ABSTRACT
Coronavirus disease 2019 (COVID-19) was extraordinarily harmful, with high rates of infection and hospitalization. This study aimed to evaluate the impact of COVID-19 vaccination status and other factors on hospitalization and disease severity, using data from Nagasaki Prefecture, Japan. Confirmed cases of COVID-19 infection with vaccination status were included and the differences in characteristics between different vaccination statuses, hospitalization or not, and patients with varying levels of disease severity were analyzed. Furthermore, logistic regression was used to calculate odds ratio (ORs) and 95% confidence intervals (CI) to evaluate the association of various factors with hospitalization and disease severity. From March 14, 2020 to August 31, 2022, 23,139 patients were unvaccinated 13,668 vaccinated the primary program with one or two doses, and 4,575 completed the booster. Vaccination reduced the risk of hospitalization with an odd ratio of 0.759 (95% CI: 0.654–0.881) and the protective effect of completed booster vaccination was more pronounced (OR: 0.261, 95% CI: 0.207–0.328). Similarly, vaccination significantly reduced the risk of disease severity (vaccinated primary program: OR: 0.191, 95% CI: 0.160–0.228; completed booster vaccination: OR: 0.129, 95% CI: 0.099–0.169). Overall, unvaccinated, male, elderly, immunocompromised, obese, and patients with other severe illness factors were all risk factors for COVID-19-related hospitalization and disease severity. Vaccination was associated with a decreased risk of hospitalization and disease severity, and highlighted the benefits of completing booster.
Introduction
Coronavirus disease 2019 (COVID-19) is caused by a coronavirus called SARS-CoV-2, first notified by the World Health Organization (WHO) on December 31, 2019.Citation1 The COVID-19 pandemic has profound health impacts, encompassing a spectrum of illness severity, including serious illness and fatality across all age groups. A Japanese observational study during the early stages of pandemic found that the median age of the patients became younger as the epidemic progressed.Citation2 And the incidence of severe illness and mortality was quite high among people >65 years old, which study showed that the incidence of severe illness reached 26.8% and the mortality rate 12.1%.Citation2 Following the emergence of the Omicron sublineage in January 2022, the number of infections had increased even more rapidly in Japan.Citation3 Overall, COVID-19 was extraordinarily harmful and imposed a significant disease burden on patients due to hospitalization for COVID-19 infection, multisystem damage, and mortality.Citation4–6
To control and minimize the COVID-19 pandemic and its impact, COVID-19 vaccines have been developed.Citation7,Citation8 Vaccination against COVID-19 was also promoted globally as the safest and most efficient strategy for preventing and controlling epidemics.Citation9 In February 2021, the Japanese government officially launched a nationwide COVID-19 vaccination campaign.Citation10 The primary vaccinations administered were Pfizer/BioNTech and Moderna/NIAID, both belong to messenger RNA (mRNA) vaccines. Data from multicenter randomized controlled trials concluded that both vaccines showed more than 90% efficacy in preventing COVID-19 diseases.Citation11,Citation12 In some Japanese real-world studies, complete mRNA vaccination was shown to have a high vaccine effectiveness (VE) against symptomatic COVID-19 infections, with a VE of 88% in the Delta-dominant period and a VE of over 68% in the Omicron-dominant period.Citation13–15 Moreover, vaccination significantly reduced the risk of hospitalization or COVID-19 related mortality and resulted in a shorter recovery period for non-hospitalized patients.Citation3,Citation16 The aforementioned studies primarily investigated the effectiveness of the mRNA vaccines on the infection COVID-19 and related mortality. However, there are limited research about the effects of vaccination and the interval after vaccination on the disease severity. Although similar studies had been reported in Western countries,Citation17,Citation18 few have been reported in Japanese region. In addition, the impact of the COVID-19 vaccine on the potential clinical symptoms is pending.
Therefore, the aim of this study was to investigate the impact of COVID-19 vaccination status on hospitalization and disease severity, using data from Nagasaki Prefecture in Japan. Furthermore, we aimed to analyze some severe illness factors such as immunocompromised status, COPD, CKD, obesity and others, contributing to the hospitalization and disease severity.
Patients and methods
Study setting and design
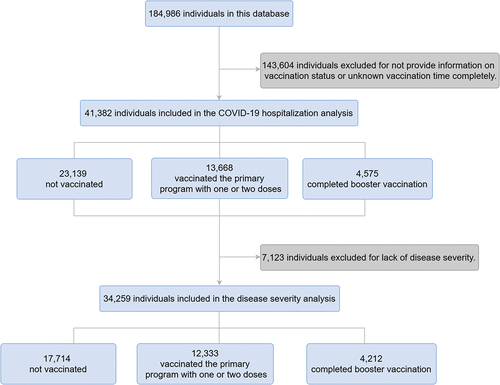
All data for this study were obtained from the information center for infectious diseases of the Nagasaki Prefectural Institute of Environment and Public Health. This center provided information on daily confirmed COVID-19 cases from March 14, 2020, to August 31, 2022. COVID-19 diagnostic tests include PCR (Polymerase Chain Reaction), antigen detection, LAMP (Loop-mediated isothermal amplification) test and so on. A COVID-19-infected patient was defined as an individual testing positive for any of the above tests. There was a total of 184,986 patients with infected COVID-19. Each subject was recorded in detail, including demographic characteristics and clinical factors such as sex, age, residence city, time of diagnosis, vaccination status and time, hospitalization, disease severity and symptoms and so on. This study aimed to analyze the vaccination status of COVID-19-infected patients and investigate the vaccine’s effects on hospitalization and the severity of disease of COVID-19. Therefore, patients whose vaccination status was not confirmed were excluded from this study. In addition, patients with unknown disease severity were also excluded in the analysis related to disease severity. Finally, we selected 41,282 individuals for hospitalization analysis, and furthermore selected 34,259 individuals for disease severity analysis ().
Due to the retrospective nature of the study, the requirement for obtaining informed consent was waived. The patients were not involved in the design, conduct, reporting, or dissemination of our studies. This study was approved by the Research Ethics Committees of Nagasaki Prefectural Institute of Environment and Public Health (No. 2021-11-1). And followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement (Table S1).Citation19
Study data
The demographic characteristics and clinical information of this study were provided by the center and used directly in the analysis. Some of the variables were categorized and processed to suit the requirements of our study.
All vaccinated patients received the mRNA vaccine. To ensure that the immunization fully exerted immune responses, we define 14 days after vaccination as the completion of this vaccination.Citation20 The specific definitions were as follows, self-reported no vaccination and self-reported vaccination with one dose of vaccine but less than 14 days interval between vaccination time and COVID-19 diagnosis were categorized as unvaccinated; Patients vaccinated with one or two doses of vaccine with an interval between vaccination and diagnosis of ≥14 days and those who completed booster vaccine but with an interval between vaccination and diagnosis of less than 14 days were classified as vaccinated the primary program with one or two doses; Patients who completed booster vaccination fulfilled the requirement of receiving three vaccinations and the third vaccination was received 14 days before diagnosis.
The disease severity was divided into four levels defined by the Ministry of Health, Labour and Welfare. Disease severity was categorized primarily by blood oxygen saturation (SpO2) and respiratory symptoms. Level 1 was SpO2 ≥ 96% with only cough or no respiratory symptoms, which cannot be recognized as pneumonia; level 2 was 93% <SpO2 < 96%, dyspnea, and pneumonia symptoms; level 3 was SpO2 ≤ 93%, requiring oxygen; and level 4 was requiring admission to the ICU or use artificial ventilator. If the oxygen saturation and clinical status severity did not coincide, the higher severity was taken. As detailed in the Diagnosis and Treatment Guidelines Manual established by the Ministry of Health, Labour and Welfare.Citation21 We combined those patients in analysis, because fewer patients had a disease severity of 2, 3 and 4. We categorized the disease severity into a binary of low level (disease severity level 1) and high level (disease severity level 2, 3 or 4). Then it was used in the analysis of the association of vaccines and other factors on disease severity.
Statistical analysis
Characteristics of patients with different vaccination statuses, hospitalization, and disease severity levels were compared using the Chi-square (χ2) test for categorical variables and the Kruskal-Wallis test or the Mann-Whitney U test for continuous variables. The associations of factors such as vaccines with hospitalization and disease severity of COVID-19 were evaluated using logistic regression, and odds ratio (ORs) were calculated. The relevant covariates were included in the regression equation. The p value of less than .05 was considered statistically significant. All analyses were done using SPSS 25.0 and data visualization was done with RStudio 2023.03.1.
Results
Characteristics of the study patients
In this database, there were 184,986 COVID-19-infected patients in Nagasaki Prefecture, Japan, from March 14, 2020 to August 31, 2022 (). 143,604 were excluded for the preliminary analysis because of lack of information.
As shown in , patients characteristics were analyzed by vaccination status as “Not vaccinated” (n = 23,139), “Vaccinated the primary program with one or two doses” (n = 13,668) and “Completed booster vaccination” (n = 4,575). It was observed that the differences in all characteristics except residence city were statistically significant. Among these COVID-19 patients, more females completed booster vaccination (2,746, 60.2%), and more males were unvaccinated (12,188, 52.8%) or vaccinated in the primary program with one or two doses (6,832, 50.1%). The vaccinated patients tended to be older (median age 11 vs. 36 vs. 45 years). The majority of the unvaccinated were patients in the under 18 age group. The interval time between vaccination and diagnosis was relatively long for those who were vaccinated in the primary program with one or two doses, with the majority >140 days (9,497, 79%). In addition, the number of symptoms indicated that the unvaccinated patients had lesser number of symptoms compared to the vaccinated patients. Similarly, the number of severe illness factors showed that unvaccinated patients were at a lower critical factors number (). The pairwise comparison was detailed in Table S2.
Table 1. Characteristics of patients with COVID-19 in different vaccination status.
Furthermore, we analyzed the association between vaccination status and hospitalization and the disease severity of COVID-19. Because the disease severity was unknown for 7,123 patients, they were excluded from the analysis of the association of disease severity, and 34,259 patients were included ().
COVID-19 hospitalization status
Of the 41,382 COVID-19-infected patients included, a total of 1,887 (4.6%) were hospitalized (Table S3). Among them, there were 995 (52.8%) males, which indicated a higher proportion of males in the hospitalized patients (p = .035). The median age was older at 67 (45, 81) years, with a tendency to those >80 years old (527, 27.9%). The number of unvaccinated patients was higher (932, 49.4%). Moreover, the interval between vaccination and diagnosed was >140 days in most of them (588, 83.8%) among the patients who were vaccinated in the primary program with one or two doses. Moreover, the hospitalized patients had more severe illness factors (1,077, 67.2%), the number of severe illness factors was larger, and the disease severity was higher than those of the non-hospitalized patients (Table S3).
From and Table S4, it was observed that among total patients, the risk of hospitalization was lower in females than in males (OR: 0.713, 95% confidence interval (CI): 0.624–0.814). The same result was observed in vaccinated patients. Vaccination further reduced the risk of hospitalization in females (OR: 0.516, 95% CI: 0.420–0.633) (Table S4). Not unexpectedly, the risk of hospitalization was higher with older age (OR: 1.051, 95% CI: 1.048–1.055), a more significant number of severe illness factors (≥4; OR:2.580, 95% CI: 1.767–3.769), and more considerable disease severity (3; OR: 13.577, 95% CI: 9.030–20.415) among total patients (Table S4). However, vaccination can reduce the risk of hospitalization with an OR of 0.759 (95% CI: 0.654–0.881) and the protective effect of completed booster vaccination was more pronounced (OR: 0.261, 95% CI: 0.207–0.328). Moreover, vaccination could lead to a decrease in the adverse effect of disease severity on hospitalization. As shown in , the ORs were decreased for each level of disease severity.
Figure 2. Odds ratios for characteristics associated with COVID-19 related hospitalization among total patients, patients of vaccinated the primary program and completed booster vaccination.
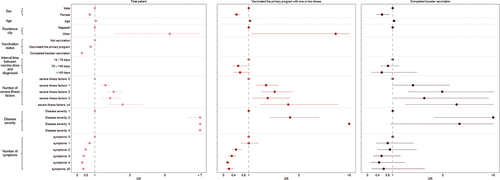
In addition, essentially all severe illness factors were associated with COVID-19 hospitalization outcomes (Table S5). In particular, among those unvaccinated patients with chronic kidney disease (CKD) (OR: 3.790, 95% CI: 2.851–5.037) and reduced immune function due to organ transplantation, use of immunosuppressants, anticancer drugs, and so on (OR: 2.581, 95% CI: 1.835–3.628). Nevertheless, vaccination did not significantly help patients with severe illness factors and even increased the risk of hospitalization in some patients with extreme illness factors. For example, patients suffering from reduced immune function due to organ transplantation, use of immunosuppressants, anticancer drugs, and so on had a substantially higher risk of hospitalization after vaccinated primary program (OR: 2.581, 95% CI:1.835–3.628) and with completed booster vaccination (OR: 4.508, 95% CI: 1.942–10.462).
Disease severity of COVID-19
Of the 34,259 COVID-19-infected patients included, the disease severity was divided into four levels: 1 (n = 33,276), 2 (n = 814), 3 (n = 153), and 4 (n = 16) (; Tables S6 and S7). Patients with higher disease severity were more male (level 3: n = 95, 62.1%; level 4: n = 14, 87.5%). Due to the small number of patients with a disease severity level of 3 and 4 were subsequently combined and analyzed. The disease severity was categorized as low level of severity (disease severity level 1) and high level of severity (disease severity level 2, 3, or 4). Compared characteristics were broadly similar to the results of the four classifications of disease severity, with only the difference in sex turning out to be not significant (p > .05) (Tables S6 and S8). In comparison to the low level of severity, patients with a high level of severity were generally older (median age 23 vs. 57 years) and with a greater proportion in the >80 years age group (189, 19.2%). Moreover, there were more unvaccinated patients in the high level of severity (573, 58.3%). In addition, patients with a high level of severity had a larger number of symptoms (≥5: 118, 12%), more patients with severe illness factors (643, 69.4%), a greater number of severe illness factors (≥4: 54, 5.4%), and more need for hospitalization (417, 42.4%) (Table S8).
The disease severity of COVID-19 was associated with age, and the severity risk increased with age (OR: 1.050, 1.046–1.055) (; Table S9). It was also noted that the risk of disease severity was increased in patients with a larger number of severe illness factors (≥4: OR: 3.367, 95% CI: 2.232–5.079), a larger number of symptoms (≥5: OR: 14.582, 95% CI: 9.015–23.587) and hospitalization (OR: 8.447, 95% CI: 7.059–10.108). However, it could be found that vaccination significantly reduced the risk of disease severity (vaccinated primary program: OR: 0.191, 95% CI: 0.160–0.228; completed booster vaccination: OR: 0.129 95% CI: 0.099–0.169). At the same time, vaccination caused a reduction in the adverse effects of the number of symptom occurrences (≥5: vaccinated primary program: OR: 8.159, 95% CI: 3.378–19.705) and hospitalization (vaccinated primary program: OR: 3.432, 95%CI: 2.493–4.725), compared to unvaccinated patients (; Table S9). What’s more, completed booster vaccination can further reduce the adverse effects of age (OR: 1.047, 95% CI: 1.031–1.063) and number of symptom occurrences (≥5: OR: 3.826, 95% CI: 1.068–13.701).
Figure 3. Odds ratios for characteristics associated with COVID-19 disease severity (binary classification) among total patients, patients of vaccinated the primary program and completed booster vaccination.
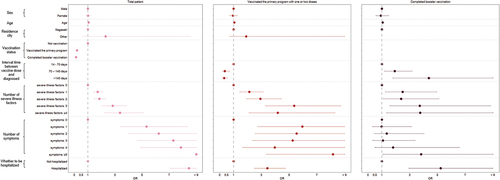
Besides, most severe illness factors were related to the disease severity (Table S10). Among unvaccinated patients, severe illness factors contributed to the disease severity, especially obesity (OR: 4.025, 95% CI: 3.055–5.303), patients with reduced immune function due to organ transplantation, use of immunosuppressants, anticancer drugs and so on (OR: 3.401, 95% CI: 2.342–4.939) and patients with chronic respiratory diseases (OR: 3.270, 95% CI: 2.125–5.033). Compared with unvaccinated patients, the vaccinated primary program and booster were found to have favorable effects in patients with malignant tumors (ORs: 2.783 vs. 2.382 vs. 2.286) and diabetes (ORs: 1.834 vs. 1.468 vs. 1.543), reducing the increased risk of disease severity. In other factors, however, the effects were not significant.
Clinical symptoms
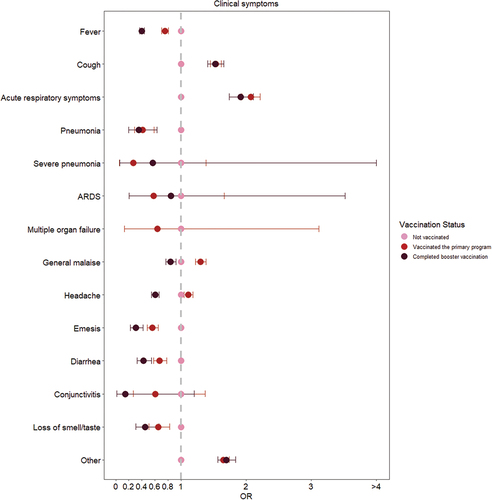
The majority of unvaccinated COVID-19 infected patients had fever (18,177, 78.6%) and cough (8,588, 37.1%) symptoms, and a high number had headache (5,238, 22.6%) and general malaise (4,623, 20.0%) (Table S11). This was also the situation in the vaccinated patients, but vaccination slightly decreased the risk of some symptoms. As can be seen in and Table S11, vaccination significantly decreased the risk of fever (vaccinated primary program: OR: 0.754, 95% CI: 0.706–0.805; completed booster vaccination: OR: 0.400, 95% CI: 0.367–0.437), pneumonia (vaccinated primary program: OR: 0.409, 95% CI: 0.286–0.585; completed booster vaccination: OR: 0.355, 95% CI: 0.200–0.631), emesis (vaccinated primary program: OR: 0.563, 95% CI: 0.484–0.655; completed booster vaccination: OR: 0.306, 95% CI: 0.223–0.418), diarrhea (vaccinated primary program: OR: 0.674, 95% CI: 0.583–0.780; completed booster vaccination: OR: 0.424, 95% CI: 0.328–0.548), and loss of smell/taste (vaccinated primary program: OR: 0.650, 95% CI: 0.511–0.826; completed booster vaccination: OR: 0.452, 95% CI: 0.305–0.668). For some symptoms, such as general malaise (OR: 0.842, 95% CI: 0.766–0.926) and headache (OR: 0.605, 95% CI: 0. 548–0.668), preventive effects were shown only after completing booster vaccination. On the contrary, it can be recognized that vaccination was ineffective in preventing all symptoms. There was no preventive effect on cough and acute respiratory symptoms other than cough in this study.
Figure 4. Odds ratios for clinical symptoms in different vaccination status.
Discussion
Our study described the vaccination characteristics of COVID-19-infected patients in Nagasaki, Japan, from March 14, 2020, to August 31, 2022. It also determined that vaccination was associated with a decreased risk of hospitalization and disease severity, and highlighting the benefits of completing booster. In addition, other risk factors associated with hospitalization and disease severity of COVID-19 were analyzed, such as male, the older (especially those over 80 years old), obesity, CKD, immunocompromised status, and other factors.
To begin with, this study showed that many infected patients were not vaccinated in Nagasaki Prefecture, Japan, with a vaccination rate of only 44.08%. On the one hand, this may be related to the delay in vaccine roll-out in Japan.Citation22 A study claimed that the hesitancy of COVID-19 vaccine was more severe in Japan, with 30% of respondents expressing hesitate to receive the vaccine due to concerns about the safety and potential side effects.Citation23 The percentage of people who had received at least one dose in other developed countries in April 2021 ranged from 28% to 51%, but this percentage was relatively low in Japan, at only 4%.Citation22 On the other hand, the low vaccination rate among infected patients may be mainly due to the vaccine’s effectiveness in decreasing the risk of infection. Clinical trials demonstrated that the VE was as high as 90%.Citation11,Citation12 Real-world studies in Japan had shown that the vaccine was still highly effective, with VE reaching more than 80% in the Delta-dominant period.Citation13,Citation14 However, in the Omicron-dominant period, VE ranged from 32% to 56%. After booster vaccination, VE improved considerably, reaching about 70%.Citation13,Citation15,Citation24 Nevertheless, some studies found an increased risk of infection of 14% after ten weeks of vaccinationCitation17 and an increased risk of infection beyond 20 weeks when the vaccine’s efficacy declined by more than 20%.Citation25–27 We also noticed that most of the patients we included were individuals vaccinated for more than 140 days. Perhaps as shown in the previous research, the level of neutralizing antibodies in the body dropped 3.9-fold after ten weeks,Citation28 and intranasal IgA was essentially back to pre-COVID-19 levels after nine months.Citation29 The chances of being infected are greatly increased by the drop in the body’s immune levels caused by the longer period of time experienced after vaccination. However, the number of symptoms and severe illness factors were higher in vaccinated patients. This may not be consistent with our expectations. It was important to consider that the vaccine may have initially been promoted among populations at higher risk of severe disease, such as the elderly. As a result, these groups naturally have a higher risk level compared to others.
In addition to preventing COVID-19 infection, studies in some Western countries had shown that vaccination decreased the risk of hospitalization, critical illness and mortality from COVID-19-related diseases.Citation17,Citation18,Citation20,Citation30 The results from our study supported these findings, as we found that vaccinated the primary program decreased the risk of COVID-19 related hospitalization by 24.1%. Completion of booster vaccination was even more effective, decreasing the risk of hospitalization by 73.9%. Similar conclusions were drawn from the results of a study of COVID-19 hospitalization and mortality outcomes in Nara Prefecture, Japan.Citation3 Moreover, the association between the vaccine and risk of disease severity was analyzed in our study. The results showed that vaccinating the primary program reduced the risk of disease severity by 80.9%, and completing the booster vaccination reduced the risk by 87.1%. Unlike other studies,Citation17,Citation18,Citation25,Citation31 we did not find an increased risk of hospitalization and disease severity with longer intervals after vaccination in our current study. There was no reduction in the protective efficacy of the vaccine long after vaccination, except for a significant decrease in protection against the risk of disease severity beyond 70 days of booster vaccination.
Vaccination can help in reducing the risk of hospitalization and severe illness in some patients with risk factors. For example, males, older age, patients who suffer from critical factors, and patients who occur with more symptoms. Differences in disease severity between males and females may be attributed to differences in the expression levels of sex hormones, ACE2 and TMPRSS2 involved in the inflammatory process, as well as lifestyle differences such as smoking.Citation32 Age was also found to be an independent risk factor after performing a multifactorial analysis. Vaccination with a booster shot could slightly reduce the risk of age-related disease severity. More comorbidities, age-related physiological changes, or aging of the immune system significantly contribute to increased susceptibility and impaired immune response to vaccination.Citation33,Citation34 Therefore, vaccines may be less protective in the elderly than in the younger, and booster vaccinations are recommended for older people as necessary.
With regard to severe illness factors, we observed that CKD, immunocompromised status, and obesity led to more than a two-fold increase in the risk of hospitalization. Obesity, immunocompromise status, and chronic respiratory diseases resulted in a more than three-fold increase in the risk of disease severity. Many studies described the mechanisms associated with these severe illness factors. Notably, obesity was one of the factors strongly associated with hospitalization and disease severity. The higher risk of thrombosis and respiratory dysfunction due to altered respiratory mechanisms contributed to increased risk in obese patients.Citation35 Moreover, it had been proposed that obesity is a pro-inflammatory disease, and inflammatory markers in COVID-19 admitted patients had been significantly associated with serious illness and mortality.Citation32,Citation35,Citation36 Similarly, it was unsurprising that immunocompromised, CKD, and chronic respiratory diseases patients were at high risk. A persistent pro-inflammatory state, elevated expression of ACE2 and TMPRSS2, airflow limitation, and use of corticosteroids would lead to an increased risk of hospitalization and severe illness.Citation36–40 Unfortunately, vaccines were less effective to help those vulnerable groups. Immunosuppressive therapies will also have a negative effect on the immune response to the COVID-19 vaccine.Citation38 Therefore, other options should be considered, such as the use of antiviral medications and anti-spiking monoclonal antibodies.Citation17 However, some studies showed that vaccination is the most important intervention for the pregnant population to protect pregnant women and even newborns.Citation41,Citation42 Whereas, this study could not draw valid conclusions due to too few pregnant people in the included patients.
In terms of clinical symptoms, vaccination decreased the risk of some symptoms in patients. Symptoms were also an important risk factor for disease severity, as fever, cough, headache, and gastrointestinal symptoms were commonly seen in infected patients. This had also been found in previous studies, and symptoms may be associated with cytokine.Citation32
This study had some limitations. First, this study only focused on COVID-19 infected patients in Nagasaki Prefecture, Japan, limiting the ability to assess the vaccine effectiveness in preventing infection and the results should be extrapolated with caution. Second, the data in this study were obtained from the COVID-19 infected patient surveillance system, and some information was self-reporting, which may have recall bias. Third, there had many missing values in this data, such as vaccination status. It had to be excluded due to too many patients with unknown doses of vaccinations. Because this did not qualify for multiple interpolation, interpolating more than a certain percentage of the data would cause a large bias.Citation43 Fourth, previous studies had shown that vaccine types had differential effects on the occurrence of adverse outcomes.Citation26,Citation44 In this study, the vaccine type was not further analyzed because most patients were vaccinated with Pfizer-BioNTech (Vaccination with Moderna was only 11 individuals). Fifth, genomic analysis of COVID-19 patients was not performed in this study, and it was not possible to estimate the impact of different strain lineages on the outcomes. Lastly, due to lack of antibody data, we were unable to rule out potential prior infections in the non-vaccinated.
Conclusion
The data shown that vaccination rates were low from March 14, 2020 to August 31, 2022 in Nagasaki Prefecture, Japan. The findings suggested that vaccination was associated with reduced risk of COVID-19-related hospitalization and disease severity, and highlighted the benefits of completing boosters. In addition, COVID-19 associated risk factors for hospitalization and disease severity were male, elderly, patients with immunocompromised, obese, and other severe illness factors. However, vaccination can help in reducing the risk of hospitalization and severe illness in some patients with risk factors. Therefore, vaccination is one of the effective ways to control the outbreaks and reduce severity.
Abbreviations
COVID-19 Coronavirus disease 2019
WHO World Health Organization
mRNA Messenger RNA
STROBE Strengthening the Reporting of Observational Studies in Epidemiology
VE Vaccine Effectiveness
ORs odds ratio
CKD Chronic Kidney Disease
Author contributions
Conceptualization, Guoxi Cai, Shiwen Liu, Kouichi Morita, Kiyoshi Aoyagi and Fei He; data curation, Guoxi Cai, Yumika Takaki, Fumiaki Matsumoto, Akira Yoshikawa, Toshitsugu Taguri and Maiko Hasegawa; formal analysis, Shiwen Liu, Guoxi Cai, Yixiao LU, Nguyen Tien Huy, Masaya Saito, Shouhei Takeuchi and Fei He; funding acquisition, Guoxi Cai, Taro Yamamoto, Kouichi Morita, Kiyoshi Aoyagi and Fei He; writing – original draft, Shiwen Liu; writing – review and editing, Guoxi Cai, Yixiao LU, Jianfen Xie, Kazuhiko Arima, Satoshi Mizukami, Jiwen Wu and Fei He; All authors have read and agreed to the published version of the manuscript.
revised Supplementary material.docx
Download MS Word (93.8 KB)Acknowledgments
We thank medical personals for checking and reporting the COVID-19 cases.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2024.2322795.
Additional information
Funding
References
- Chams N, Chams, S, Badran, R, Shams, A, Araji, A, Raad, M, Mukhopadhyay, S, Stroberg, E, Duval, EJ, Barton, LM, et al. COVID-19: A multidisciplinary review. Front Public Health. 2020 Jul 29;8:383. doi:10.3389/fpubh.2020.00383B3.
- Miyashita K, Hozumi H, Furuhashi K, Nakatani E, Inoue Y, Yasui H, Karayama M, Suzuki Y, Fujisawa T, Enomoto N, et al. Changes in the characteristics and outcomes of COVID-19 patients from the early pandemic to the delta variant epidemic: a nationwide population-based study. Emerg MIcrobes Infec. 2023 Dec 1;12(1):2155250. doi:10.1080/22221751.2022.2155250.
- Tomioka K, Uno K, Yamada M. Association between vaccination status and severe health consequences among community-dwelling COVID-19 patients during omicron BA.1/BA.2 and BA.5-predominant periods in Japan. Environ Health Prev. 2023 Jan 20;28:35. doi:10.1265/ehpm.23-00061.
- Peramo-Alvarez FP, Lopez-Zuniga MA, Lopez-Ruz MA. Medical sequels of COVID-19. Med Clin-Barcelona. 2021 Oct 22;157(8):388–10. doi:10.1016/j.medcli.2021.04.023.
- Tsuzuki S, Miyazato Y, Terada M, Morioka S, Ohmagari N, Beutels P. Impact of long-COVID on health-related quality of life in Japanese COVID-19 patients. Health Qual Life Out. 2022 Aug 19;20(1):125. doi:10.1186/s12955-022-02033-6.
- WHO. 2023 [accessed 9 Sep 2023]. https://www.who.int/teams/health-care-readiness/post-covid-19-condition
- Li M, Wang H, Tian L, Pang Z, Yang Q, Huang T, Fan J, Song L, Tong Y, Fan H. COVID-19 vaccine development: milestones, lessons and prospects. Signal Transduct Tar. 2022 May 3;7(1):146. doi:10.1038/s41392-022-00996-y.
- Tregoning JS, Flight KE, Higham SL, Wang Z, Pierce BF. Progress of the COVID-19 vaccine effort: viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat Rev Immunol. 2021 Oct 1;21(10):626–36. doi:10.1038/s41577-021-00592-1.
- Our World in data coronavirus (COVID-19) vaccinations. 2023 [accessed 2023 Sep 9]. https://ourworldindata.org/covid-vaccinations
- National Institute of Infectious Diseases. COVID-19 vaccine (as of May 10, 2021). https://www.tandfonline.com/doi/full/10.1080/14760584.2023.2188950; 2021 [accessed 2023 Sep 9].
- Baden LR, El SH, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021 Feb 4;384(5):403–16. doi:10.1056/NEJMoa2035389.
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C. et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020 Dec 31;383(27):2603–15. doi:10.1056/NEJMoa2034577.
- Arashiro T, Arima Y, Muraoka H, Sato A, Oba K, Uehara Y, Arioka H, Yanai H, Kuramochi J, Ihara G, et al. Coronavirus disease 19 (COVID-19) vaccine effectiveness against symptomatic severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection during delta-dominant and omicron-dominant periods in Japan: a multicenter prospective case-control study (Factors Associated with SARS-CoV-2 infection and the effectiveness of COVID-19 vaccines study). Clin Infect Dis. 2023 Feb 8;76(3):e108–15.
- Maeda H, Saito N, Igarashi A, Ishida M, Suami K, Yagiuchi A, Kimura Y, Komino M, Arai H, Morikawa T. et al. Effectiveness of messenger RNA coronavirus disease 2019 vaccines against symptomatic severe acute respiratory syndrome coronavirus 2 infections during the delta variant epidemic in Japan: vaccine effectiveness real-time surveillance for SARS-CoV-2 (VERSUS). Clin Infect Dis. 2022 Nov 30;75(11):1971–9. doi:10.1093/cid/ciac292.
- Maeda H, Saito N, Igarashi A, Ishida M, Terada M, Ito T, Ikeda H, Kamura H, Motohashi I, Kimura Y. et al. Effectiveness of mRNA COVID-19 vaccines against symptomatic SARS-CoV-2 infections during the SARS-CoV-2 omicron BA.1 and BA.2 epidemic in Japan: vaccine effectiveness real-time surveillance for SARS-CoV-2 (VERSUS). Expert Rev Vaccines. 2023 Jan 1;22(1):288–98. doi:10.1080/14760584.2023.2188950.
- Tomioka K, Uno K, Yamada M. Association between vaccination status and COVID-19-related health outcomes among community-dwelling COVID-19 patients in Nara, Japan. Environ Health Prev. 2023 Jan 20;28:7. doi:10.1265/ehpm.22-00199.
- Agrawal U, Bedston S, McCowan C, Oke J, Patterson L, Robertson C, Akbari A, Azcoaga-Lorenzo A, Bradley DT, Fagbamigbe AF, et al. Severe COVID-19 outcomes after full vaccination of primary schedule and initial boosters: pooled analysis of national prospective cohort studies of 30 million individuals in England, Northern Ireland, Scotland, and Wales. Lancet. 2022 Oct 15;400(10360):1305–20. doi:10.1016/S0140-6736(22)01656-7.
- Tenforde MW, Self WH, Adams K, Gaglani M, Ginde AA, McNeal T, Ghamande S, Douin DJ, Talbot HK, Casey JD, et al. Association Between mRNA Vaccination and COVID-19 Hospitalization and disease severity. Jama-J Am Med Assoc. 2021 Nov 23;326(20):2043–54. doi:10.1001/jama.2021.19499.
- von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007 Oct 20;370(9596):1453–7. doi:10.1016/S0140-6736(07)61602-X.
- Agrawal U, Katikireddi SV, McCowan C, Mulholland RH, Azcoaga-Lorenzo A, Amele S, Fagbamigbe AF, Vasileiou E, Grange Z, Shi T, et al. COVID-19 hospital admissions and deaths after BNT162b2 and ChAdOx1 nCoV-19 vaccinations in 2·57 million people in Scotland (EAVE II): a prospective cohort study. Lancet Resp Med. 2021 Dec 1;9(12):1439–49. doi:10.1016/S2213-2600(21)00380-5.
- COVID-19 clinical guidelines these guidelines (Version 5.3); 2021. https://www.mhlw.go.jp/content/000829136.pdf.
- Kosaka M, Hashimoto T, Ozaki A, Tanimoto T, Kami M. Delayed COVID-19 vaccine roll-out in Japan. Lancet. 2021 Jun 19;397(10292):2334–5. doi:10.1016/S0140-6736(21)01220-4.
- Okamoto S, Kamimura K, Komamura K. COVID-19 vaccine hesitancy and vaccine passports: a cross-sectional conjoint experiment in Japan. BMJ Open. 2022 Jun 16;12(6):e60829. doi:10.1136/bmjopen-2022-060829.
- Hotta K, Suzuki E, Ichihara E, Kiura K. Three doses of mRNA COVID-19 vaccine protects from SARS-CoV-2 infections in Japan. J Intern Med. 2022 Oct 1;292(4):687–9. doi:10.1111/joim.13526.
- Feikin DR, Higdon MM, Abu-Raddad LJ, Andrews N, Araos R, Goldberg Y, Groome MJ, Huppert A, O’Brien KL, Smith PG. et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet. 2022 Mar 5;399(10328):924–44. doi:10.1016/S0140-6736(22)00152-0.
- Fiolet T, Kherabi Y, MacDonald CJ, Ghosn J, Peiffer-Smadja N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: a narrative review. Clin Microbiol Infec. 2022 Feb 1;28(2):202–21. doi:10.1016/j.cmi.2021.10.005.
- Wu N, Joyal-Desmarais K, Ribeiro P, Vieira AM, Stojanovic J, Sanuade C, Yip D, Bacon SL. Long-term effectiveness of COVID-19 vaccines against infections, hospitalisations, and mortality in adults: findings from a rapid living systematic evidence synthesis and meta-analysis up to december, 2022. Lancet Resp Med. 2023 May 1;11(5):439–52. doi:10.1016/S2213-2600(23)00015-2.
- Levin EG, Lustig Y, Cohen C, Fluss R, Indenbaum V, Amit S, Doolman R, Asraf K, Mendelson E, Ziv A, et al. Waning immune humoral response to BNT162b2 covid-19 vaccine over 6 months. N Engl J Med. 2021 Dec 9;385(24):e84. doi:10.1056/NEJMoa2114583.
- Liew F, Talwar S, Cross A, Willett BJ, Scott S, Logan N, Siggins MK, Swieboda D, Sidhu JK, Efstathiou C, et al. SARS-CoV-2-specific nasal IgA wanes 9 months after hospitalisation with COVID-19 and is not induced by subsequent vaccination. EBioMedicine. 2023 Jan 1;87:104402. doi:10.1016/j.ebiom.2022.104402.
- Haas EJ, Angulo FJ, McLaughlin JM, Anis E, Singer SR, Khan F, Brooks N, Smaja M, Mircus G, Pan K, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021 May 15;397(10287):1819–29. doi:10.1016/S0140-6736(21)00947-8.
- Tenforde MW, Self WH, Naioti EA, Ginde AA, Douin DJ, Olson SM, Talbot HK, Casey JD, Mohr NM, Zepeski A, et al. Sustained effectiveness of pfizer-BioNTech and moderna vaccines against COVID-19 associated hospitalizations among adults — United States, March–July 2021. MMWR-Morbid Mortal W. 2021 Aug 27;70(34):1156–62. doi:10.15585/mmwr.mm7034e2.
- Gao YD, Ding M, Dong X, Zhang JJ, Kursat AA, Azkur D, Gan H, Sun YL, Fu W, Li W, et al. Risk factors for severe and critically ill COVID-19 patients: a review. Allergy. 2021 Feb 1;76(2):428–55. doi:10.1111/all.14657.
- Chen Y, Klein SL, Garibaldi BT, Li H, Wu C, Osevala NM, Li T, Margolick JB, Pawelec G, Leng SX. Aging in COVID-19: vulnerability, immunity and intervention. Ageing Res Rev. 2021 Jan 1;65:101205. doi:10.1016/j.arr.2020.101205.
- Hyams C, Marlow R, Maseko Z, King J, Ward L, Fox K, Heath R, Tuner A, Friedrich Z, Morrison L. et al. Effectiveness of BNT162b2 and ChAdOx1 nCoV-19 COVID-19 vaccination at preventing hospitalisations in people aged at least 80 years: a test-negative, case-control study. Lancet Infect Dis. 2021 Nov 1;21(11):1539–48. doi:10.1016/S1473-3099(21)00330-3.
- Jaisinghani P, Kumar R. Obesity and viral infections. Gastroenterol Clin N. 2023 Jun 1;52(2):393–402. doi:10.1016/j.gtc.2023.03.012.
- Radzikowska U, Ding M, Tan G, Zhakparov D, Peng Y, Wawrzyniak P, Wang M, Li S, Morita H, Altunbulakli C, et al. Distribution of ACE2, CD147, CD26, and other SARS-CoV-2 associated molecules in tissues and immune cells in health and in asthma, COPD, obesity, hypertension, and COVID-19 risk factors. Allergy. 2020 Nov 1;75(11):2829–45. doi:10.1111/all.14429.
- Fitzgerald KC, Mecoli CA, Douglas M, Harris S, Aravidis B, Albayda J, Sotirchos ES, Hoke A, Orbai A-M, Petri M, et al. Risk factors for infection and health impacts of the coronavirus disease 2019 (COVID-19) pandemic in people with autoimmune diseases. Clin Infect Dis. 2022 Feb 11;74(3):427–36. doi:10.1093/cid/ciab407.
- Galmiche S, Luong NL, Tartour E, de Lamballerie X, Wittkop L, Loubet P, Launay O. Immunological and clinical efficacy of COVID-19 vaccines in immunocompromised populations: a systematic review. Clin Microbiol Infec. 2022 Feb 1;28(2):163–77. doi:10.1016/j.cmi.2021.09.036.
- Higham A, Mathioudakis A, Vestbo J, Singh D. COVID-19 and COPD: a narrative review of the basic science and clinical outcomes. Eur Respir Rev. 2020 Dec 31;29(158). doi:10.1183/16000617.0199-2020.
- Singh J, Malik P, Patel N, Pothuru S, Israni A, Chakinala RC, Hussain MR, Chidharla A, Patel H, Patel SK. et al. Kidney disease and COVID-19 disease severity—systematic review and meta-analysis. Clin Exp Med. 2022 Feb 1;22(1):125–35. doi:10.1007/s10238-021-00715-x.
- Jorgensen S, Burry L, Tabbara N. Role of maternal COVID-19 vaccination in providing immunological protection to the newborn. Pharmacotherapy. 2022 Jan 1;42(1):58–70. doi:10.1002/phar.2649.
- Nana M, Nelson-Piercy C. COVID-19 in pregnancy. Clin Med. 2021 Sept 1;21(5):e446–50. doi:10.7861/clinmed.2021-0503.
- Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, Wood AM, Carpenter JR. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ-Brit Med J. 2009 Jun 29;338:b2393. doi:10.1136/bmj.b2393.
- Self WH, Tenforde MW, Rhoads JP, Gaglani M, Ginde AA, Douin DJ, Olson SM, Talbot HK, Casey JD, Mohr NM, et al. Comparative effectiveness of moderna, Pfizer-BioNTech, and Janssen (Johnson & Johnson) vaccines in preventing COVID-19 hospitalizations among adults without immunocompromising conditions — United States, March–August 2021. MMWR-Morbid Mortal W. 2021 Sept 24;70(38):1337–43. doi:10.15585/mmwr.mm7038e1.