ABSTRACT
Seasonal influenza significantly affects both health and economic costs in children and adults. This narrative review summarizes published cost-effectiveness analyses (CEAs) of cell-based influenza vaccines in children and adults <65 years of age, critically assesses the assumptions and approaches used in these analyses, and considers the role of cell-based influenza vaccines for children and adults. CEAs from multiple countries demonstrated the cost-effectiveness of cell-based quadrivalent influenza vaccines (QIVc) compared with egg-based trivalent/quadrivalent influenza vaccines (TIVe/QIVe). CEA findings were consistent across models relying on different relative vaccine effectiveness (rVE) estimate inputs, with the rVE of QIVc versus QIVe ranging from 8.1% to 36.2% in favor of QIVc. Across multiple scenarios and types of analyses, QIVc was consistently cost-effective compared with QIVe, including in children and adults across different regions of the world.
Introduction
Seasonal influenza causes considerable health and economic costs for children and adults annually.Citation1,Citation2 In an average influenza season, attack rates between 20–35% can be seen in children,Citation3 and 5–15% of the population will be affected.Citation4 Among children, rates of hospitalization and complications are typically higher in those <5 years of age,Citation5 and the disease burden in children <2 years of age can be substantial.Citation6 Economic costs associated with influenza in early childhood cohorts are often high, in part due to substantial indirect caregiver costs, such as lost working days for parents; with direct costs in Europe reported as up to €252 per child per episode and indirect costs of up to €251 per episode for workdays lost by parents.Citation2 As such, protection of young children from influenza may be beneficial. Certain inactivated vaccines are indicated for children as young as 6 months of age in the United States,Citation7,Citation8 although pediatric indications for seasonal influenza vaccines may begin from 2 years of age in other regions,Citation9,Citation10 and from 2 years of age for live attenuated influenza vaccines (LAIV) in the United States.Citation8
Vaccination protects against influenza infection through the induction of humoral antibodies largely against the hemagglutinin (HA) and neuraminidase (NA) glycoproteins.Citation11 Annual vaccination against seasonal influenza is required because this segmented negative-strand RNA virus continually changes over time to avoid immune detection.Citation12 Each influenza season is different from the last because the relative prevalence of drifted circulating influenza A strains and influenza B lineages causing human disease is also continuously evolving.Citation13 Of note, the Yamagata lineage of influenza B viruses has not been isolated in recent years following the coronavirus disease 2019 (COVID-19) pandemic, and the World Health Organization (WHO) Influenza Vaccine Composition Advisory Committee, along with the US Food and Drug Administration (FDA), have recommended that B/Yamagata lineage antigens be removed from influenza vaccines for use internationally as soon as reasonably possible.Citation14,Citation15 This update will lead to changes in vaccine composition and the re-introduction of trivalent influenza vaccines (TIVs) or alternative quadrivalent options in regions that currently recommend Yamagata-containing quadrivalent influenza vaccines (QIVs).Citation16,Citation17
Multiple factors affect vaccine effectiveness (VE), including complex host–virus interactions that may influence the level of vaccine protection experienced by individuals.Citation18 Many of the factors affecting VE are difficult to control; however, vaccine mismatch due to egg adaptation (which has the greatest impact on the A/H3N2 antigen) can potentially be eliminated by optimizing the technology of vaccine production. In traditional egg-based vaccine manufacture, vaccine virus propagation occurs in fertilized chicken eggs. As a result of this process, viral HA and NA sequences may undergo avian-adaptive amino acid changes that may affect the antigenicity of the vaccine against WHO-selected strains prepared prior to each influenza season, and thus potentially limit the protection that egg-based influenza vaccines provide against eventual circulating influenza strains, including A/H3N2.Citation19 Mismatched seasons are associated with reduced VE and tend to be associated with higher rates of hospitalization.Citation19 Cell-based influenza vaccine manufacturing avoids egg adaptation and, consequently, potential mutations at antigenic sites.Citation20 There is a potential for increased VE if egg-adaptive changes that arise when using the traditional egg-based manufacturing process are avoided.Citation21
Seasonal variation in circulating influenza strains, the potential for vaccine mismatch during influenza seasons, and a need for continual monitoring and data collection on vaccine performance in the real-world, justifies the requirement for the ongoing collection of real-world evidence (RWE). Compared with data from randomized controlled trials (RCTs), RWE enables the timely collection of data on additional endpoints that may not be evaluated in RCTs, VE estimates from multiple seasons, and estimates of relative VE (rVE) between different vaccines.Citation22,Citation23 RWE may also enable the evaluation of data from different settings, age groups, and risk groups that complement those assessed in RCTs. RWE is of particular importance to cost-effectiveness analysis (CEA) modeling and to National Immunization Technical Advisory Groups (NITAGs) requiring comparative economic evaluations.Citation24
Analysis of trends seen across CEAs is of interest as CEAs and budget impact modeling contribute to NITAG recommendations and/or reimbursement decisions, thus influencing patient access to influenza vaccines. This narrative review provides an overview of published CEAs on cell-based influenza vaccines in children and adults <65 years of age, critically assesses assumptions and approaches in identified CEAs, and provides an expert opinion about the role of cell-based influenza vaccines for adults and children. The review focuses on individuals <65 years of age because, although cell-based influenza vaccines are approved for older adults,Citation7 country guidelines may preferentially recommend higher-dose or adjuvanted vaccines in those ≥65 years of age.Citation8
Methods
A targeted literature search was performed in September 2023 in MEDLINE (PubMed) to identify CEAs of cell-based influenza vaccines in children and adults <65 years of age. Search terms included: influenza, cost-effectiveness, cell-based TIV/QIV (TIVc/QIVc), egg-based TIV/QIV (TIVe/QIVe), LAIV, and recombinant QIV (QIVr). Systematic search methodology was not used and studies were not graded for quality. Outputs retrieved from database searches were hand-searched for populations and study types of interest. English language publications were prioritized by the application of a filter on search outputs. Additional references were gathered by searching the reference lists of identified publications and via author recommendations. Search strings focused on the cost-effectiveness analysis of cell-based influenza vaccines yielded approximately 30 hits. Searches focused on the cost-effectiveness of LAIV and QIVr returned between approximately 10 to 50 results, the majority of which were excluded based on lack of comparison with TIVc/QIVc. Supplemental searches on the cost-effectiveness of TIVe/QIVe returned more than 700 hits, most of which were excluded based on lack of comparison with TIVc/QIVc.
Results
Eleven CEAs from nine countries were analyzed, of which nine CEAs evaluated QIVc versus TIVe/QIVe (; ).Citation25,Citation27,Citation29–35 In addition, one study estimated the cost impact of extending QIVc to a broader age group.Citation36 Finally, one CEA analyzed QIVc versus QIVr.Citation37 Although no formal quality analysis was conducted, all studies were transparent in their methodology and reporting, presented uncertainty analyses, and discussed study limitations. Model input parameters were similar between analyses from the payer/healthcare system perspective, and between those from the societal perspective.
Figure 1. CE of QIVc vs QIVe, itemized by study perspective, time horizon, population and estimated local willingness-to-pay threshold.Citation25,Citation27,Citation29–35
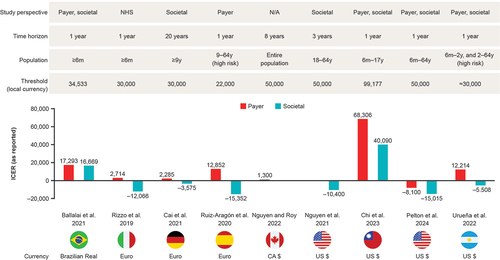
Table 1. CEA of QIVc versus QIVe.
Cost-effectiveness studies of QIVc and QIVe
Nine CEAs of QIVc versus TIVe/QIVe were analyzed.Citation25,Citation27,Citation29–35 CEAs were performed in eight countries. CEA models included a range of rVE estimates, vaccine costs, study designs, time horizons, and study perspectives ().Citation25,Citation27,Citation29–35 Nearly all studies evaluated cost-effectiveness from both payer and societal perspectives; of these, the societal perspective was the base case scenario in two analyses, and a dual base case design was used in four studies, including the three most recently published CEAs.Citation25,Citation27,Citation29–35 Four studies included susceptible–exposed–infectious–recovered (SEIR) dynamic-transmission modeling as part of their CEAs, and one study used an adapted dynamic 4Flu transmission combined with a decision-tree model ().Citation25,Citation27,Citation29–35
Estimates of rVE of QIVc versus QIVe applied in the CEA studies ranged from 8.1% to 36.2%, with a trend toward lower estimates applied in more recent CEAs. The three most recent CEAs used rVE data from meta-analysis estimates,Citation33–35 and several other analyses included a base case rVE point estimate originating from the 2017–2018 egg-adapted seasonCitation25,Citation27,Citation29,Citation30,Citation32 (), which was characterized as a high-severity season with documented evidence of egg-adapted mutations in the egg-based vaccine A(H3N2) strain dominated by A(H3N2) and B strains.
Table 2. Variation in rVE estimates of QIVc versus QIVe as part of sensitivity/scenario analyses.Citation25,Citation27,Citation29–35
Estimates from all eight countries demonstrated the cost-effectiveness of QIVc compared with QIVe (or TIVe, used in one analysisCitation25) in both children and adults, with base case incremental cost-effectiveness ratios (ICERs) ranging from CA$1,300 to US$68,306 (less than pre-defined thresholds) or QIVc found to be dominant in all instances (). QIVc was frequently a dominant strategy from a societal perspective. ICERs in analyses where QIVc was dominant ranged from −€3,575 to −€15,352 (). Although QIVc had a higher vaccine acquisition cost than QIVe, its use was associated with direct and indirect savings, owing to higher rVE.Citation25,Citation27,Citation29–35
Cost-effectiveness studies of QIVc and other comparators
One CEA of QIVc versus QIVr was identified.Citation37 A dynamic transmission model was applied and calibrated to United Kingdom (UK) infection data across 10 influenza seasons. The model estimated that the rVE of QIVr must be ≥25% versus QIVc to be cost-effective (incremental cost-effectiveness ratio of £20,000) at list prices for UK adults <65 years of age.Citation37 A different study in the UK found that extending QIVc to all individuals 50–64 years of age, rather than to at-risk individuals 50–64 years of age only, was cost-effective or cost-saving.Citation36 No CEA comparisons were found between QIVe and QIVc, QIVc and LAIV, and QIVc and adjuvanted or higher-dose vaccines.
Discussion
We performed a narrative review of published CEAs on cell-based influenza vaccines in children and adults <65 years of age. Despite variations in model structure, perspective, and base case parameters used, we found broad consistency in estimates of cost-effectiveness, with QIVc identified as cost-effective relative to egg-based vaccines.
Effectiveness inputs
CEAs are highly sensitive to estimates of benefit, such as rVE inputs.Citation33,Citation40,Citation41 The WHO recommends the use of VE estimates from systematic reviews or meta-analyses, or a range of values, subject to sensitivity analyses representative of extreme circumstances.Citation33,Citation40,Citation42,Citation43 Best available rVE estimates should be inputted into CEA models, and, depending on local needs, a range of rVE values can be justified with respect to WHO guidance.
The analyzed CEAs used a range of rVE values in models. Some variation in rVE point estimates between models can be attributed to differences in populations assessed, different influenza endpoints assessed, and different methodologies used to generate estimates, including data from single-season retrospective cohorts, data from different influenza seasons, pooled RWE estimates from multiple seasons, and point estimates from meta-analyses. The highest rVE values of 26.8% and 36.2% used in five CEAsCitation25,Citation27,Citation29,Citation30,Citation32 were based on RWE from the 2017–2018 season,Citation26,Citation28 which was characterized as a high-severity influenza season with egg-adapted vaccine-mismatch dominated by A(H3N2) and B strains. Influenza A(H3N2) can be particularly affected by egg adaptation and is a strain that can be associated with high rates of illness, medical visits, and hospitalization, especially in those ≥50 years of age.Citation44–47 Seasons with adaptive mutations in the A(H3N2) egg-based vaccine strain are anticipated to provide the greatest differences in VE between QIVc and QIVe, as avoidance of mismatch related to egg adaptation underpins a rationale for cell-based manufacture.
Findings of cost-effectiveness of QIVc over QIVe are not surprising, considering rVE estimates favoring QIVc from rigorous sources, such as meta-analyses, large RWE studies including multiple seasons, and test-negative designs.Citation34,Citation38,Citation43,Citation48–51 Meta-analysis data used in three CEAsCitation33–35 summarized the rVE of QIVc versus QIVe against influenza-related medical encounters as 8.1% in children ≥4 years of age and 11.4% in adults based on four studies.Citation34 In RWE studies, the rVE of QIVc versus QIVe for the prevention of influenza-related hospitalizations/emergency room visits was 14.4%, 6.5%, and 5.3% in children and adults for the 2017–2018, 2018–2019, and 2019–2020 influenza seasons, respectively. The rVE of QIVc versus QIVe for the prevention of overall inpatient or outpatient influenza-related medical encounters was 19.3%, 7.6%, and 17.2% in children and adults for the 2017–2018, 2018–2019, and 2019–2020 influenza seasons, respectively. This RWE shows that QIVc consistently provided similar or better protection against influenza-related medical encounters, with benefits seen in both children and adults, and in both matched and mismatched seasons.Citation38,Citation48–50 A recent study using a retrospective test-negative design to evaluate the rVE of QIVc versus QIVe for preventing test-confirmed influenza in outpatients 4–64 years of age during the 2017–2018, 2018–2019, and 2019–2020 seasons found rVE estimates of 14.8%, 12.5%, and 10.0%, respectively.Citation51 These findings, based on estimates from approximately 3000–4000 individuals administered QIVc and approximately 30,000 individuals administered QIVe that attended outpatient clinics and were tested for influenza due to acute respiratory or febrile illness, are similar to rVE estimates obtained from much larger retrospective cohort studies involving millions of individuals.Citation38,Citation48–50 Test-negative study designs are increasingly used to efficiently evaluate VE ensuring a robust selection of appropriate test-negative controls, thereby minimizing selection bias and supporting internal validity.Citation52 Finally, a recent review of the rVE of QIVc versus QIVe in children identified point estimates ranging from 2.1% to 33.0%, suggesting an incremental benefit of QIVc versus QIVe in this population.Citation45 To enhance the outputs of future CEAs, additional effectiveness and rVE data from children <4 years of age should be included, as should rVE estimates by disease severity. RWE from more countries and generated using different study designs is also desirable.
Study design, study perspective, and sensitivity/scenario analysis
CEA model type can influence cost-effectiveness estimates. The use of dynamic models, wherein the estimated force of infection changes over time, is preferred over static models in scenarios when an intervention affects disease transmissionCitation53; however, the use of dynamic models is dependent on adequate data on infection rates, an adequate reflection of the population, and the impact of vaccination and rVE on infection and transmission, which can be difficult to estimate. Cost-effectiveness of QIVc over QIVe has been shown in both static and dynamic models.Citation25,Citation27,Citation29–35
Certain NITAGs recommend the use of a societal base case analysis, wherein both direct and indirect costs, such as absenteeism and parental work loss, are included in the cost-effectiveness estimate,Citation29,Citation54 whereas other decision-makers may find payer perspectives more actionable to their needs and more straightforward to interpret. In the current analysis, cost-effectiveness of QIVc over QIVe was seen in both payer and societal analyses.Citation25,Citation27,Citation29–35 Furthermore, it is interesting to observe the trend of cost-effectiveness and cost-savings (ie, QIVc as a dominant strategy over QIVe) extended across both high-income and low-/middle-income countries (LMICs), as indirect costs in LMICs may have less influence on societal perspective analyses than in wealthier countries as, for example, the cost of absenteeism may have less impact in countries with lower average salaries.
The WHO recommends that base case findings should be viewed as one of many potential outcomes. Sensitivity and scenario analysis can be of great help to decision-makers, as meaningful information can be found in outcomes modeled under extreme scenarios, which is relevant to decisions made for long-term programs. Probabilistic sensitivity analysis (PSA) may enable decision-makers to judge the impact of results across a broad range of potential outcome estimates.Citation55 In the current analysis, PSA was performed in all studies and deterministic sensitivity analysis was performed in most studies.Citation25,Citation27,Citation29–35 Multiple studies report that cost-effectiveness estimates are validated by sensitivity and scenario analyses, with some studies reporting that QIVc remains highly cost-effective, even in the worst-case scenarios.
Other considerations
In our analysis, no CEA comparisons were found in the direction of QIVe versus QIVc, between QIVc and LAIV, and between QIVc and adjuvanted or higher-dose vaccines. As LAIVs are approved for individuals 2–49 years of age without underlying medical conditions,Citation8 and noninvasive intranasal formulations may offer administration advantages for children, a CEA of QIVc and LAIV may be of interest in some settings, particularly as LAIVs are produced with egg-based manufactureCitation56 and thus may be susceptible to egg adaptation. Large country datasets reporting VE with egg-based vaccines may not stratify VE findings by those produced from QIVe or egg-based LAIV alternatives, which may confound analysis, but suggests that economic comparisons of QIVc versus LAIV may result in estimates similar to those seen when comparing QIVc versus QIVe, although this assumption must undergo evidence-based validation. Adjuvanted or higher-dose vaccines are most commonly recommended in older adults,Citation8 rather than in individuals <65 years of age, which is the focus of this review. Data support improved VE for adjuvanted or higher-dose influenza vaccines relative to standard-dose unadjuvanted egg-based vaccines in adults ≥65 years of age, and economic analyses confirm the cost-effectiveness of these comparisons.Citation23,Citation57 However, a clear benefit of QIVc over QIVe has not been established in adults ≥65 years of age,Citation19 and data comparing QIVc with adjuvanted or higher-dose influenza vaccines in this older population is not available. As data allow, CEAs comparing cell-based vaccines to alternative strategies in older adults may also be of interest in the future, especially as adjuvanted cell-based influenza vaccines are currently in development.
When making a vaccine recommendation, NITAGs consider multiple factors, such as whether a specific vaccine or platform is associated with higher VE, whether costs are more reasonable, and whether cost-effectiveness can be demonstrated. Our review has identified that many CEAs rely on rVE estimates from populations primarily comprised of adults, and/or apply a single rVE to estimate cost-effectiveness across mixed pediatric/adult datasets, as rVE values in children have been lacking. Recently, it has been suggested that it may be beneficial for infants to receive their first dose of influenza vaccine from a vaccine without egg adaptations.Citation58 First and subsequent exposures to recurrent egg-adapted epitopes may result in a long-lasting reduction in immune response to circulating influenza viruses (through imprinting).Citation59,Citation60 As imprinted responses to egg-adapted antigens may have long-term consequences, further study of the occurrence of immune imprinting, and consideration of how this effect may be translated into a measurable health outcome, is encouraged. This may allow for greater recognition of cell-based vaccines by NITAGs as an option to avoid egg adaptation and greater acknowledgment of the improvements in effectiveness and value for money associated with cell-based vaccines. The selection of first vaccines for children requires careful consideration, as better protection of young children protects older adults and may spare parents from costly work absences.Citation61
Finally, although RCTs are considered the gold-standard source of evidence in many contexts, including regulatory approvals, RWE can be of high quality and application of evidence-based medicine plus (EBM+) approaches may be of particular value to seasonal influenza VE assessment and CEAs.Citation62 Furthermore, different forms of statistical interrogation can be used to interpret the validity of estimates obtained from non-randomized observations, such as RWE. Quantitative bias analysis can be performed to determine how much plausible uncertainty is needed to invalidate an effect estimate.Citation63 Meta-regression techniques for the assessment of heterologous within-effect variability can identify sources of variation within an effect estimate from a meta-analysis.Citation64,Citation65 These and other forms of quantitative bias analysis may provide enhanced confidence to committees considering observational data to make and justify policy decisions. Both RCTs and RWE provide complementary sources of data for inclusion within evidence appraisal. Indeed, as influenza vaccination may transition from quadrivalent to trivalent vaccines as early as Northern Hemisphere season 2024/2025 in some countries in-line with WHO composition recommendations, new RWE will be required to assess vaccine performance following regulatory approvals; although, we do not anticipate any change in the value of cell-based vaccines over egg-based vaccines.Citation14,Citation15,Citation66
As with any targeted literature review there are several limitations associated with our review. Although search terms were recorded to allow reproducibility, only one database, MEDLINE (PubMed), was searched and so, relevant sources not captured by MEDLINE may have been missed. The reference lists of identified publications were searched in order to limit this restriction. Further, analyses from gray literature, such as non-published models from government agencies were not considered.
Conclusions
This review of published CEAs highlights the consistency of cost-effectiveness and cost-saving findings of QIVc over QIVe across multiple analyses using different designs, inputs, perspectives, and when applied to children and adults in different world regions, including wealthy countries and LMICs. Our review identified published evidence that supports both the increased effectiveness of QIVc versus QIVe against disease outcomes and the cost-effectiveness of QIVc over QIVe, despite higher acquisition costs.
Author contributions
All authors made substantial contributions to the design, conception, analysis, and interpretation of literature review findings; critically reviewed draft manuscripts for important intellectual content and provided input into draft manuscripts; and provided final approval of the version to be published. All authors agree to be accountable for all aspects of the work.
Acknowledgments
Medical writing support was provided by Helene Wellington, MS, of Scion (a division of Prime, London, UK), supported by CSL Seqirus according to Good Publication Practice guidelines (Link).
Disclosure statement
D.F. has received honoraria for participating in advisory boards with Merck, Sanofi-Pasteur, CSL Seqirus, Pfizer, and AstraZeneca related to influenza, SARS-CoV-2, and pneumococcal vaccines.
N. G. received funding for investigator-led studies from GSK, MSD, CSL Seqirus, Takeda, and Sanofi-Pasteur and Global Vaccine Data Network. N.G. has received honoraria from CSL Seqirus, Takeda, and Pfizer for acting as a speaker in congresses, and from GSK, Takeda, and CSL Seqirus for taking part in advisory boards.
M.J.L. received honoraria from CSL Seqirus, GSK, Pfizer, Moderna, Curevo, AstraZeneca, and Dynavax for taking part in advisory boards. M.J.L. has received institution funding for investigator-led studies from GSK.
V.H.N. received funding for conducting RWE and CEAs on vaccines from Takeda, CSL Seqirus, Pfizer, and Moderna. V.H.N. has also received honoraria from those companies for taking part in advisory boards.
S.I.P. has received honoraria from CSL Seqirus as a consultant on research design and analysis and participation in advisory boards on influenza vaccines.
M.P. has received honoraria from CSL Seqirus for taking part in advisory boards.
J.R.A. has received honoraria from CSL Seqirus, GSK, and MSD for taking part in advisory boards.
A.U. received institution funding for investigator-led studies from GSK, MSD, CSL Seqirus, Takeda, and Sanofi-Pasteur. A.U. has also received honoraria from CSL Seqirus and Takeda for acting as a speaker in congresses, and from GSK, Takeda, Sanofi, and CSL Seqirus for taking part in advisory boards. She is a member of the Directory board of Sociedad Argentina de Vacunología y Epidemiologia (SAVE).
J.M.Q. is an employee of CSL Seqirus.
Additional information
Funding
References
- de Courville C, Cadarette SM, Wissinger E, Alvarez FP. The economic burden of influenza among adults aged 18 to 64: a systematic literature review. Influenza Other Respir Viruses. 2022;16(3):376–12. doi:10.1111/irv.12963.
- Villani L, D’Ambrosio F, Ricciardi R, de Waure C, Calabrò GE. Seasonal influenza in children: costs for the health system and society in Europe. Influenza Other Respir Viruses. 2022;16(5):820–31. doi:10.1111/irv.12991.
- World Health Organization. Influenza. https://www.who.int/teams/health-product-policy-and-standards/standards-and-specifications/vaccine-standardization/influenza#:~:text=Influenza%20occurs%20all%20over%20the,disruption%20across%20all%20age%20groups.
- Paget WJ, Balderston C, Casas I, Donker G, Edelman L, Fleming D, Larrauri A, Meijer A, Puzelli S, Rizzo C, et al., EPIA collaborators. Assessing the burden of paediatric influenza in Europe: the European Paediatric Influenza Analysis (EPIA) project. Eur J Pediatr. 2010;169(8):997–1008. doi:10.1007/s00431-010-1164-0.
- Ruf BR, Knuf M. The burden of seasonal and pandemic influenza in infants and children. Eur J Pediatr. 2014;173(3):265–76. (In eng). doi:10.1007/s00431-013-2023-6.
- Tillard C, Chazard E, Faure K, Bartolo S, Martinot A, Dubos F. Burden of influenza disease in children under 2 years of age hospitalized between 2011 and 2020 in France. J Infect. 2022;84(2):145–50. doi:10.1016/j.jinf.2021.11.006.
- Seqirus. FLUCELVAX QUADRIVALENT (influenza vaccine) prescribing information. 2021 Oct. https://www.fda.gov/media/115862/download?attachment.
- Advisory Committee on Immunization Practices. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States, 2023–24. Sum. recom. 2023 Aug 23. https://www.cdc.gov/flu/pdf/professionals/acip/acip-2023-24-Summary-Flu-Vaccine-Recommendations.pdf.
- European Medicines Agency. Flucelvax Tetra. 2023 Dec 15. https://www.ema.europa.eu/en/medicines/human/EPAR/flucelvax-tetra.
- European Medicines Agency. Fluenz Tetra. 2023 Aug 7. https://www.ema.europa.eu/en/medicines/human/EPAR/fluenz-tetra.
- Gomez Lorenzo MM, Fenton MJ. Immunobiology of influenza vaccines. Chest. 2013;143(2):502–10. (In eng). doi:10.1378/chest.12-1711.
- Bouvier NM, Palese P. The biology of influenza viruses. Vaccine. 2008;26(Suppl 4):D49–53. (In eng). doi: 10.1016/j.vaccine.2008.07.039.
- Zanobini P, Bonaccorsi G, Lorini C, Haag M, McGovern I, Paget J, Caini S. Global patterns of seasonal influenza activity, duration of activity and virus (sub)type circulation from 2010 to 2020. Influenza Other Respir Viruses. 2022;16(4):696–706. doi:10.1111/irv.12969.
- World Health Organization. Recommended composition of influenza virus vaccines for use in the 2024 southern hemisphere influenza season. 2023 Sept 29. https://www.who.int/publications/m/item/recommended-composition-of-influenza-virus-vaccines-for-use-in-the-2024-southern-hemisphere-influenza-season.
- Greenberg D. Industry Perspective: Challenges and Opportunities for Vaccine Strain Composition with the Reduced Public Health Threat from Influenza B/Yamagata Lineage Viruses. Vaccines and Related Biological Products Advisory Committee; 2023 Oct 5. https://www.fda.gov/media/172764/download.
- Paget J, Caini S, Del Riccio M, van Waarden W, Meijer A. Has influenza B/Yamagata become extinct and what implications might this have for quadrivalent influenza vaccines?. Euro Surveill. 2022;27(39):2200753. doi:10.2807/1560-7917.ES.2022.27.39.2200753.
- Vajo Z, Torzsa P. Extinction of the influenza B Yamagata line during the COVID pandemic—implications for vaccine composition. Viruses. 2022;14(8):1745. doi:10.3390/v14081745.
- McLean HQ, Belongia EA. Influenza vaccine effectiveness: new insights and challenges. Cold Spring Harb Perspect Med. 2021;11(6):a038315. (In eng). doi: 10.1101/cshperspect.a038315.
- Rockman S, Laurie K, Ong C, Rajaram S, McGovern I, Tran V, Youhanna J. Cell-based manufacturing technology increases antigenic match of influenza vaccine and results in improved effectiveness. Vaccines. 2022;11(1):52. doi:10.3390/vaccines11010052.
- Rajaram S, Boikos C, Gelone DK, Gandhi A. Influenza vaccines: the potential benefits of cell-culture isolation and manufacturing. Ther Adv Vaccines Immunother. 2020;8:2515135520908121. (In eng). doi:10.1177/2515135520908121.
- Ortiz de Lejarazu-Leonardo R, Montomoli E, Wojcik R, Christopher S, Mosnier A, Pariani E, Trilla Garcia A, Fickenscher H, Gärtner BC, Jandhyala R, et al. Estimation of reduction in influenza vaccine effectiveness due to egg-adaptation changes—systematic literature review and expert consensus. Vaccines. 2021;9(11):1255. doi:10.3390/vaccines9111255.
- Tenforde MW, Kondor RJG, Chung JR, Zimmerman RK, Nowalk MP, Jackson ML, Jackson LA, Monto AS, Martin ET, Belongia EA. et al. Effect of antigenic drift on influenza vaccine effectiveness in the United States—2019–2020. Clin Infect Dis. 2021;73(11):4244–50. doi:10.1093/cid/ciaa1884.
- Postma M, Fisman D, Giglio N, Márquez-Peláez S, Nguyen VH, Pugliese A, Ruiz-Aragón J, Urueña A, Mould-Quevedo J. Real-world evidence in cost-effectiveness analysis of enhanced influenza vaccines in adults ≥ 65 years of age: literature review and expert opinion. Vaccines. 2023;11(6):1089. (In eng). doi: 10.3390/vaccines11061089.
- Chicoye A, Crépey P, Nguyen VH, Márquez-Peláez S, Postma M, Pugliese A, Ruiz-Aragón J, Mould-Quevedo J. Contributions of cost-effectiveness analyses (CEA) to influenza vaccination policy for older adults in europe. Vaccine. 2023;41(38):5518–24. (In eng). doi: 10.1016/j.vaccine.2023.07.073.
- Ballalai I, Toniolo J, Kfouri R, Vespa G, Magneres C, Mould-Quevedo J, Pires B, Angerami R. Cost-effectiveness of cell-based quadrivalent versus egg-based trivalent influenza vaccination in the Brazilian National Immunization Program. J Bras Econ Saúde. 2021;13(2):136–44. doi:10.21115/JBES.v13.n2.p136-44.
- Boikos C, Sylvester GC, Sampalis JS, Mansi JA. Relative effectiveness of the cell-cultured quadrivalent influenza vaccine compared to standard, egg-derived quadrivalent influenza vaccines in preventing influenza-like illness in 2017–2018. Clin Infect Dis. 2020;71(10):665–71. (In eng). doi:10.1093/cid/ciaa371.
- Rizzo C, Trentini F, Capri S, Merler S. Valutazione economica dell’introduzione del nuovo vaccino antinfluenzale quadrivalente da coltura cellulare (Flucelvax® Tetra) nel contesto di cura italiano. Ital J Public Health. 2019;8(5):113–43. https://vaccinoinfluenza.iss.sm/doc/impatto-influenza-valore-vaccinazione.pdf.
- Boikos T, Sylvester G, Sampalis J, Mansi J. Effectiveness of the cell culture-and egg-derived, seasonal influenza vaccine during the 2017–2018 northern hemisphere influenza season. Bethesda, MD: NFID Clinical Vaccinology Course; 2018.
- Cai R, Gerlier L, Eichner M, Schwehm M, Rajaram S, Mould-Quevedo J, Lamotte M. Cost-effectiveness of the cell-based quadrivalent versus the standard egg-based quadrivalent influenza vaccine in Germany. J Med Econ. 2021;24(1):490–501. doi:10.1080/13696998.2021.1908000.
- Ruiz-Aragón J, Gani R, Márquez S, Alvarez P. Estimated cost-effectiveness and burden of disease associated with quadrivalent cell-based and egg-based influenza vaccines in Spain. Hum Vaccin Immunother. 2020;16(9):2238–44. doi:10.1080/21645515.2020.1712935.
- Nguyen VH, Roy B. Modelling the economic impact of influenza vaccine programs with the cell-based quadrivalent influenza vaccine and adjuvanted trivalent influenza vaccine in Canada. Vaccines. 2022;10(8):1257. (In eng). doi:10.3390/vaccines10081257.
- Nguyen VH, Hilsky Y, Mould-Quevedo J. The epidemiological and economic impact of a cell-based quadrivalent influenza vaccine in adults in the US: a dynamic modeling approach. Vaccines. 2021;9(10):1095. (In eng). doi:10.3390/vaccines9101095.
- Chi C-Y, Cheng M-F, Ko K. Mould JF, Chen C-J, Huang Y-C, Lee P-I. Cost-effectiveness analysis of cell-based versus egg-based quadrivalent influenza vaccines in the pediatric population in Taiwan. J Med Virol. 2024;96(1):e29279. (In eng). doi:10.1002/jmv.29279.
- Urueña A, Micone P, Magneres MC, McGovern I, Mould-Quevedo J, Sarmento TTR, Giglio N. Cost-effectiveness analysis of cell versus egg-based seasonal influenza vaccination in children and adults in Argentina. Vaccines. 2022;10(10):1627. doi:10.3390/vaccines10101627.
- Pelton SI, Mould-Quevedo JF, Nguyen VH. Modelling the population-level benefits and cost-effectiveness of cell-based quadrivalent influenza vaccine for children and adolescents aged 6 months to 17 years in the US. Expert Rev Vaccines. 2024;23(1):82–7. (In eng). doi:10.1080/14760584.2023.2295014.
- Kohli MA, Maschio M, Mould-Quevedo JF, Drummond M, Weinstein MC. The cost-effectiveness of an adjuvanted quadrivalent influenza vaccine in the United Kingdom. Hum Vaccin Immunother. 2021;17(11):4603–10. doi:10.1080/21645515.2021.1971017.
- Maschio M, Kohli MA, Ashraf M, Drummond MF, Weinstein MC, Mould-Quevedo JF. An economic comparison of influenza vaccines recommended for use in eligible adults under 65 years in the United Kingdom. Vaccines. 2022;10(4):599. doi:10.3390/vaccines10040599.
- Krishnarajah G, Divino V, Postma MJ, Pelton SI, Anupindi VR, DeKoven M, Mould-Quevedo J. Clinical and economic outcomes associated with cell-based quadrivalent influenza vaccine vs. standard-dose egg-based quadrivalent influenza vaccines during the 2018–19 influenza season in the United States. Vaccines. 2021;9(2):80. (In eng). doi: 10.3390/vaccines9020080.
- Boikos C, Fischer L, O’Brien D, Vasey J, Sylvester GC, Mansi JA. Relative effectiveness of the cell-derived inactivated quadrivalent influenza vaccine versus egg-derived inactivated quadrivalent influenza vaccines in preventing influenza-related medical encounters during the 2018–2019 influenza season in the United States. Clin Infect Dis. 2021;73(3):692–8. In eng. doi:10.1093/cid/ciaa1944.
- Alvarez FP, Petitjean A, Nealon J, Hollingsworth R, López-Belmonte JL. Cost-effectiveness analysis has to consider all available evidence when informing inputs. Hum Vaccin Immunother. 2021;17(3):694–5. (In eng). doi:10.1080/21645515.2020.1799670.
- Husereau D, Drummond M, Augustovski F, de Bekker-Grob E, Briggs AH, Carswell C, Caulley L, Chaiyakunapruk N, Greenberg D, Loder E. et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) 2022 explanation and elaboration: a report of the ISPOR CHEERS II Good Practices Task Force. Value in Health. 2022;25(1):10–31. doi:10.1016/j.jval.2021.10.008.
- World Health Organization. WHO guide for standardization of economic evaluations of immunization programmes, 2nd ed. WHO-IVB-19.10. Geneva: World Health Organization; 2019 Oct 17. https://www.who.int/publications/i/item/who-guide-for-standardization-of-economic-evaluations-of-immunization-programmes-2nd-ed
- Coleman BL, Gutmanis I, McGovern I, Haag M. Effectiveness of cell-based quadrivalent seasonal influenza vaccine: a systematic review and meta-analysis. Vaccines. 2023;11(10):1607. (In eng). doi: 10.3390/vaccines11101607.
- Thompson WW, Shay DK, Weintraub E. Brammer L, Bridges CB, Cox NJ, Fukuda K. Influenza-associated hospitalizations in the United States. JAMA. 2004;292(11):1333–40. (In eng). doi:10.1001/jama.292.11.1333.
- Mould-Quevedo JF, Pelton SI, Nguyen VH. Vaccine effectiveness of cell-based quadrivalent influenza vaccine in children: a narrative review. Vaccines. 2023;11(10):1594. (In eng). doi: 10.3390/vaccines11101594.
- Zost SJ, Parkhouse K, Gumina ME, Kim K, Diaz Perez S, Wilson PC, Treanor JJ, Sant AJ, Cobey S, Hensley SE. et al. Contemporary H3N2 influenza viruses have a glycosylation site that alters binding of antibodies elicited by egg-adapted vaccine strains. Proc Natl Acad Sci USA. 2017;114(47):12578–83. (In eng). doi:10.1073/pnas.1712377114.
- Rolfes MA, Flannery B, Chung JR, O’Halloran A, Garg S, Belongia EA, Gaglani M, Zimmerman RK, Jackson ML, Monto AS, et al., US Influenza Vaccine Effectiveness (Flu VE) Network, the Influenza Hospitalization Surveillance Network, and the Assessment Branch, Immunization Services Division, Centers for Disease Control and Prevention. Effects of influenza vaccination in the United States during the 2017–2018 influenza season. Clin Infect Dis. 2019;69(11):1845–53. (In eng). doi:10.1093/cid/ciz075.
- Boikos C, McGovern I, Molrine D, Ortiz JR, Puig-Barberà J, Haag M. Review of analyses estimating relative vaccine effectiveness of cell-based quadrivalent influenza vaccine in three consecutive US influenza seasons. Vaccines. 2022;10(6):896. (In eng). doi:10.3390/vaccines10060896.
- Divino V, Ruthwik Anupindi V, DeKoven M, Mould-Quevedo J, Pelton SI, Postma MJ, Levin MJ. A real-world clinical and economic analysis of cell-derived quadrivalent influenza vaccine compared to standard egg-derived quadrivalent influenza vaccines during the 2019–2020 influenza season in the United States. Open Forum Infect Dis. 2022;9(1):ofab604. (In eng). doi: 10.1093/ofid/ofab604.
- Divino V, Krishnarajah G, Pelton SI, Mould-Quevedo J, Anupindi VR, DeKoven M, Postma MJ. A real-world study evaluating the relative vaccine effectiveness of a cell-based quadrivalent influenza vaccine compared to egg-based quadrivalent influenza vaccine in the US during the 2017–18 influenza season. Vaccine. 2020;38(40):6334–43. (In eng). doi: 10.1016/j.vaccine.2020.07.023.
- Stein A. Superior effectiveness of cell-based versus egg-based quadrivalent influenza vaccines against test-confirmed influenza over three consecutive seasons in the United States. The Ninth ESWI Influenza Conference; 17–20 September, 2023; Valencia, Spain. 2023 Sept 19.
- Chua H, Feng S, Lewnard JA, Sullivan SG, Blyth CC, Lipsitch M, Cowling BJ. The use of test-negative controls to monitor vaccine effectiveness: a systematic review of methodology. Epidemiology. 2020;31(1):43–64. (In eng). doi: 10.1097/ede.0000000000001116.
- Pitman R, Fisman D, Zaric GS, Postma M, Kretzschmar M, Edmunds J, Brisson M, ISPOR-SMDM Modeling Good Research Practices Task Force. Dynamic transmission modeling: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-5. Value Health. 2012;15(6):828–34. (In eng). doi: 10.1016/j.jval.2012.06.011.
- German Standing Committee on Vaccination (STIKO). 16 Mar 2016. Modelling methods for predicting epidemiological and health economic effects of vaccinations guidance for analyses to be presented to the German Standing Committee on Vaccination (STIKO): Version 1.0. https://www.nitag-resource.org/sites/default/files/fcf579b0fd106551e22cbebabb28c56ae0a058e2_1.pdf. 2016 (Berlin: Robert Koch Institut)
- World Health Organization. Guidance on the economic evaluation of influenza vaccination. WHO/IVB/16.05. Geneva: World Health Organization; 2016 Sept. https://apps.who.int/iris/bitstream/handle/10665/250086/WHO-IVB-16.05-eng.pdf
- Milián E, Kamen AA. Current and emerging cell culture manufacturing technologies for influenza vaccines. Biomed Res Int. 2015;2015:504831. (In eng). doi:10.1155/2015/504831.
- Coleman BL, Sanderson R, Haag MDM, McGovern I. Effectiveness of the MF59-adjuvanted trivalent or quadrivalent seasonal influenza vaccine among adults 65 years of age or older, a systematic review and meta-analysis. Influenza Other Respir Viruses. 2021;15(6):813–23. doi:10.1111/irv.12871.
- Liu F, Gross FL, Jefferson SN, Holiday C, Bai Y, Wang L, Zhou B, Levine MZ. Age-specific effects of vaccine egg adaptation and immune priming on A(H3N2) antibody responses following influenza vaccination. J Clin Invest. 2021;131(8):e146138 (In eng). doi:10.1172/jci146138.
- Kelvin AA, Zambon M. Influenza imprinting in childhood and the influence on vaccine response later in life. Euro Surveill. 2019;24(48):1900720. (In eng). doi: 10.2807/1560-7917.Es.2019.24.48.1900720.
- Francis T. On the doctrine of original antigenic sin. Proc Am Philos Soc. 1960;104(6):572–8. http://www.jstor.org/stable/985534.
- Blanchet Zumofen M-H, Frimpter J, Hansen SA. Impact of influenza and influenza-like illness on work productivity outcomes: a systematic literature review. Pharmacoeconomics. 2023;41(3):253–73. (In eng). doi:10.1007/s40273-022-01224-9.
- Greenhalgh T, Fisman D, Cane DJ, Oliver M, Macintyre CR. Adapt or die: how the pandemic made the shift from EBM to EBM+ more urgent. BMJ Evid Based Med. 2022;27(5):253–60. doi:10.1136/bmjebm-2022-111952.
- Petersen JM, Ranker LR, Barnard-Mayers R, MacLehose RF, Fox MP. A systematic review of quantitative bias analysis applied to epidemiological research. Int J Epidemiol. 2021;50(5):1708–30. (In eng). doi: 10.1093/ije/dyab061.
- Mathur MB, VanderWeele TJ. Meta-regression methods to characterize evidence strength using meaningful-effect percentages conditional on study characteristics. Res Synth Methods. 2021;12(6):731–49. (In eng). doi:10.1002/jrsm.1504.
- Deeks JJ, Higgins JPT, Altman DG. on behalf of the Cochrane Statistical Methods Group. Chapter 10: Analysing data and undertaking meta-analyses. Cochrane Handbook for Systematic Reviews of Interventions version 6.4. Cochrane; 2023 Aug. www.training.cochrane.org/handbook
- NHS England. National flu immunisation programme 2024 to 2025 letter. 2024 Mar 12. https://www.gov.uk/government/publications/national-flu-immunisation-programme-plan-2024-to-2025/national-flu-immunisation-programme-2024-to-2025-letter
