immunization practices (ACIP). MMWR. 2009;58:1–25.
- Yen C, Tate JE, Hyde TB, Cortese MM, Lopman BA, Jiang B, Glass RI, Parashar UD. Rotavirus vaccines: current status and future considerations. Hum Vaccin Immunother. 2014;10:1436–48. doi:10.4161/hv.28857.
- Yoshikawa T, Matsuki T, Sato K, Mizuno M, Shibata M, Hasegawa S, Morita M, Iwasa M, Gopala K, Holl K. Impact of rotavirus vaccination on the burden of acute gastroenteritis in Nagoya city, Japan. Vaccine. 2018;36:527–34. doi:10.1016/j.vaccine.2017.12.006.
- Asada K, Kamiya H, Suga S, Nagao M, Ichimi R, Fujisawa T, Umemoto M, Tanaka T, Ito H, Tanaka S. et al. Rotavirus vaccine and health-care utilization for rotavirus gastroenteritis in Tsu city, Japan. Western Pac Surveill Response J. 2016;7:28–36. doi:10.5365/WPSAR.2016.7.3.005.
- Ito H, Kawakatsu H, Yoshioka H, Takeuchi K. The study of introduction effects of rotavirus vaccine in Kyoto prefecture. J Kyoto Med Assoc. 2015;62:89–95. [ Japanese].
- Yamamoto K, Tokuda K, Kawano Y, Nishi J. The surveillance of rotavirus gastroenteritis hospitalization in Kagoshima Prefecture, Japan during a 6-year period spanning the introduction of the rotavirus vaccine. The 46th Annual meeting of the Japanese Society for Pediatric Infectious Diseases; 2014 Oct 18–19. Tokyo, Japan [Japanese].
- Oishi T, Tsukano S, Nakano T, Sudo S, Kuwajima H, for the Shibata RVGE Study Group. Impact of rotavirus vaccination in severe rotavirus gastroenteritis outpatient visits at three pediatric primary care clinics in Shibata City, Niigata Prefecture, Japan. Open J Pediatr. 2014;4:291–9. doi:10.4236/ojped.2014.44040.
- Vesikari T, Karvonen A, Ferrante SA, Ciarlet M. Efficacy of the pentavalent rotavirus vaccine, RotaTeq®, in Finnish infants up to 3 years of age: the Finnish extension study. Eur J Pediatr. 2010;169:1379–86. doi:10.1007/s00431-010-1242-3.
- Vesikari T, Karvonen A, Ferrante SA, Kuter BJ, Ciarlet M. Sustained efficacy of the pentavalent rotavirus vaccine, RV5, up to 3.1 years following the last dose of vaccine. Pediatr Infect Dis J. 2010;29:957–63. doi:10.1097/INF.0b013e3181e28e6e.
- Phua KB, Lim FS, Lau YL, Nelson EAS, Huang LM, Quak SH, Lee BW, van Doorn LJ, Teoh YL, Tang H, et al. Rotavirus vaccine RIX4414 efficacy sustained during the third year of life: a randomized clinical trial in an Asian population. Vaccine. 2012;30:4552–7. doi:10.1016/j.vaccine.2012.03.030.
- Oishi T, Matsunaga M, Nakano T, Sudo S, Kuwajima H, Tokuriki S, Study SR. Occurrence of severe rotavirus gastroenteritis in children younger than three years of age before and after the introduction of rotavirus vaccine: a prospective observational study in four pediatric clinics in Shibata City, Niigata Prefecture, Japan. Hum Vaccin Immunother. 2020;16:2495–501. doi:10.1080/21645515.2020.1720435.
- Freedman SB, Eltorky M, Gorelick M, Pediatric Emergency Research Canada Gastroenteritis Study Group. Evaluation of a gastroenteritis severity score for use in outpatient settings. Pediatrics. 2010;125:1278–85. doi:10.1542/peds.2009-3270.
- Tamura T, Nishikawa M, Anh DD, Suzuki H. Molecular epidemiological study of rotavirus and norovirus infections among children with acute gastroenteritis in Nha Trang, Vietnam, December 2005–June 2006. Jpn J Infect Dis. 2010;63(6):405–11. doi:10.7883/yoken.63.405.
- WHO. Immunization coverage. [accessed 2023 Jun 16]. https://www.who.int/news-room/fact-sheets/detail/immunization-coverage.
- Abou-Nader AJ, Sauer MA, Steele AD, Tate JE, Atherly D, Parashar UD, Santosham M, Nelson EAS. Global rotavirus vaccine introductions and coverage: 2006 - 2016. Hum Vaccin Immunother. 2018;14:2281–96. doi:10.1080/21645515.2018.1470725.
- Pitzer VE, Atkins KE, de Blasio BF, Effelterre TV, Atchison CJ, Harris JP, Shim E, Galvani AP, Edmunds WJ, Viboud C. et al. Direct and indirect effects of rotavirus vaccination: comparing predictions from transmission dynamic models. PLoS One. 2012;7:e42320. doi:10.1371/journal.pone.0042320.
- Li W, Zhu Y, Lou J, Chen J, Xie X, Mao J. Rotavirus, and adenovirus infections in children during COVID-19 outbreak in Hangzhou, China. Transl Pediatr. 2021;10:2281–6. doi:10.21037/tp-21-150.
- Fukuda Y, Tsugawa T, Nagaoka Y, Ishii A, Nawa T, Togashi A, Kunizaki J, Hirakawa S, Iida J, Tanaka T. et al. Surveillance in hospitalized children with infectious diseases in Japan: pre- and post-coronavirus disease 2019. J Infect Chemother. 2021;27:1639–47. doi:10.1016/j.jiac.2021.07.024.
- Yamaguchi H, Nozu K, Hanafusa H, Nambu Y, Kido T, Kondo A, Tamura A, Awano H, Morioka I, Nagase H, et al. Impact after the change from voluntary to universal oral rotavirus vaccination on consecutive emergency department visits for acute gastroenteritis among children in Kobe City, Japan (2016-2022). Vaccines (Basel). 2022;10:1831. doi:10.3390/vaccines10111831.
- Tsugawa T, Akane Y, Honjo S, Kondo K, Kawasaki Y. Rotavirus vaccination in Japan: efficacy and safety of vaccines, changes in genotype, and surveillance efforts. J Infect Chemother. 2021;27:940–8. doi:10.1016/j.jiac.2021.04.002.
- Hoa-Tran TN, Nakagomi T, Vu HM, Nguyen TTT, Takemura T, Hasebe F, Dao ATH, Anh PHQ, Nguyen AT, Dang AD, Nakagomi O. Detection of three independently-generated DS-1-like G9P[8] reassortant rotavirus a strains during the G9P[8] dominance in Vietnam, 2016-2018. Infect Genet Evol. 2020;80:104194. doi:10.1016/j.meegid.2020.104194.
- Amit LN, John JL, Mori D, Chin AZ, Mosiun AK, Ahmed K. Increase in rotavirus prevalence with the emergence of genotype G9P[8] in replacement of genotype G12P[6] in Sabah, Malaysia. Arch Virol. 2023;168(6):173. doi:10.1007/s00705-023-05803-9.
- Omatola CA, Ogunsakin RE, Prevalence OA. Pattern and genetic diversity of rotaviruses among children under 5 years of age with acute gastroenteritis in South Africa: a systematic review and meta-analysis. Viruses. 2021;13(10):1905. doi:10.3390/v13101905.
- Matthijnssens J, Ciarlet M, Rahman M, Attoui H, Bányai K, Estes MK, Gentsch JR, Iturriza-Gómara M, Kirkwood CD, Martella V, et al. Recommendations for the classification of group a rotaviruses using all 11 genomic RNA segments. Arch Virol. 2008;153:1621–9. doi:10.1007/s00705-008-0155-1.
- Ono M, Tsugawa T, Nakata S, Kondo K, Tatsumi M, Tsutsumi H, Kawasaki Y. Rotavirus genotype and Vesikari score of outpatients in Japan in the vaccine era. Pediatr Int. 2020;62:569–75. doi:10.1111/ped.14150.
- Yamamoto SP, Kaida A, Kubo H, Iritani N. Gastroenteritis outbreaks caused by a DS-1-like G1P[8] rotavirus strain, Japan, 2012-2013. Emerg Infect Dis. 2014;20:1030–3. doi:10.3201/eid2006.131326.
- Akane Y, Tsugawa T, Fujii Y, Honjo S, Kondo K, Nakata S, Fujibayashi S, Ohara T, Mori T, Higashidate Y, et al. Molecular and clinical characterization of the equine-like G3 rotavirus that caused the first outbreak in Japan, 2016. Gen Virol. 2021;102(3). doi:10.1099/jgv.0.001548.
- Fukuda S, Akari Y, Hatazawa R, Negoro M, Tanaka T, Asada K, Nakamura H, Sugiura K, Umemoto M, Kuroki H, et al. Rapid spread in Japan of unusual G9P[8] human rotavirus strains possessing NSP4 genes of E2 genotype. Jpn J Infect Dis. 2022;75(5):466–75. doi:10.7883/yoken.JJID.2022.020.
- Kamiya H, Tanaka-Taya K, Kono Y, Oishi K, Okabe N, Sunagawa T. Trends in intussusception hospitalization among Japanese infants before and after the introduction of the rotavirus vaccine: 2007-2014. The 12th International Rotavirus Symposium; 2016 Sep 7–9; Melbourne, Australia.
