ABSTRACT
COVID-19 vaccine uptake varied across countries, in part due to vaccine hesitancy fueled by a lack of trustworthy information. To help health workers provide evidence-based answers to common questions about COVID-19 vaccines and vaccination, and thereby, assist individuals´ decisions on vaccine acceptance, COVID-19 InfoVaccines, a joint WHO-EU project, was launched in February 2021 to support COVID-19 vaccine rollout in 6 Eastern European countries. COVID-19 InfoVaccines was made available in seven languages and shared on social media networks. A total of 262,592 users accessed COVID-19 InfoVaccines.com between February 11, 2021, and January 31st, 2023. The users were most interested in: general questions; vaccine efficacy and duration of protection; vaccine safety; vaccine co-administration, and dose-interval and interchangeability; though the interest in a specific theme varied in function of the epidemiological situation. A total of 118,510 (45.1%) and 46,644 (17.7%) users scrolled up to 35% and 75% of the COVID-19 InfoVaccines webpage, respectively. The average engagement rate was 71.61%. The users accessed COVID-19 InfoVaccines from 231 countries and territories, but the majority were in Ukraine (N = 38,404; 14.6%), Spain (N = 23,327; 8.9%), and Argentina (N = 21,167; 8.1%). Older Facebook users were more interested in COVID-19 information than younger individuals (X2 p-value < .0001). Two hundred twenty-eight videos were shared on YouTube. The average Click-Through-Rate on Facebook was 7.82%, and that on YouTube was 4.4%, with 60 videos having a Click-Through-Rate >5%, falling in the range of average YouTube video Click-Through-Rate (2% – 10%). As misinformation about vaccines and vaccination spreads easily and can negatively impact health-related decisions, initiatives like COVID-19 InfoVaccines are crucial to facilitate access to reliable information.
Introduction
The COVID-19 pandemic has been a major threat to public health. By greatly reducing the risk of severe illness and death, vaccination against SARS-CoV-2 is the most powerful tool to combat the virus. Vaccination program success lies not only in making safe vaccines equitably available but also in ensuring their acceptance and uptake by the target population.
COVID-19 vaccine acceptance rates widely vary across countries. A meta-analysis of 172 studies from 50 countries estimated a pooled COVID-19 vaccine acceptance rate of 61%.Citation1 Determinants of vaccine hesitancy encompass, among others, socioeconomic, and psychosocial factors, including knowledge of the vaccine.Citation2 A shift in how patients seek health information has been reported for more than a decade, with more using the internet as a first source of information.Citation3,Citation4 The quality of online information can vary from peer-reviewed online health information to personal blogs and opinions that are not fact-checked, raising the need for increasing the population’s awareness of trustful health information sources. Basch and colleagues concluded that most videos on vaccine information on YouTube are published by non-experts in vaccines, and that more than 65% of those videos discouraged vaccination.Citation5 A study in the United States reported a significant correlation between the sentiments of social media (tweets) and vaccine acceptance.Citation6
During the COVID-19 pandemic, social media has been particularly influential. A study undertaken in Philadelphia reported that individuals who use social media as the only source of vaccine information have double the chance of rejecting a COVID-19 vaccine.Citation7 Anti-vaccination efforts on social media contribute to population beliefs on the safety of vaccinations. Disinformation campaigns are substantially associated with a drop in vaccination coverage over time, with each point shift upwards in a five-point disinformation scale associated with a two percentage point drop in mean vaccination coverage year over year.Citation8 A randomized controlled trial in the United Kingdom and the United States found that recent misinformation lowers the intent to accept a COVID-19 vaccine by 6.2% points and 6.4% points in the United Kingdom and the United States, respectively, with “scientific-sounding” misinformation leading to a further decline in vaccination intent.Citation9 Another study in the United States also found a negative association between misinformation and vaccination uptake rates.Citation10 Information published through unscientific communications substantially influences socioeconomically vulnerable populations as they have difficulties finding and understanding health information.Citation11 Social media platforms vary in the degree of vaccine disinformation shared with the public.Citation12 If the information on social media originates from trusted sources, it can be a useful tool to increase and disseminate information on vaccines.Citation7 A study undertaken in Thailand revealed that the quality of a health website affects the intention to use it, especially when users trust the website and perceive its usefulness.Citation13 Accordingly, educational interventions, especially in increasing awareness of the benefits of vaccination and enhancing public confidence and trust in vaccination, play a fundamental role in minimizing vaccine hesitancy and thereby increasing uptake.Citation2
In light of the speed in which evidence was developing on COVID-19 and the importance of making WHO evidence-based recommendations on vaccination available as widely as possible to both health professionals and the general public, the WHO Collaborating Center for Vaccine Safety at the University de Santiago de Compostela, together with the WHO Regional Office for Europe, developed Covid19infovaccines.com, a multimedia content website that offers extensive educational information in the form of scientific videos, podcasts, and text content related to COVID-19 vaccination and COVID-19 vaccines. COVID-19 InfoVaccines is partially funded through a European Union-WHO joint project to support COVID-19 vaccine deployment and vaccination in six countries in Eastern Europe.Citation14 In this report, using data collected between February 2021 and January 2023, we aimed to analyze the adoption of COVID-19 Infovaccines as an educational tool. We also pretended to uncover the ways and means of reaching the COVID-19 Infovaccines website to help plan the dissemination of other educational tools in the future. Lastly, we examined COVID-19 Infovaccines content usefulness by exploring the users’ interest in a specific topic published in COVID-19 Infovaccines and the trend of interest in that specific topic in function of time and geographical location.
Methods
WHO collaborating center for vaccine safety
The WHO Collaborating Center for Vaccine Safety is located at the University Hospital Complex of Santiago de Compostela, Spain. It has been designated as a collaborating center since 2018, and its main function is to assist WHO in developing and updating training materials for health workers on vaccine safety, contraindications, and clinical management of adverse events following immunization; to contribute to WHO work in training of health workers on vaccine safety, contraindications, and introduction of new vaccines; and to contribute to WHO work in training national immunization technical advisory groups of experts (NITAGs) on developing recommendations on COVID-19 vaccines and vaccination strategies.Citation15
COVID-19 InfoVaccines project creation
COVID-19 InfoVaccines was created by the WHO Collaboration Center for Vaccine Safety in collaboration with the WHO Regional Office for Europe primarily to improve the knowledge and increase the confidence of healthcare workers in advising their patients about COVID-19 vaccines in order to enhance vaccine acceptance and avoid missed opportunities to vaccinate patients due to unjustified contraindications. COVID-19 InfoVaccines secondary aim was to inform members of the general public looking for thorough evidence-based answers to common questions related to COVID-19 vaccines and associated WHO recommendations.
COVID-19 InfoVaccines was established on 11 February 2021 as a result of the need for comprehensive and up-to-date information for health professionals in the context of a rapidly evolving epidemiological situation and several new vaccines being developed and introduced globally. A working group was established with experts from the WHO Collaborating Center, the WHO Regional Office for Europe, several WHO country offices, and independent experts in various fields.
COVID-19 InfoVaccines working group consists of 15 multidisciplinary members from Czechia, Georgia, Romania, Russian Federation, Spain and Ukraine. The working group, co-led by an international expert in vaccines and infectious diseases (F M-T) from the WHO Collaborating Center, and a Regional Advisor of the Vaccine-preventable diseases and immunization program at the WHO Regional Office for Europe (S S-D), includes content creators, translators, dubbers, project coordinators, video editors, designers, website developers and communications professionals.
COVID-19 InfoVaccines content creation and update
The working group is responsible for selecting the evidence-based content most relevant to COVID-19 vaccination and that could be of interest to healthcare professionals and the general public.
By WHO request, the WHO Collaborating Center for Vaccine Safety trained hundreds of frontline medical workers from Azerbaijan and Georgia on all aspects of COVID-19 vaccines and vaccination via webinars in April, June and October 2021. In February 2022, the WHO Collaborating Center also conducted an online training on vaccine safety and contraindications against vaccination for leading clinicians and immunization program staff from Armenia, Azerbaijan, Belarus, Georgia, Kazakhstan, Kyrgyzstan, Republic of Moldova, Tajikistan, Ukraine and Uzbekistan. During the webinars, the most updated information on the value and safety of COVID-19 vaccines, and recommendations on contraindications against vaccination as well as the risk–benefit ratio of vaccinating patients with comorbidities and children were discussed. Questions raised by the participants were noted and informed in part by the selection of relevant content for COVID-19 InfoVaccines. Other sources of questions included media and individual requests received by the WHO Regional Office for Europe. All answers were developed based on available, robust scientific evidence and WHO recommendations, with input provided by all members of the working group as per their individual areas of expertise.
The content was made available in the form of questions and the answers were recorded in video format and provided as a downloadable PDF. In addition, the WHO Collaborating Center for Vaccine Safety collaborated with experts in hygiene, infectious disease prevention, immunization and vaccines to organize a series of expert opinion videos addressing common concerns about COVID-19 and vaccination. The expert opinion series was in the format of short videos, from 1 to 5 minutes in duration.
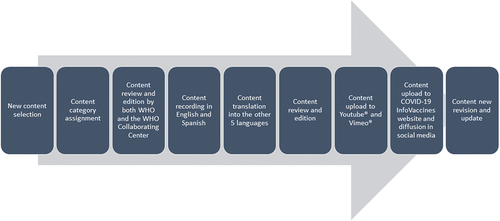
The process of content creation and publication is summarized in .
COVID-19 InfoVaccines dissemination
To increase the utility and visibility of COVID-19 InfoVaccines, the website as well as the transcripts of each video were posted in seven languages: Czech, English, Georgian, Romanian, Russian, Spanish, and Ukrainian. The content was originally prepared in English and Spanish, and then translated and adapted to the other five languages. COVID-19 InfoVaccines was promoted within professional networks and on social media, namely via Instagram, Facebook, Twitter and Google Ads.
The English and Ukrainian versions of the videos were transmitted via specific channels on YouTube® (@covid19infovaccines and @covid-19infovaccinesUA respectively), while the Spanish and Russian versions were disseminated through Vimeo® (covid19infovaccines). The content was also made available as a guide entitled “Learn about COVID-19 vaccines and vaccination on the go” which can be downloaded in PDF format and printed. All videos can be shared by e-mail or via social media networks.
Data analysis
To explore the reach of COVID-19 InfoVaccines, metadata were drawn from Google Analytics, Google Data Studio, and YouTube Studio between 11 February 2021 and 31 January 2023. The data included the number of users over time; undertaken events such as page viewing, starting sessions and scrolling; engagement; acquisition; traffic channels; users´ geographic distribution; and content viewed.
User demographic characteristics were not available for webpage use due to restrictions in the data use agreement; however, it was available for Facebook and YouTube channels. When available, differences in COVID-19 InfoVaccines use according to gender and age were analyzed using a chi-squared (X2) test.
Results
COVID-19 InfoVaccines use
A total of 262,592 users accessed the COVID-19 InfoVaccines between February 11, 2021, and January 31, 2023. Around 46,000 users visited COVID-19 InfoVaccines more than once. The users undertook 3,258,588 events. A total of 118,510 (45.1%) and 46,644 (17.7%) users scrolled up to 35% and 75% of the COVID-19 InfoVaccines webpage, respectively. The average engagement rate was 71.61% with an average engagement time of 1 min. and 27 seconds.
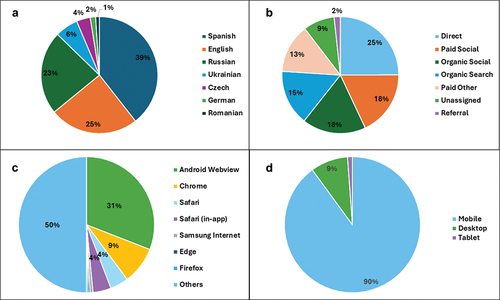
The users were located in 231 countries and territories (). Most were based in Ukraine (N = 38,404; 14.6%), Spain (N = 23,327; 8.9%), Argentina (N = 21,167; 8.1%), Venezuela (N = 11,478; 4.4%), Nicaragua (N = 10,625; 4.0%), Mexico (N = 10,473; 4.0%), Czechia (N = 9,932; 3.8%), the United States (N = 8,917; 3.4%), the United Kingdom (N = 7,985; 3.0%) and Latvia (N = 7,079; 2.7%). The most consulted languages on COVID-19 InfoVaccines were Spanish (N = 98,240; 37.3%), English (N = 61,482; 23.4%), Russian (N = 57,640; 22.0%) and Ukrainian (N = 15,940; 6.06%) ().
Figure 2. Distribution of COVID-19 InfoVaccines users worldwide. The bubble size reflects the number of users, the largest the bubble is, the greatest the number of users. Most of the users were located in Ukraine, Spain, Argentina, Venezuela, Nicaragua, Mexico, Czechia, United States, United Kingdom and Latvia; 46 countries and territories had more than 1000 users, 16 provided between 986 and 505 users, while the rest of the 233 had less than 500 users.
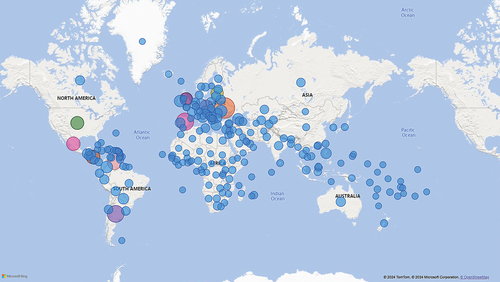
Figure 3. Acquisition characteristics of COVID-19 InfoVaccines. a) distribution of the languages used by the visitors to consult COVID-19 InfoVaccines. b) distribution of the traffic channel by which the user was first acquired. c) distribution of the browsers used by COVID-19 InfoVaccines visitors. d) distribution of the devices used by COVID-19 InfoVaccines visitors.
User acquisition
Device and traffic channel
The majority of the users accessed COVID-19 InfoVaccines through mobile phones (N = 236,180; 90%); 9% (N = 23,331) accessed through desktop, while the rest used tablets. Following Android Webview (31%), Google Chrome (9%) was the most commonly used browser (). The most frequent traffic channel by which the user was first acquired was direct search (25%) followed by paid social (18%), organic social (traffic from social media platforms) (18%), and organic search (unpaid listings on search engine results pages) (15%) () .
Organic Google search
A total of 43,877 organic Google search clicks drove the users to COVID-19 InfoVaccines. Users mainly inquired about COVID-19 vaccine doses, composition, and function. The most common search queries in English language were “janssen vaccine how many doses” (N = 1,441), “how many doses of johnson and johnson vaccine do you need” (N = 482), “what is considered fully vaccinated for COVID” (N = 434), “inactivated vaccine” (N = 396), “comirnaty bivalent” (N = 359), “protein-based vaccines” (N = 322), “sterilized vaccine” (N = 316), and “how do inactivated vaccines work” (N = 313).
The organic Facebook page generated 1,097 followers, 12,128 lifetime post reach and 2,548,541 post interaction, an indicator of user engagement with the published content. To increase page visibility, 34 campaigns were launched on Facebook in English, Georgian, Russian Federation, Spanish, and Ukrainian. Eighteen of the 34 campaigns were undertaken exclusively in Ukraine by the WHO Country Office in Ukraine. Overall, the campaigns generated 38,849,849 impressions that reached 10,517,410 users, who clicked 3,037,098 times, generating a Click-Through-Rate (CTR) of 7.82%. CTR is a ratio of how often people who see an ad or free product listing end up clicking on it. Females were more likely than males to click on an advertisement on COVID-19 as it was reflected by click-to-reach (females: 33.12%; males: 20.41%; unknown: 29.59%; X2 p-value <.0001) and CTR (females: 8.57%; males: 6.09%; unknown: 7.55%; X2 p-value <.0001) ratios. Older Facebook users were also more interested in COVID-19 information than younger individuals (X2 p-value <.0001). The click-to-reach ratio increased with age reaching 37.99% in users older than 65 years. Nonetheless, the CTR was slightly higher in individuals aged between 35 and 44 than in the older age categories, probably due to the higher activity of this age group on social media ().
Figure 4. Statistics of COVID-19 InfoVaccines use through Facebook platforms. a) Distribution of clicks to reach ratio according to age. b) Distribution of clicks to reach ratio according to gender. c) Distribution of clickthrough rate according to age. d) Distribution of clickthrough rate according to gender.
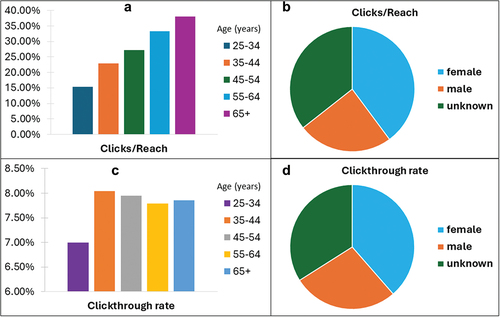
Restricting the analysis to Facebook campaigns launched by WHO in Ukraine also revealed a higher CTR value for females (3.13%) than males (2.34%). In Ukraine, in individuals aged 25 or older, the CTR increased with age, with individuals older than 65 years having the highest CTR values (3.94%) compared to other age categories.
Stratifying the campaigns by trimestral period showed that the highest CTR values were obtained in the 2nd trimester (May 16th - August 14th, 2021: CTR = 12.37%), 3rd trimester: (August 15th - November 13th, 2021: CTR = 13.96%) and 4th trimester (November 14th, 2021–February 12th, 2022: CTR = 11.91%).
Youtube
COVID-19 InfoVaccines YouTube channel included 228 videos published in English with an overall number of views of 115,593. The average impressions CTR was 4.4%, with 60 videos having CTR greater than 5, falling in the range of average YouTube video CTR (2%–10%).Citation16,Citation17 More than half of the audience were males (55.15%). Videos were more watched by older than younger adults (). The most-viewed videos are summarized in . The audience was mainly in the United States (N = 20,393; 17.6%), Australia (N = 9,127; 7.9%), the Philippines (N = 6,678; 5.8%), Canada (N = 5,556; 4.8%) and the United Kingdom (N = 5,147; 4.5%) (). About 43.0% of the audience did not use subtitles, while 42.0% used English subtitles. Eight hundred sixty-eight video shares and 702 video likes were registered.
Figure 5. Statistics of COVID-19 InfoVaccines adoption in YouTube. a) distribution of the number of views as a percentage of total views and the average view duration according to age. b) distribution of total watch time according to countries.
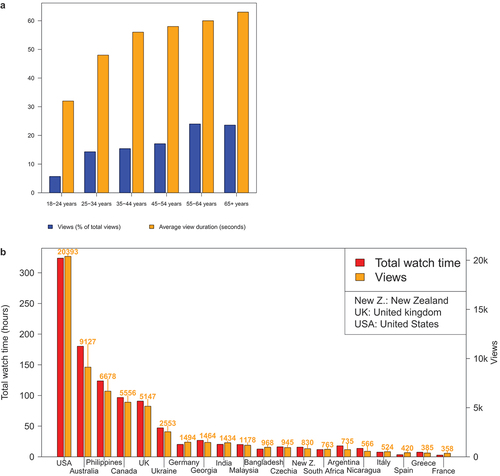
Table 1. Most-viewed videos on COVID-19 InfoVaccines YouTube channel.
The viewers mainly found the videos by browse features (30.5%), external search (27.4%), suggested videos (18.1%) and YouTube search (16.0%). About 65.1% (N = 73,016) viewers watched the videos on mobile phone, while 22.5% (N = 25,209) used computer, 7.2% (N = 8,805) used tablet and 5.1%(N = 5,730) used TV. The average view duration was the highest on TV (1 min., 14 sec.) and the lowest on mobile (1 min., 01 sec.).
Additionally, within the framework of promotional campaigns of COVID-19 vaccination in Ukraine the WHO Country Office in Ukraine launched a separate channel with Ukrainian dubbing. Covid-19infovaccinesUA YouTube channel includes 127 videos with an overall number of views of 1,359,144 (99% of which are from Ukraine). The average impressions CTR was 1.18%. More than half of the audience were males (56.8%). The audience aged 35 to 65+ accounted for 61% of viewers. The 10 most viewed videos are shown in . Only 19.4% of viewers used Ukrainian subtitles. The distribution by device showed that 53.7% (729,812) of viewers used TV, 25.6% (347,593) used mobile phone, 12,8% used computer (174,457) and 4.6% used tablet (62,412). The average view duration was the same for all devices.
Table 2. Most-viewed videos in “COVID-19 infovaccines Україна” YouTube channel.
Vimeo
Videos published in Vimeo COVID-19 InfoVaccines channel were seen 22,080 times and watched in full 7,300 (33.06%) times. The majority of these views took place in Ukraine (N = 5524; 25%), followed by Republic of Moldova (N = 1334; 6.0%), Latvia (N = 1015; 4.6%), Argentina (N = 949; 4.3%) and Georgia (N = 946; 4.28%). A total of 21582 (97.7%) views originated from the COVID-19 InfoVaccines webpage (covid19infovaccines.com). 17292 (78.3%) views took place on a mobile phone and 4323 (19.57%) were on a desktop. The most-viewed video on COVID-19 InfoVaccines Vimeo channel was “How does the Oxford-AstraZeneca COVID-19 vaccine work?” (N = 1,847; 1,261 views in English and 586 views in Russian).
Lifetime use of COVID-19 InfoVaccines
The lifetime user acquisition is presented in . Before initiating the advertisement campaigns in April 2021, most of the new users accessed COVID-19 InfoVaccines through direct search. The traffic of new users through paid social channels varied in function of the periodicity of the advertisement campaigns (); nonetheless, new users continued to adopt COVID-19 InfoVaccines through non-paid channels (direct, organic social, and organic search) ().
Figure 6. Lifetime adoption of COVID-19 InfoVaccines represented in weekly units (W) and stratified by the traffic channel (direct, organic search, paid social, organic social and paid other). W1 refers to the first week of COVID-19 InfoVaccines launching, and successively until week 101 (W101).
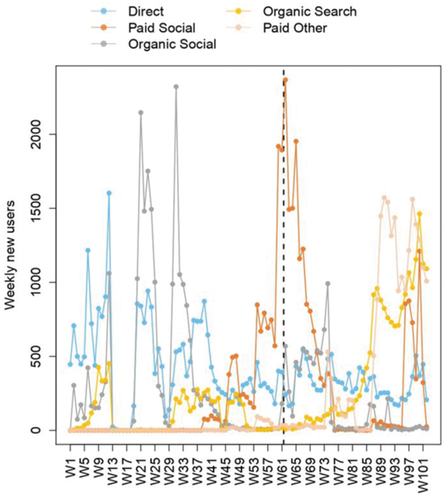
The greatest adoption of COVID-19 InfoVaccines was observed between the weeks of June 20th, 2021, and that of November 14th, 2021 (total N = 104,041). Most of those users were in Ukraine (N = 15,859; 15.2%), Nicaragua (N = 9,368; 9.0%), Venezuela (N = 7,464; 7.2%), Mexico (N = 5,879; 5.7%) and Argentina (N = 5,302; 5.1%). The seven advertisement campaigns launched during that period in Czech, English, Russian, Spanish, and Ukrainian could have contributed to COVID-19 InfoVaccines adoption during that period. These campaigns generated a CTR value of 13.43%, which is higher than the average values for advertising campaigns in the health as well as education sectors for the year 2022.Citation18
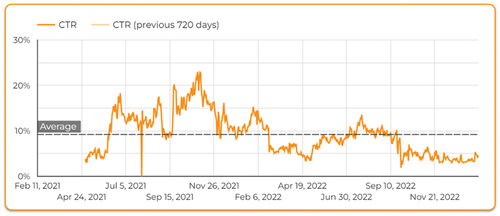
The organic Google search CTR was influenced by the marketing campaigns such as paid social ads. The CTR increased sharply on September 27th, 2021 from 0% to 5.39% and reached a maximum of 22.90% on November 18th, 2021, exceeding the average Google search CTR during the COVID-19 pandemic (5.90%).Citation19 Subsequently, the CTR started to fluctuate according to advertisement intensity and the evolution of the COVID-19 pandemic ().
COVID-19 InfoVaccines content
As of January 2023, the content of COVID-19 InfoVaccines was organized into 19 categories and videotaped after validation into almost four hours of video (). The content was regularly updated by removing videos with outdated information and updating others, and updating the downloadable PDF accordingly.
Table 3. COVID-19 InfoVaccines content (as of February 2023).
respectively summarize the most visualized pages of COVID-19 InfoVaccines in English and Spanish, the most common languages used. Inquiries about a specific topic related to COVID-19 vaccination varied with time and location, probably due to changes in the epidemiological situation and the availability of new information about the pandemic and vaccines. For instance, in the 1st trimester (February 11th - May 15th, 2021) Spanish speakers were interested in “Should I do a test to check that I am protected after getting vaccinated?” while those who use the English language were curious about “How does the Oxford-AstraZeneca COVID-19 vaccine work?.” Certain inquiries such as “Why are some vaccinated people getting sick with COVID-19 and in some cases being hospitalized?” remained of high interest for more than one trimester. The number of views per page could be influenced by the public interest in a topic, the epidemiological situation as well as by the advertisement campaigns.
Table 4. Most-viewed content published in English in COVID-19 InfoVaccines, stratified by trimester period since the website launch in February 2021.
Table 5. Most-viewed content published in Spanish in COVID-19 InfoVaccines, stratified by trimester period since the website launch in February 2021.
The videos were categorized into themes on the main page “COVID-19 vaccines and vaccination explained.” The users were most interested in the following themes: general questions; vaccine efficacy and duration of protection; vaccine safety; vaccine co-administration, dose-interval and interchangeability; Janssen COVID-19 Vaccine (Johnson & Johnson); vaccine and infection; and Vaxzevria – Oxford-AstraZeneca vaccine.
The compiled document “COVID-19 vaccines and vaccination explained,” which includes scripts of all videos address common questions about COVID-19 vaccines was downloaded 3,879 times.
The series of experts’ opinions collected a total of 36,378 views. It encompassed the following eight topics between May 2 and November 1, 2021, that are listed by descending number of views: 1) Can you be fully vaccinated against COVID-19 and get infected? (9,731 views), 2) Will we need further COVID-19 vaccine doses? (8,072 views), 3) Are vaccines against COVID-19 equal or different? (7,497 views), 4) The paradox of vaccination success and opposition to vaccination (3,259 views), 5) Safety of vaccines: a question of understanding risks and statistics (2,932 views), 6) How can we deal with vaccine hesitancy? (2,403 views), 7) What important lesson should we have learned from this pandemic for future pandemics? (1,686 views), and 8) How does COVID-19 pandemic influence vaccine value perception? (798 views).
COVID-19 InfoVaccines includes a portal dedicated to WHO questions and answers (WHO Q&A), and that links a user´s specific inquiry on COVID-19 infection and vaccination to the specific portal on the WHO website. The website also addresses common questions related to COVID-19 and vaccination among vulnerable populations, such as people with cancer, AIDS, diabetes and stroke patients, as well as questions about health risks like antibiotic resistance. The “Featured” section provides specific portals linking to the international days of various underlying health conditions and the corresponding content in COVID-19 InfoVaccines. For example, the portal of “December 1st, World AIDS Day” brings the user to content related to COVID-19 and vaccination in AIDS and immunocompromised individuals such as “How quickly does the vaccine work and how long does the protection last?” and “Can COVID-19 vaccines cause AIDS?.” The portal of World Diabetes Day links to content like “Is COVID-19 vaccination safe and recommended for people who have diabetes?” and “Why are people with diabetes a priority group for COVID-19 vaccination?.”
Discussion
COVID-19 InfoVaccines initiative represents a vital intersection of public health advocacy, digital innovation, and global collaboration. The data-driven insights derived from website analytics may help to guide future endeavors in health communication, emphasizing the need for responsive, accessible, and reliable digital health resources.
COVID-19 InfoVaccines is a multimedia content website that was created as a digital initiative supported by the WHO Regional Office in Europe to serve as trustful information on COVID-19 and related vaccinations. It was initiated to help provide scientific-based information on COVID-19 vaccines presented by health professionals who have expertise in vaccines and infectious disease prevention. COVID-19 InfoVaccines was shared on the most commonly used social media applications Facebook and YouTube, which count over 2.9 billion and 2.5 billion active users according to 2022 statistics.Citation20 The website reached five continents, yet most of the users were in Ukraine, Spain, Argentina, Venezuela, Nicaragua, Mexico, Czechia, the United States, the United Kingdom, and Latvia; 46 countries and territories had more than 1000 users, 16 provided between 986 and 505 users, while the rest of the 233 had less than 500 users. Social media statistics revealed that COVID-19 InfoVaccines was well-received by the population based on CTR values.Citation18,Citation19 COVD-19 InfoVaccines could have reached more users that cannot be measured, such as those who viewed the content by co-viewing, which has become a trend.Citation21
Several factors underpin COVID-19 InfoVaccines’ adoption and diffusion: 1) initiation by a trustworthy source – WHO, together with the WHO Collaborating Center for Vaccine Safety; 2) variety and continuous updating of content; 3) availability in six languages; 4) accessibility, as it was designed to be able to be used on any device, especially mobile phones; and 5) advertisement campaigns, which helped attract a large fraction of the users.
COVID-19 InfoVaccines has been a useful tool in informing more than 200,000 individuals about COVID-19 vaccination. The tool, in combination with training courses undertaken by the WHO and the WHO Collaborating Center, helped to increase frontline doctors’ knowledge of vaccine safety and contraindications against vaccination and improved their confidence in recommending vaccination to their patients. The utility of the tool is ongoing as the general public and healthcare professionals need to be continuously informed on evolving evidence and recommendations related to COVID-19 vaccines including safety and effectiveness, protection duration, and new vaccine formulations.
The COVID-19 InfoVaccines website emerged in the context of misinformation and infodemics,Citation22 illuminating a path for over quarter-million users seeking trustworthy information on COVID-19 vaccines. The detailed analytics of the website’s use reveal profound insights into the public’s engagement with digital health information sources, transcending geographic boundaries. Notably, the device and access trends – with a predominant use of mobile phones and discovery via organic Google search – underscore the public’s preference for readily accessible and searchable health information. The tendency toward mobile access also highlights the importance of optimizing health communication for mobile platforms to enhance reach and user engagement. Furthermore, the specific inquiries users made reveal a dynamic landscape of vaccine-related concerns that correlate with the evolving epidemiological situation, demonstrating the website’s role as a responsive and timely health resource.
Our findings that most users accessed the site via mobile devices and that older individuals engaged more with COVID-19 information on Facebook have crucial implications for tailoring communication strategies. The viewership patterns indicate that while the site was effective in reaching a broad audience, the video content had a significant impact, particularly among older users. This engagement with multimedia content highlights the importance of diverse content formats in health communication strategies.
The COVID-19 InfoVaccines website has functioned not only as an informational resource but also as a means for public health engagement, shaping the vaccine discourse in a rigorous scientific-based manner. The meticulous effort to present content in various languages and formats reflects a strong commitment to global health education and an understanding of the diverse needs of the website’s international audience. From these insights, the efforts of numerous individuals under this WHO-Europe initiative in maintaining and updating the COVID-19 InfoVaccines website seem well-justified. However, the discussion should also reflect on the resource allocation for such initiatives, with substantial human effort and funding behind the COVID-19 InfoVaccines project.
The pandemic has demonstrated the critical role of digital health tools in public health responses, and although the success of COVID-19 InfoVaccines reiterates the value of reliable online health resources, the magnitude of the impact of this initiative has to be carefully balanced against the important number of resources involved in the performance. The affirmative engagement metrics and widespread international use suggest that the website has been a valuable tool in combatting vaccine misinformation, and the website has likely contributed to informed decision-making among individuals considering COVID-19 vaccination. The success of such endeavors should be evaluated not only by the number of hits or views but also by the cascade effects this information may actually have when reaching the appropriate stakeholders and by their contribution to the overarching public health goal of increasing vaccine acceptance and uptake.
Conclusions
The endeavor to develop and maintain the COVID-19 InfoVaccines project, a collaborative effort between the WHO Collaborating Center on Vaccine Safety and the WHO Regional Office for Europe, has proven to be a substantial yet worthwhile undertaking. The analysis indicates that the website has effectively served its purpose of disseminating evidence-based information, as reflected by high engagement rates and widespread reach. The project’s ability to adapt content in real-time to the changing landscape of the pandemic has underscored the intrinsic value of such digital tools in public health communication.
The success of the COVID-19 InfoVaccines website lends credence to the potency of digital platforms in addressing public health challenges. However, it also opens a dialogue about the allocation of resources and the efficiency of digital campaigns. The substantial investment in creating and promoting accurate health information via the COVID-19 InfoVaccines website is a testament to the commitment to public health education and the fight against misinformation.
As the digital age ushers in a new paradigm for health communication, the experiences and lessons learned from COVID-19 InfoVaccines provide valuable insights. These should inform future strategies to enhance the reach and impact of online health resources, ensuring they remain a cornerstone of public health initiatives in the ongoing and future health crises.
Author contributions statement
Narmeen Mallah: investigation; literature review; data analysis and interpretation and writing original draft of the manuscript. Jacobo Pardo-Seco: Figures. Irene Rivero-Calle, Ouhao Zhu-Huang, María Fernández Prada, Catharina Reynen-de Kat, Oleg Benes, Liudmila Mosina, Siddhartha Sankar-Datta, Olga Aleksinskaya, Vusala Allahverdiyeva, Yevgenii Grechukha, Tamara Jobava, Mariia Savchyna, Pavla Kortusova, Ioana Novac: investigation; literature review; resources; and project administration. David Díaz: software; and resources. Federico Martinón-Torres: conceptualization; investigation; literature review; supervision; resources; funding; and project administration. All authors reviewed and revised the manuscript, approved it for publication and count responsible for its content.
Disclosure statement
Irene Rivero-Calle reports a relationship with GSK that includes speaking and lecture fees. Irene Rivero-Calle reports a relationship with Pfizer Inc that includes speaking and lecture fees. Irene Rivero-Calle reports a relationship with Sanofi Pasteur Inc that includes speaking and lecture fees. Irene Rivero-Calle reports a relationship with MSD Vaccins that includes speaking and lecture fees. Federico Martinon-Torres reports a relationship with GSK Vaccines SRL that includes consulting or advisory. Federico Martinon-Torres reports a relationship with Pfizer Inc that includes consulting or advisory. Federico Martinon-Torres reports a relationship with Sanofi Pasteur Inc that includes consulting or advisory. Federico Martinon-Torres reports a relationship with Janssen Pharmaceuticals Inc that includes consulting or advisory. Federico Martinon-Torres reports a relationship with MSD that includes consulting or advisory. Federico Martinon-Torres reports a relationship with Seqirus Pty Ltd that includes consulting or advisory. Federico Martinon-Torres has received support for the present work from the Instituto de Salud Carlos III (Proyecto de Investigación en Salud, Acción Estratégica en Salud): Fondo de Investigación Sanitaria (FIS; PI070069/PI1000540/PI1601569/PI1901090) del plan nacional de I+D+I and ‘fondos FEDER’ and Proyectos GaIN Rescata-Covid_IN845D 2020/23 (GAIN, Xunta de Galicia). Irene Rivero-Calle has acted as a sub-investigator in randomized controlled trials of Ablynx, Abbot, Seqirus, Sanofi Pasteur MSD, Sanofi Pasteur, Cubist, Wyeth, Merck, Pfizer, Roche, Regeneron, Jansen, MedImmune, Novavax, Novartis and GSK.
Additional information
Funding
References
- Norhayati MN, Che Yusof R, Azman YM. Systematic review and meta-analysis of COVID-19 vaccination acceptance. Front Med. 2021;8:783982. doi:10.3389/fmed.2021.783982.
- Anakpo G, Mishi S. Hesitancy of COVID-19 vaccines: rapid systematic review of the measurement, predictors, and preventive strategies. Hum Vaccin Immunother. 2022;18(5):2074716. doi:10.1080/21645515.2022.2074716.
- Hesse BW, Nelson DE, Kreps GL, Croyle RT, Arora NK, Rimer BK, Viswanath K. Trust and sources of health information: the impact of the Internet and its implications for health care providers: findings from the first health information national trends survey. Arch Intern Med. 2005;165(22):2618–13. doi:10.1001/archinte.165.22.2618.
- Tan SS, Goonawardene N. Internet health information seeking and the patient-physician relationship: a systematic review. J Med Internet Res. 2017;19(1):e9. doi:10.2196/jmir.5729.
- Basch CH, Zybert P, Reeves R, Basch CE. What do popular YouTube(TM) videos say about vaccines? Child Care Health Dev. 2017;43(4):499–503. doi:10.1111/cch.12442.
- Aleksandric A, Obasanya MJ, Melcher S, Nilizadeh S, Wilson GM. Your tweets matter: how social media sentiments associate with COVID-19 vaccination rates in the US. Online J Public Health Inform. 2022;14(1):e2. doi:10.5210/ojphi.v14i1.12419.
- Al-Uqdah L, Franklin FA, Chiu CC, Boyd BN. Associations between social media engagement and vaccine hesitancy. J Community Health. 2022;47(4):577–87. doi:10.1007/s10900-022-01081-9.
- Wilson SL, Wiysonge C. Social media and vaccine hesitancy. BMJ Glob Health. 2020;5(10):e004206. doi:10.1136/bmjgh-2020-004206.
- Loomba S, de Figueiredo A, Piatek SJ, de Graaf K, Larson HJ. Measuring the impact of COVID-19 vaccine misinformation on vaccination intent in the UK and USA. Nat Hum Behav. 2021;5(3):337–48. doi:10.1038/s41562-021-01056-1.
- Pierri F, Perry BL, DeVerna MR, Yang KC, Flammini A, Menczer F, Bryden J. Online misinformation is linked to early COVID-19 vaccination hesitancy and refusal. Sci Rep. 2022;12(1):5966. doi:10.1038/s41598-022-10070-w.
- Ruedin D, Probst J, Wanner P, Efionayi-Mader D, Bodenmann P. COVID-19-Related health literacy of socioeconomically vulnerable migrant groups. Int J Public Health. 2022;67:1604664. doi:10.3389/ijph.2022.1604664.
- Puri N, Coomes EA, Haghbayan H, Gunaratne K. Social media and vaccine hesitancy: new updates for the era of COVID-19 and globalized infectious diseases. Hum Vaccin Immunother. 2020;16(11):2586–93. doi:10.1080/21645515.2020.1780846.
- Boon-Itt S. Quality of health websites and their influence on perceived usefulness, trust and intention to use: an analysis from Thailand. J Innov Entrep. 2019;8(4). doi:10.1186/s13731-018-0100-9.
- World Health Organization. Partnering with the European Union to support deployment of COVID-19 vaccines and vaccination. https://www.who.int/europe/activities/partnering-with-the-european-union-to-support-deployment-of-covid-19-vaccines-and-vaccination.
- World Health Organization. WHO Collaborating Center for Vaccine Safety. https://apps.who.int/whocc/Detail.aspx?NKqHaG2CXWNiJMIqXqvhig==.
- YouTube.Impressions & click-through-rate FAQs 2023. https://support.google.com/youtube/answer/7628154?hl=en#zippy=%2Chow-do-i-know-if-my-impressions-click-through-rate-is-high-or-low.
- Databox. Improve YouTube click-through rate (CTR) and grow your channel with these 11 pro tips 2022.
- Heitman S. Updated search advertising benchmarks for 2022 (+Expert tips to improve results): LoacliQ; 2022. https://localiq.com/blog/search-advertising-benchmarks/.
- Irvine M. Updated Google ads benchmarks for your industry during COVID-19: word stream; 2022. https://www.wordstream.com/blog/ws/2020/04/06/google-ads-benchmarks-during-covid-19.
- Statista. Most popular social networks worldwide as of January 2023, ranked by number of monthly active users 2022. https://www.statista.com/statistics/272014/global-social-networks-ranked-by-number-of-users/.
- Strike Social. The rise of co-viewing and connected TV. https://www.dropbox.com/s/1j9vz14qz74stww/2021%20YouTube%20Insight%20-%20Strike%20Social.pdf?dl=0&utm_source=insight+page&utm_medium=button&utm_campaign=2021+YouTube+Insight+Report.
- World Health Organization. Managing the COVID-19 infodemic: promoting healthy behaviours and mitigating the harm from misinformation and disinformation 2020. https://www.who.int/news/item/23-09-2020-managing-the-covid-19-infodemic-promoting-healthy-behaviours-and-mitigating-the-harm-from-misinformation-and-disinformation.