?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Pertussis is a vaccine-preventable infectious disease; however, data on pertussis antibody levels in a nationwide population are still limited in China. We aimed to pool the seropositivity rates of IgG antibodies against pertussis toxin (PT-IgG) across the country. We systematically searched PubMed, Web of Science, Embase, and the China National Knowledge Infrastructure Database for studies published between January 1, 2010, and June 30, 2023. Studies reporting the seroprevalence of PT-IgG among a healthy Chinese population were included. Pooled estimates were obtained using random-effects meta-analyzes. The meta-analysis included 39 studies (47,778 participants) reporting anti-PT IgG seropositivity rates. The pooled rate for all ages was 7.06% (95% CI, 5.50%-9.07%). Subgroup analyzes showed rates ranging from 6.36% to 12.50% across different age groups. This meta-analysis indicated a low anti-PT IgG seropositivity rate in the Chinese population, particularly among school-aged children and young adults. This finding underscores the urgent need to refine immunization strategies.
Introduction
Pertussis is a highly contagious respiratory infection caused by the bacterium Bordetella pertussis.Citation1,Citation2 The disease can occur in all age groups, but young infants are at the highest risk of severe symptoms, complications, and death.Citation3 According to the World Health Organization (WHO), more than 3 million children died of pertussis annually worldwide before 1970.Citation4 Since the introduction of the pertussis vaccine in the 1950s and the implementation of the Expanded Program on Immunization in the 1970s, the morbidity and mortality rates of pertussis have decreased significantly.Citation5 However, pertussis has never been completely eliminated, and the epidemic peaks continued every 3–5 years, even in countries with high immunization rates.Citation6 In 2018, the WHO reported more than 151 000 cases of pertussis, despite an estimated global 90% diphtheria tetanus-pertussis (DTP) vaccine coverage with 3 doses.Citation7
The current immunization schedule for pertussis in China, which was established in 1982, includes 4 doses of combined DTP vaccine at 3,4,5,18 months of age.Citation8 A combined diphtheria, tetanus, and whole-cell pertussis vaccine (DTwP) was used before 2007; DTwP was replaced with diphtheria, tetanus, and acellular pertussis vaccine (DTaP) throughout the country in 2013.Citation9 Although DTP coverage has remained above 95% over the last 20 years, a resurgence of pertussis has occurred in China during the past decade.Citation10 The reported incidence of pertussis in China is expected to increase from 0.12/100000 in 2013 to 2.69/100000 in 2022.Citation11
Pertussis is a vaccine-preventable infectious disease in which the corresponding antibody levels are a crucial factor in the spread of the disease, and serological surveys are widely used to measure immunity to the disease in the population.Citation12–14 Pertussis toxin (PT) is the only specific antigen for pertussis, and the immunoglobulin G (IgG) of PT is believed to be an important indicator of clinical immunity to pertussis.Citation15,Citation16 A comprehensive understanding of the seroprevalence of pertussis is fundamental to guide the implementation of prevention and control.Citation17 Several seroprevalence studies on pertussis have been conducted in limited areas of China. Nevertheless, relevant synthesized data and nationally representative studies in China are lacking. To fill these gaps, we conducted a systematic review and meta-analysis to estimate the seroprevalence of anti-PT IgG in the general Chinese population and explore variations across different subgroups.
Materials and methods
We conducted this systematic review and meta-analysis according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guidelines.Citation18 The study protocol was prospectively registered in the Prospective Register of PROSPERO (CRD42023456254).
Search strategy and study selection
We systematically searched PubMed, Web of Science, Embase, and the China National Knowledge Infrastructure Database for studies published between January 1, 2010, and June 30, 2023, without language restrictions. The keywords used were “serology or serological or serologic,” “pertussis,” and “China or Chinese.” Additionally, the reference lists of related reviews and included articles were screened manually to identify additional relevant studies. The detailed search strategies are shown in Supplementary (Table S1).
Two authors (YZ and WZ) independently identified observational studies on IgG antibodies against pertussis toxin (PT-IgG) in a healthy Chinese population, including data on sample size, the number of seropositivity of anti-PT IgG or seropositivity rate of anti-PT IgG. Where the same study population was described in more than one article, the article with the largest sample size and most detailed information was used. The exclusion criteria were as follows: the study participants were cases or suspected cases of pertussis, the study had no data or was not distinguishable on PT-IgG, the method of antibody assays was unclear, the serum sample was cord blood, or no seropositivity rate of anti-PT IgG was calculated. Reviews, case reports, conference abstracts, and commentaries were also excluded.
We excluded studies that used enzyme-linked immunosorbent assay (ELISA) B. pertussis IgG kits rather than B. pertussis toxin IgG kits. There are two antigens in B. pertussis IgG kits: filamentous hemagglutinin (FHA) and PT.Citation19 FHA is a component of the cell wall in all Bordetella species, and it is unclear whether antibodies against FHA offer protection from disease.Citation16 Therefore, we included only studies targeting anti-PT IgG to obtain more precise estimates.
Duplicate articles between databases were removed from the selection. Against the inclusion and exclusion criteria, two authors (YZ and WZ) independently reviewed all titles and abstracts and conducted a full-text review to determine potential relevance. Disagreements during the review process were resolved through discussions with a third author (JH).
Data extraction
Both authors (YZ and WZ) independently extracted the following data from the included articles: author, title, publication year, study region, location, study design, investigation year, definition of seropositivity rate of anti-PT IgG, ELISA kits, cutoff values for seropositivity of anti-PT IgG, sample size, and seropositivity of anti-PT IgG. The study regions were classified as North China, Eastern China, Northeast China, South China, Central China, Northwest China, and Southwestern China. We imputed the investigation year by subtracting 3 years from the year of publication when the studies did not provide data for the year investigated (Supplementary Table S2). The outcome was the seropositivity rate for anti-PT IgG, which was defined as the proportion of participants seropositive for anti-PT IgG. Wherever applicable, data on the year-, age-, or sex-specific seropositivity rates of anti-PT IgG were separately extracted.
Quality assessment
Two authors (YZ and SL) independently evaluated the risk of bias in the included studies using a quality scale based on the Joanna Briggs Institute (JBI) Prevalence Critical Appraisal Tool.Citation20 Each criterion can be assessed as “Yes,” “No,” “Unclear,” or “Not applicable” (Supplementary Table S3). Any discrepancies were resolved through consultation and group discussions. “Yes” was assigned 1 score, and others were assigned 0 score. The total score, ranging from 0 to 10, represented the overall quality of each study.
Statistical analysis
The variance in the raw rates from each study was stabilized using logit transformation before synthesizing the seropositivity rate of anti-PT IgG. The estimates with 95% confidence intervals (CIs) were pooled separately using a random-effects (DerSimonian) meta-analysis.Citation21 The total variation due to heterogeneity was evaluated using the Cochran Q test (p < .05 represented significant heterogeneity), and I2 index (a value > 50% may indicate substantial heterogeneity).Citation22,Citation23 Publication bias was evaluated using the Peter test.Citation24 Leave-one-out sensitivity analysis was conducted to examine whether single studies had a disproportionately excessive influence.Citation25
To determine the potential sources of heterogeneity, we performed subgroup analyzes according to age, sex, study region, investigation year, ELISA kit, and cutoff value for seropositivity for anti-PT IgG.
Furthermore, to assess the association between various sample characteristics and seropositivity rates for anti-PT IgG, we performed a meta-regression analysis. For the seropositivity rate, multiple data points (age-specific or investigation year-specific rate) were generally reported in a single study; therefore, we used a multilevel mixed-effects meta-regression approach to account for the occurrence of different data points from the same study. Given that the seropositivity rate (p) = cases/number of participants, we stabilized the rate using the logit link as follows:
Due to the availability of relevant information, we assessed the effects of age, investigation year, study region, ELISA kit, and cutoff value using univariate and multivariate meta-regression (Supplementary Table S4). To take the region-specific context of socio-economic development, the study region identification number () was employed as the random effect. To allow for a nonlinear relationship between age and rate, we adopted five-knot restricted cubic regression splines in the multivariable meta-regression model. The final model is as follows:
then,
The age-specific seropositivity rates of anti-PT IgG in 2012, 2016, and 2020 were based on the fixed ELISA kit (Institute Virion/Serion Würzburg GmbH, Germany) and cutoff value (40 IU/mL) owing to the limited data points for other kits and cutoff values.
A two-sided p value of ≤ 0.05 was considered statistically significant. All analyzes were performed using R version 4.3.1 (R Foundation for Statistical Computing), and R packages “meta” was used for mate-analysis.
Role of the funding source
The funding source had no role in the study design, data collection, data analysis, data interpretation, or the writing of the manuscript. The corresponding authors have the final responsibility for the decision to submit this manuscript for publication.
Results
Study selection
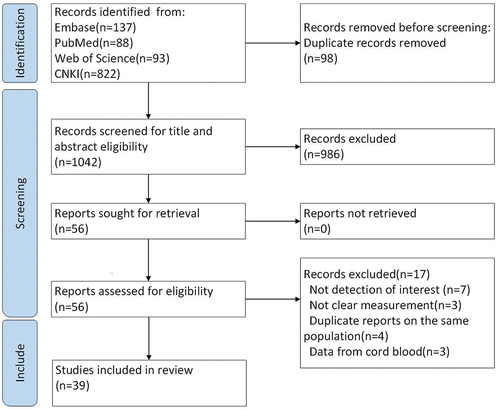
We identified 1 140 records from the initial literature search. After removing duplicate records and initial screening of titles and abstracts, 56 full-text articles were retrieved. Furthermore, 17 studies were excluded because of insufficient data, unclear measurements, duplicate studies based on the same population, or data from cord blood. Finally, 39 studies that provided 44 data points were considered eligible and included in the current systematic review and meta-analysis ( and Table S5).
Study characteristics
presents the study characteristics. All the studies were cross-sectional, of which 12 were conducted in East China, 12 in North China, 8 in South China, 4 in Central China, and 3 in Southwest China (Figure S1). Among the included studies, 8 targeted pregnant women or individuals of childbearing age, 6 were confined to children and adolescents, and the other 25 enrolled the general population, covering a wider age range. The anti-PT IgG of all 39 included studies were measured quantitatively by ELISA kits, in which 35 studies adopted commercial ELISA kits (24 manufactured by the Institute Virion/Serion Würzburg GmbH, Germany, 9 by Euroimmun Medizinische Labordiagnostika AG, Germany, 2 by Zhengzhou Etebio, China), other 4 studies were established by in-house biological laboratory and calibrated by WHO Human International Standard for Pertussis Antiserum (NIBSC 06/140). Therefore, the cutoff values for seropositivity for anti-PT IgG varied across studies, of which 23 studies were 40 IU/mL, 6 studies were 30 IU/mL, 6 studies were 20 IU/mL, 3 studies were 30 FDA-U/mL (equivalent to 40 IU/mL), and 1 study was 28 IU/mL. Performance characteristics of test kits were shown in the supplementary materials (Table S8).The average quality score of the studies was 8.33. Among these, 46% had a quality score of 9, 41% had a quality score of 8, and 13% had a quality score of 7. The quality scores of each eligible study are shown in Supplementary Table S6.
Table 1. Characteristics of included studies.
Pooled and stratified seropositivity rate of anti-PT IgG
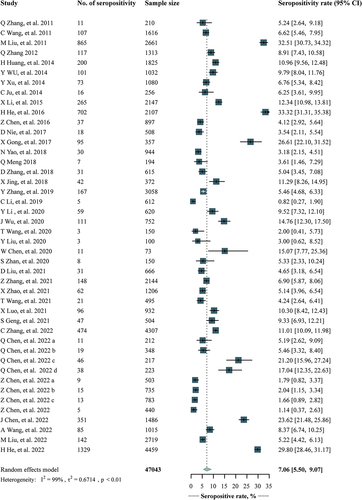
A total of 39 studies involving 47 778 participants reported the seropositivity rates of anti-PT IgG. The pooled estimate was 7.06% (95% CI, 5.50%-9.07%), with study-specific rates ranging from 0.82% to 33.32% (). The sensitivity analysis showed that the pooled estimates were relatively robust, fluctuating between 6.81% (95% CI, 5.33%-8.71%) and 7.39% (95% CI, 5.81%-9.40%) (Table S7). No obvious publication bias was found using the Peter test (p = .103).
shows the pre-specified subgroup meta-analyzes for six variables considering the seropositivity rates of anti-PT IgG. The estimates varied across ages (p = .022), being the highest among children aged 1–2 years old (12.50%, 95% CI, 9.31%-16.78%); higher among those 5–9 years old (8.96%, 95% CI, 7.25%-11.08%), 10–19 years old (8.90%, 95% CI, 7.46%-10.63%), and 40 years and older (8.96%, 95% CI, 7.20%-11.16%); slightly high among those younger than 1 year old (8.71%, 95% CI, 5.04%-15.08%); and the lowest among those 20–39 years old (6.36%, 95% CI, 5.17%-7.83%). The estimates also varied across study regions (p < .001), with the lowest in Southwestern China (4.22%, 95% CI, 3.20%-5.56%) but the highest in East China (11.89%, 95% CI, 8.17%-17.29%). As for ELISA kits, the estimates were 6.31% (95% CI, 4.55%-8.75%) in studies that tested by Virion/Serion, 6.77% (95% CI, 4.10%-11.18%) in those that tested by Euroimmun, and 12.79% (95% CI, 8.33%-19.63%) in those that tested by other types. As for the anti-PT IgG cutoff values, the estimates decreased with the cutoff values, from 13.64% (95% CI, 7.43%–25.04%) in studies that used 20 IU/mL, 12.34% (95% CI, 11.03%-13.82%) in 28 IU/mL, 10.59% (95% CI, 6.83%-16.43%) in 30 IU/mL, and to 5.59% (95% CI, 4.15%-7.54%) in those that used 40 IU/mL.
Table 2. Subgroup analysis for seropositivity rate of anti-PT IgG.
The estimated age-specific seropositivity rate of anti-PT IgG from 2012 to 2020
Based on the 238 data points extracted from the included studies, we first conducted univariate and multivariate meta-regressions because of the availability of a substantial number of age- and investigation-year-specific data points. The results of the univariate meta-regression analyzes demonstrated that age, investigation year, ELISA kit, and cutoff value (all p < .0001) were significantly associated with the seropositivity rate of anti-PT IgG (Supplementary Table S4). To control for the association of different ELISA kits and cutoff values with estimates, we chose only studies that used Virion/Serion and a cutoff value of 40 IU/mL, which had the largest dataset (134 data points). The relationship between age, investigation year, and the seropositivity rate of anti-PT IgG is demonstrated in Supplementary Figure S2, showing that the estimates gradually decreased with age, then slowly rose, and gradually became stable. Moreover, the seropositivity rates were higher in 2012 and lower in 2020.
The estimated age-specific seropositivity rates of anti-PT IgG among individuals in 2012, 2016, and 2020 are shown in . The seropositivity rates decreased from 7.22% (95% CI, 4.62%-11.11%) among children younger than 1 year old to 4.48% (95% CI, 2.92%-6.82%) among those aged 10–14 years, and then gradually increased to 5.99% (95% CI, 3.94%-9.02%) among adults aged 40 years and older in 2020. During the 8 years from 2012 to 2020, the decreasing seropositivity rates of anti-PT IgG were similar across the entire age range, fluctuating from 56.82% to 58.50%.
Table 3. The estimated age-specific seropositivity rate of anti-PT IgG in 2012, 2016, and 2020.
Discussion
This systematic review and meta-analysis comprehensively describes the seroprevalence of pertussis based on anti-PT IgG levels in the general Chinese population, with an overall seropositivity rate of 7.06%. Children aged 1–2 years had the highest rate at 12.5%, while adults of childbearing age (20–39 years) had the lowest rate at 6.36%.
Pertussis is a highly infectious disease with a basic regeneration number (R0) of 12 ~ 17.Citation1 The calculated immune protection of the population must reach 91.67% ~ 94.12% to block the transmission of pertussis. PT is a specific component of B. pertussis and is regarded as a strong immunogen.Citation63 Antibodies against PT represent clinical immunity against pertussis and are believed to be the most important mediators of clinical protection.Citation15 Our results revealed that the overall seropositivity rate of anti-PT IgG in the Chinese population was only 7.06%, which was lower than those reported by studies conducted in Korea, Brazil and Thailand.Citation64–66 This discrepancy may be caused by different immunization strategies, types of vaccines and antibody detection methods. Up to now, there is no universally accepted threshold for seropositivity of antibodies against pertussis and the definition of this threshold varies across countries.Citation64,Citation65,Citation67,Citation68 In this meta-analysis, we pooled the seropositive rate based on thresholds adopted in each studies, which was defined according to the corresponding manufacturer’s recommendation. These thresholds were relatively higher than those adopted in other countries.Citation64,Citation65 It also should be noted that, the relationship between serological level and protection has not yet been well established,Citation16 and the levels of antibodies positivity cannot fully represent their clinical protection effectiveness.
A relatively higher seropositivity rate of 12.50% was observed in children aged 1–2 years than in other age groups. However, this rate was lower than expected. The current immunization strategy for pertussis in China was 4-dose series of DTP administered at 3, 4, 5, and 18 months of age.Citation8,Citation69 According to the report by the World Health Organization (WHO), administrative and official DTP-containing vaccination (3rd dose) coverage has been maintained more than 99% in China for many years,Citation70 therefore, children aged 1 to 2 years old were expected to have an ideal antibody level. Such low seropositivity rate might be due to the unsatisfactory performance of the vaccines. Currently, DPT vaccines produced domestically adopted a co-purification process during their preparation,Citation9 which may, to a certain extent, affect the stability of antigen content among different vaccine batches. In addition, the seropositivity rate of anti-PT IgG declined to approximately 8% in the population older than 5 years, indicating a decay of antibodies. These could partially explain the current increase in pertussis incidence in the context of high childhood pertussis vaccination coverage rates.Citation69 A similar phenomenon was observed in seroepidemiological studies conducted in other Asian countries.Citation66,Citation71,Citation72 A nationwide Japanese study reported that Japanese children aged 3–6 years had significantly lower titers and avidities of anti-PT IgG than young children aged 1–2 years.Citation71 A cross-sectional study conducted in Russia demonstrated that Russian children became susceptible to infection soon after entering school.Citation72
Meanwhile, our study showed that adults of childbearing age (20 ~ 39 years) had the lowest seropositivity rate of 6.36%, highlighting the high susceptibility of this population. Once pregnant women and their families are infected with pertussis, they are at high risk of transmitting the bacterium to their newborns, who suffer from severe symptoms and complications.Citation2 A dose of the tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine is strongly recommended for pregnant women in some developed countries because it can help protect the mother from transmitting pertussis to her infant and provide passive immunity to the infant.Citation73,Citation74 However, no Tdap is available for teenagers or adults in China.
Interestingly, studies conducted after 2012 reported a relatively lower seropositivity rate than those conducted before 2012, although this difference was not statistically significant. However, after adjusting for age, ELISA kits, and cutoff values by multivariable meta-regression, the seropositivity rate showed a significant downward trend with the investigation years. Between 2012 and 2020, seropositivity rates of anti-PT IgG decreased by 56.82% to 58.50% across age groups. This can be partially explained by vaccine switching. Since 2007, DTwP vaccines have gradually replaced DTaP vaccines, and their replacement was completed throughout the country in 2013.Citation9 It has been reported that DTaP vaccines are significantly less reactogenic, mainly in terms of immune persistence than DTwP vaccines.Citation75 The European study also indicated that adolescents who received the DTwP vaccine in childhood were more protected during pertussis outbreaks than those who received the DTaP vaccine.Citation76 Additionally, a meta-analysis evaluating the duration of pertussis immunity after DTaP immunization found that the odds of pertussis increased 1.33 times fold every year after the last dose of DTaP.Citation77 Therefore, booster vaccination for pertussis at school-entry age or in adolescents is needed after the use of DTaP.
This study also revealed geographical variations in the seropositivity rate for anti-PT IgG. In general, people living in East China had a relatively higher seropositivity rate for anti-PT IgG than those in other regions of China. This gap may be related to discrepancies in the coverage and types of pertussis vaccines.Citation32 In addition, laboratory techniques, such as different detection reagents and corresponding cutoff values, can affect the results. Although the anti-PT IgG antibodies in the included studies were assayed using ELISA, different ELISA kits were used. Most studies employed commercial ELISA kits (Virion/Serion, Euroimmun, or Zhengzhou Etio), while a few studies used in-house ELISA methods. Two commercial ELISA kits (Virion/Serion or Euroimmun) yielded similar results, whereas other ELISA methods presented a higher rate. This was mainly due to the lower cutoff value adopted in other ELISA methods. These factors should be considered when interpreting the results of antibody-level studies, and the reagents should be unified in subsequent studies to increase comparability.
To the best of our knowledge, this is the first systematic review and meta-analysis on the seroprevalence of pertussis in a Chinese population. This study fills an important gap in the literature and will inform policymakers and advocacy communities regarding the epidemiological magnitude and profiles of pertussis. Furthermore, we conducted multiple subgroup analyzes and multivariate meta-regression based on the available evidence of the study characteristics, facilitating a broader assessment of pertussis seroprevalence in the Chinese population.
Our study had a few limitations. Although we restricted antibodies to specific antigens (PT), substantial heterogeneity was detected among the studies included in our meta-analysis. However, if the predefined inclusion criteria are met, and the sensitivity analysis shows that the pooled estimates are robust, the heterogeneity may already be acceptable. Additionally, the data in this meta-analysis were not evenly distributed throughout the country; thus, the pooled seropositivity rate of anti-PT IgG for regions with limited or no data might not reflect the actual situation. This may be the case for estimates in Western China, where data are particularly limited due to low population densities.
Pertussis is a pressing health issue in the Chinese population. In addition to the low seropositivity rate of anti-PT IgG, a downward trend has been observed in recent years. Furthermore, seroprotection in school-aged children and young adults is of particular concern. These findings highlight the urgent need for great efforts to optimize immunization strategies and promote future policy formation and programmatic implementation. Additionally, more high-quality epidemiological studies on the effectiveness of pertussis-containing vaccines are required.
Author’s contributions
Hanqing He, Yao Zhu, and Wanting Zhang conceptualized the study. Yao Zhu, Wanting Zhang, and Jie Hu contributed to literature review and data extraction. Yao Zhu and Shuying Luo evaluated the quality of the included studies. Yang Zhou, Xuewen Tang, and Xuan Deng performed statistical analyzes. Yao Zhu, Wanting Zhang and Rui Yan wrote the first draft of the manuscript. Hanqing He and Shuying Luo accessed and verified the data. All authors interpreted the results, commented on the drafts of the manuscript, and approved the final version. All authors had full access to all data in the study and were ultimately responsible for the decision to submit the manuscript for publication.
supplementary materials_clean.docx
Download MS Word (530.1 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data required to evaluate the conclusions of this study are presented in the paper, Supplementary Materials, or both.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2024.2341454
Additional information
Funding
References
- Decker MD, Edwards KM. Pertussis (Whooping Cough). J Infect Dis. 2021;224(12 Suppl 2):S310–11. doi:10.1093/infdis/jiaa469.
- Zhang C, Zong Y, Wang Z, Wang L, Li Y, Yang Y. Risk factors and prediction model of severe pertussis in infants < 12 months=”” of=”” age=”” in=”” Tianjin,=””>. BMC Infect Dis. 2022;22(1):24. doi:10.1186/s12879-021-07001-x.
- Nguyen VTN, Simon L. Pertussis: the whooping cough. Prim Care. 2018;45(3):423–31. doi:10.1016/j.pop.2018.05.003.
- Guiso N. Bordetella pertussis and pertussis vaccines. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. Clin Infect Dis. 2009;49(10):1565–9. doi:10.1086/644733.
- Guiso N, Meade BD, Wirsing von König CH. Pertussis vaccines: the first hundred years. Vaccine. 2020;38(5):1271–6. doi:10.1016/j.vaccine.2019.11.022.
- World Health Organization (WHO). Pertussis vaccines: WHO position paper—August 2015 [R]; 2015.
- World Health Organization (WHO). Pertussis[EB/OL]; 2022 Jul 23. https://www.who.int/health-topics/pertussis#tab=tab_2.
- Zhang Z, Pan J, Chen M, Zhang T, Li J, Lu L. Seroepidemiology of pertussis in China: a population-based, cross-sectional study. Vaccine. 2021;39(12):1687–92. doi:10.1016/j.vaccine.2021.02.032.
- Yu J, He H, Zhang Y, Gao Y, Chen C, Xu J, Xu L, Zhang X, Zhou Q, Zhu Y. et al. Burden of whooping cough in China (PertussisChina): study protocol of a prospective, population-based case–control study. BMJ Open. 2022;12(3):e053316. doi:10.1136/bmjopen-2021-053316.
- Zhang Y, Bambrick H, Mengersen K, Tong S, Feng L, Zhang L, Liu G, Xu A, Hu W. Resurgence of pertussis infections in Shandong, China: space-time cluster and trend analysis. Am J Trop Med Hyg. 2019;100(6):1342–54. doi:10.4269/ajtmh.19-0013.
- World Health Organization (WHO). Pertussis reported cases and incidence[EB/OL]; 2023 Nov 4. https://immunizationdata.who.int/pages/incidence/PERTUSSIS.html?CODE=Global+CHN&YEAR=.
- Chen Z, Zhang J, Cao L, Zhang N, Zhu J, Ping G, Zhao J, Li S, He Q. Seroprevalence of pertussis among adults in China where whole cell vaccines have been used for 50 years. J Infect. 2016;73(1):38–44. doi:10.1016/j.jinf.2016.04.004.
- Hughes MM, Englund JA, Edwards K, Yoder S, Tielsch JM, Steinhoff M, Khatry SK, LeClerq SC, Katz J. Pertussis seroepidemiology in women and their infants in Sarlahi District, Nepal. Vaccine. 2017;35(48):6766–73. doi:10.1016/j.vaccine.2017.09.074.
- C KC, Huang YC, Hsieh YC, Huang Y-L, Huang Y-C, Hung Y-T. Seroepidemiology of pertussis among elementary school children in northern Taiwan. J Microbiol Immunol Infect. 2017;50(3):327–32. doi:10.1016/j.jmii.2015.07.006.
- Kapil P, Merkel TJ. Pertussis vaccines and protective immunity. Curr Opin Immunol. 2019;59:72–8. doi:10.1016/j.coi.2019.03.006.
- Plotkin SA, Orenstein WA, Offit PA. Vaccines [M]. 7th ed. Philadelphia, PA: Elsevier; 2018.
- Cutts FT, Hanson M. Seroepidemiology: an underused tool for designing and monitoring vaccination programmes in low- and middle-income countries. Trop Med Int Health. 2016;21(9):1086–98. doi:10.1111/tmi.12737.
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi:10.1371/journal.pmed.1000097.
- Zhang Y, Huang H, Gao Z, Liu Y, Liu P, Ding Y, Wang L, Chen D, Wu S. A sera-epidemiological study on pertussis immunity levels among community populations and an analysis of the underlying factors in Tianjin China. Vaccine. 2015;33(51):7183–7. doi:10.1016/j.vaccine.2015.10.133.
- Munn Z, Moola S, Riitano D, Lisy K. The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. Int J Health Policy Manag. 2014;3(3):123–8. doi:10.15171/ijhpm.2014.71.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. doi:10.1016/0197-2456(86)90046-2.
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis[J]. Stat Med. 2002;21(11):1539–58. doi:10.1002/sim.1186.
- Higgins JP, Thompson SG, Deeks JJ, Altman, DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. doi:10.1136/bmj.327.7414.557.
- Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta-analysis. JAMA. 2006;295(6):676–80. doi:10.1001/jama.295.6.676.
- Wallace BC, Schmid CH, Lau J, Trikalinos TA. Meta-analyst: software for meta-analysis of binary, continuous and diagnostic data. BMC Med Res Methodol. 2009;9(1):80. doi:10.1186/1471-2288-9-80.
- Zhang Q, Han F, Nie Q, Ren H, Zhang B, Liu Q, He Q, Shao Z. Seroprevalence of antibodies to pertussis and diphtheria among healthy adults in China. J Infect. 2011;63(6):441–6. doi:10.1016/j.jinf.2011.07.018.
- Wang CQ, Zhu QR. Seroprevalence of Bordetella pertussis antibody in children and adolescents in China. Pediatr Infect Dis J. 2011;30(7):593–6. doi:10.1097/INF.0b013e31820eaf88.
- Liu M, Zheng H, Xu N. Surveillance of antibody levels of pertussis, diphtheria in children from different areas in Guangdong Province during 2007-2008. Chin J Vaccines Immun. 2011;17(5):436–9.
- Zhang Q, Zheng H, Liu M, Han K, Shu J, Wu C, Xu N, He Q, Luo H. The seroepidemiology of immunoglobulin G antibodies against pertussis toxin in China: a cross sectional study. BMC Infect Dis. 2012;12(1):138. doi:10.1186/1471-2334-12-138.
- Huang H, Liu Y, Zhang Z, Gao Z, Liu P, Li Y, Zhang Y, Cao W. A sero-epidemiological study on pertussis among the community-based populations in Tianjin during 2010–2012. Chin J Epidemiol. 2014;35(12):1354–7.
- Wu Y, Zhu B, Gao Y, Shi Z, Wang J, Wang H, Shao Z. Clustered cases of Bordetella pertussis infection cause high levels of IgG antibodies against pertussis toxin in adolescents in Gaobeidian city, China. Epidemiol Infect. 2014;142(4):738–43. doi:10.1017/S0950268813003099.
- Xu Y, Wang L, Xu J, Wang X, Wei C, Luo P, Ma X, Hou Q, Wang J. Seroprevalence of pertussis in China: need to improve vaccination strategies. Hum Vaccines Immunother. 2014;10(1):192–8. doi:10.4161/hv.26335.
- Ju C, Duan Y, Chen H. Seroepidemiology of immunoglobulin G antibodies against pertussis toxin and diphtheria in healthy people in Nanshan District, Shenzhen City. China Trop Med. 2014;14:1466–8.
- Li X, Chen M, Zhang T, Li J, Zeng Y, Lu L. Seroepidemiology of diphtheria and pertussis in Beijing, China: a cross-sectional study. Hum Vaccines Immunother. 2015;11(10):2434–9. doi:10.1080/21645515.2015.1062954.
- He H, Yao P, Zhou Y, Deng X, Pan J. Is pertussis infection neglected in China? Evidence from a seroepidemiology survey in Zhejiang, an Eastern Province of China. PLOS ONE. 2016;11(5):e0155965. doi:10.1371/journal.pone.0155965.
- Nie D. The research about pertussis infection and epidemic status in Suizhou, Hubei Province [D]. Beijing: Chinese Centre for Disease Control and Prevention; 2017.
- Gong X, Yin Z, Lan Y, Zhong J, Fang Q. Epidemiological characteristics and immune surveillance of pertussis in Quzhou City. Zhonghua Yu Fang Yi Xue Za Zhi. 2017;18(6):470–2.
- Yao N, Zeng Q, Wang Q. Seroepidemiology of diphtheria and pertussis in Chongqing, China: serology-based evidence of Bordetella pertussis infection. Public Health. 2018;156:60–6. doi:10.1016/j.puhe.2017.12.009.
- Meng QH, Liu Y, Yu JQ, Li L-J, Shi W, Shen Y-J, Li L, Zhan S-N, Yang F, Wang Y-J. et al. Seroprevalence of maternal and cord antibodies specific for diphtheria, tetanus, pertussis, measles, mumps and rubella in Shunyi, Beijing. Sci Rep. 2018;8(1):13021. doi:10.1038/s41598-018-31283-y.
- Zhang D, Liu C, Feng J, Duan H, Mi F, Liu M, Guan Q, Yu T, Liu Q, Li Z, et al. Epidemiological features of pertussis and population antibody levels in two regions of Guizhou province,2006–2016. Chin J Vaccines Immun. 2018;24(4):383–6+2.
- Jing X, Lu M. The seroepidemiology of anti-Bordetella pertussis toxin IgG antibodies among children in Shanghai. Chin J Infect Chemother. 2018;18:585–91.
- Zhang Y, Chen Z, Zhao J, Zhang N, Chen N, Zhang J, Li S, He Q. Increased susceptibility to pertussis in adults at childbearing age as determined by comparative seroprevalence study, China 2010–2016. Journal Of Infection. 2019;79(1):1–6. doi:10.1016/j.jinf.2019.04.011.
- Li C, Guo J, Guan L. Guo F, Mi R, Fu J, Cui X, Xiao F, Ma G, Lyu Y, Geng S. Pertussis antibody titers in pregnant women′s venous blood and cord blood—a survey from three women and children′s hospitals in Beijing. Chin J Neonatol. 2019;5:338–42.
- Wu J, Chen Q, Leng H, Guo X. IgG antibody levels against pertussis toxin and filamentous hemagglutinin among healthy people of Jiangsu province in 2018. Chin J Vaccines Immun. 2020;26(2):173–6.
- Li YT, Luo XQ, Zhong XB, Cai L-M, Zhu L-P, Chen X-Q, Wang K-C, Chen Z-G. Seroprevalences of antibodies against pertussis, diphtheria, tetanus, measles, mumps and rubella: a cross-sectional study in children following vaccination procedure in Guangzhou, China. Vaccine. 2020;38(23):3960–7. doi:10.1016/j.vaccine.2020.03.056.
- Wang T, Gao Y, Duan Y, Chen H, Yuan M, Shao Z, Wang C. Investigation on antibody level of pertussis diphtheria tetanus in healthy population in Nanshan District in Shenzhen in 2018. J Prev Med Intell. 2020;36(3):326–31.
- Liu Y, Wang L, Yu H, Yang C, Wang B, Sun J, Han Y, Ma X. The level of antibodies to Bordetella pertussis among pregnant women and their newborns in Jinan area. J Clin Pediatr. 2020;38(10):726–9.
- Chen W, Zhang M, Hu Y, Lei NC, Bai Y, Li PS. Detection and analysis of antibodies against pertussis toxin and filamentous hemagglutinin among healthy adults. Chin J Vaccines Immun. 2020;26(2):169–72.
- Zhan S, Yi H, Li L, Zhang Q, Li N, Zhang A, Wang X, Yang F, Wei J, Wang Y. Serum antibody levels of pertussis, diphtheria and tetanus toxins in healthy pregnant women in Shunyi District, Beijing. Beijing Med Sci. 2019;41(11):1046–8.
- Liu D, Cheng X, Wei S, Yuan L, Chen C, Yao K. Decline of serologic immunity to diphtheria, tetanus and pertussis with age suggested a full life vaccination in mainland China. Human Vaccines & Immunotherapeutics. 2021;17(6):1757–62. doi:10.1080/21645515.2020.1840253.
- Zhao X, Ye S, Ma R, Dong HJ, Fang T, Xu GZ. [Seroepidemiology of pertussis in healthy population in Ningbo, 2019]. Chin J Epidemiol. 2021;42(4):638–42. doi:10.3760/cma.j.cn112338-20200629-00894.
- Wang T, Yuan M, Gao Y, Chen H, Zhu BQ, Shao ZJ, Duan YX. [Survey of antibody levels of pertussis, diphtheria and tetanus in 495 pregnant women in Nanshan District of 2019, Shenzhen[J]. Chi J Prev Med. 2021;55(4):521–7. doi:10.3760/cma.j.cn112150-20200331-00481.
- Luo X, Zheng L, Zheng J, Chen Z, Wu S. Seroepidemiological survey of pertussis among community population in Sanming city in 2019. Straits J Prev Med. 2021;27(4):31–3.
- Geng S, Zhang D, Zhao M, Guo S, Jia G, Xia H, Cong K, Zheng H. In 2019, healthy people in Xuchang City pertussis, diphtheria, analysis of tetanus antibody level monitoring results. South China J Prev Med. 2021;47(7):933–6.
- Zhang C, Hu W, Wang R, Li Y, Lv Y, Li W, Si Y, Zhang S. Seroepidemiology of pertussis and diphtheria among healthy adults in Shaanxi Province, northwest China: A large - scale cross-sectional study. Hum Vaccines Immunother. 2022;18(6):2133913. doi:10.1080/21645515.2022.2133913.
- Chen Q, Wang W, Shi X, Xu Y, Zhu Y, Wu Y, Wang Z, Sun H, Sun X. Seroepidemiology of pertussis in the east of China: estimates of incidence of infection in adolescents and adults pre- and post-COVID-19. Front Public Health. 2022;10:1054617. doi:10.3389/fpubh.2022.1054617.
- Chen Z, Pang J, Zhang Y, Ding Y, Chen N, Zhang N, He Q. Seroprevalence of pertussis in adults at childbearing age pre- and post- COVID-19 in Beijing, China. Chi J Prev Med. 2022;10(6):872. doi:10.3390/vaccines10060872.
- Chen Z, Pang J, Zhang N, Chen N, Ding Y, He Q. Seroprevalence study of pertussis in adults at childbearing age and young infants reveals the necessity of booster immunizations in adults in China. Vaccines. 2022;10(1):84. doi:10.3390/vaccines10010084.
- Chen J, Jiying X, Chen LI, Jun ZH, Suling WU. Investigation on serum pertussis toxin antibody levels in 0-14 year old children in Hangzhou. Chin Clin J Pediatr. 2022;37(24):1895–8.
- Wang A, He Q, Tao X, Ma Y, He P, Wu X. Survey of pertussis antibody levels in healthy people in Guangzhou in 2019. J Trop Med. 2022;22(12):1734–7.
- Liu M, Peng X, Zhang W, Li Z, Li B, Ke B, He D. Survey on pertussis toxin IgG antibody levels in Guangdong Province in 2018. South China J Prev Med. 2022;48(2):210–3.
- He H, Zhu Y, Jin M, Zhou Y, Tang X, Yan R, Deng X, Chen K. The decline in immunity and circulation of pertussis among Chinese population during the COVID-19 pandemic: A cross-sectional sero-epidemiological study. Vaccine. 2022;40(48):6956–62. doi:10.1016/j.vaccine.2022.10.020.
- Gregg KA, Merkel TJ. Pertussis toxin: a key component in pertussis vaccines? Toxins. 2019;11(10):557. doi:10.3390/toxins11100557.
- Cardona RSB, Weckx LY, de Moraes-Pinto MI, Ramos BCF, dos Santos ARA, Spina FG, de Araújo BC, Clemens R, Clemens SAC. Pertussis antibodies and vaccination coverage among healthcare professionals in Brazil is inadequate: a cross-sectional serological study. Vaccine. 2023;41(39):5769–74. doi:10.1016/j.vaccine.2023.08.008.
- Lee SY, Han SB, Bae EY, Kim J-H, Kang JH, Park Y-J, Ma SH. Pertussis seroprevalence in Korean adolescents and adults using anti-pertussis toxin immunoglobulin G. J Korean Med Sci. 2014;29(5):652–6. doi:10.3346/jkms.2014.29.5.652.
- Hanvatananukul P, Prasarakee C, Sarachai S, Aurpibul L, Sintupat K, Khampan R, Saheng J, Sudjaritruk T. Seroprevalence of antibodies against diphtheria, tetanus, and pertussis among healthy Thai adolescents. Int J Infect Dis. 2020;96:422–30. doi:10.1016/j.ijid.2020.04.088.
- Son S, Thamlikitkul V, Chokephaibulkit K, Perera J, Jayatilleke K, Hsueh P-R, Lu C-Y, Balaji V, Moriuchi H, Nakashima Y. et al. Prospective multinational serosurveillance study of Bordetella pertussis infection among 10- to 18-year-old Asian children and adolescents. Clin Microbiol Infect. 2019;25(2):250.1–.7. doi:10.1016/j.cmi.2018.04.013.
- Torzsa P, Devadiga R, Tafalla M. Seroprevalence of Bordetella pertussis antibodies in adults in Hungary: results of an epidemiological cross-sectional study. BMC infectious diseases. BMC Infect Dis. 2017;17(1):242. doi:10.1186/s12879-017-2356-2.
- Zhang J, Deng J, Yang Y. Pertussis vaccination in Chinese children with increasing reported pertussis cases. Lancet Infect Dis. 2022;22(1):21–2. doi:10.1016/S1473-3099(21)00752-0.
- World Health Organization (WHO). Diphtheria tetanus toxoid and pertussis (DTP) vaccination coverage[EB/OL]; 2024 Mar 29. https://immunizationdata.who.int/global/wiise-detail-page/diphtheria-tetanus-toxoid-and-pertussis-(dtp)-vaccination-coverage?CODE=CHN&ANTIGEN=DTPCV3&YEAR=.
- Fumimoto R, Otsuka N, Sunagawa T, Tanaka-Taya K, Kamiya H, Kamachi K. Age-related differences in antibody avidities to pertussis toxin and filamentous hemagglutinin in a healthy Japanese population. Vaccine. 2019;37(18):2463–9. doi:10.1016/j.vaccine.2019.03.055.
- Kurova N, Timofeeva EV, Guiso N, Macina D. A cross-sectional study of Bordetella pertussis seroprevalence and estimated duration of vaccine protection against pertussis in St. Petersburg, Russia. Vaccine. 2018;36(52):7936–42. doi:10.1016/j.vaccine.2018.11.007.
- Centers for Disease Control and Prevention of the United States. Pregnancy and whooping cough: [EB/OL]; 2023 Dec 19. https://www.cdc.gov/pertussis/pregnant/hcp/pregnant-patients.html.
- Department of Health and Aged Care,Australian Government. Influenza and pertussis vaccination for pregnant women[EB/OL]; 2023 Dec 19. https://www.health.gov.au/news/influenza-and-pertussis-vaccination-for-pregnant-women.
- Weigl JA, Bock HL, Clemens R, Zepp F, Habermehl P, Beutel K, Müschenborn S, Sümenicht G, Schuind A, von König CH. et al. Safety and efficacy of acellular pertussis vaccines: the Mainz study and other recent studies. Ann Acad Med Singap. 1997;26(3):320–5.
- Klein NP, Bartlett J, Fireman B, Rowhani-Rahbar A, Baxter R. Comparative effectiveness of acellular versus whole-cell pertussis vaccines in teenagers. Pediatrics. 2013;131(6):e1716–22. doi:10.1542/peds.2012-3836.
- McGirr A, Fisman DN. Duration of pertussis immunity after DTaP immunization: a meta-analysis. Pediatrics. 2015;135(2):331–43. doi:10.1542/peds.2014-1729.