ABSTRACT
The ZF2001 vaccine has demonstrated high efficacy in preventing coronavirus disease 2019 (COVID-19). However, the clinical characteristics of breakthrough infections in vaccinated individuals and the risk factors for adverse outcomes in COVID-19 patients remain unclear. We conducted a retrospective single-center cohort study at Xiangya Hospital of Central South University, including 210 fully vaccinated COVID-19 inpatients from December 5, 2022, to January 31, 2023. Data on clinical characteristics, laboratory findings, disease severity, treatment, and prognosis were collected and analyzed. Our findings revealed that COVID-19 inpatients still experienced common symptoms at the onset of illness, but most laboratory findings were within the normal range, except for white blood cell count (WBC), lymphocyte count, and lactate dehydrogenase (LDH) levels. Following standard treatment, 95.7% of patients were discharged from the hospital. We identified seven variables significantly associated with a higher risk of adverse outcomes, including age over 65, elevated WBC count, reduced lymphocyte count, higher levels of blood urea nitrogen (BUN), LDH, troponin, D-dimer, and procalcitonin. This study supports the substantial clinical benefits of the ZF2001 vaccine for COVID-19 patients. Additionally, age over 65, elevated WBC count, reduced lymphocyte count, and higher blood levels of BUN, LDH, D-dimer, and procalcitonin may be used as predictive factors for disease progression in fully vaccinated COVID-19 inpatients.
Introduction
Vaccination plays a crucial role in controlling the spread of COVID-19 and reducing the risk of severe infection and poor outcomes. An epidemiological survey involving 62,395 eligible participants revealed that the coverage of SARS-CoV-2 vaccination in China was 98.9% for at least one dose and 70.1% for booster shots.Citation1 The ZF2001 vaccine is a protein subunit COVID-19 vaccine that utilizes the receptor-binding domain (RBD) of the SARS-CoV-2 spike protein as its antigen. The antigen protein is produced in Chinese hamster ovary (CHO) cells and then adjuvanted with aluminum hydroxide to create the vaccine.Citation2 The ZF2001 vaccine requires a three-dose regimen administered at day 0, 30, and 60. It has been shown to achieve seroconversion of neutralizing antibodies in 93–100% of recipients, with geometric mean titers surpassing those found in convalescent serum samples from RT-PCR-confirmed COVID-19 patients.Citation3 Clinical trials have demonstrated the safety and effectiveness of the ZF2001 vaccine. It has shown an acceptable side-effect profile and has been proven effective against symptomatic and severe-to-critical COVID-19 for at least 6 months after full vaccination in adults,Citation3–5 and has received approval in China.Citation6 However, there is a lack of data regarding the clinical characteristics of breakthrough infections in individuals fully vaccinated with the ZF2001 vaccine. Additionally, the risk factors for disease progression in these patients have not been elucidated. This retrospective study aims to provide insights into the clinical characteristics of ZF2001 fully vaccinated patients who experience breakthrough infections requiring hospitalization, as well as to identify risk factors associated with disease progression outcomes.
Materials and methods
Patient and public involvement
Our research did not involve patient or public participation in the design, implementation, reporting, or dissemination plans. However, we are committed to providing the final results to all stakeholders, including study participants, through various channels such as media and magazines.
Study design and patients
This retrospective cohort study was conducted at Xiangya Hospital of Central South University, focusing on patients admitted between December 5, 2022, and January 31, 2023. In total, 2118 hospitalized patients with confirmed SARS-CoV-2 infection were initially enrolled.Citation7–10 Eligible patients for inclusion in this study were those who had received the full three doses of ZF2001and were above 18 years of age. The study protocol was approved by the institutional review committee of Xiangya Hospital of Central South University. As the study used anonymized data, individual informed consent was not required.
Data source
We collected electronic health records of COVID-19 patients from the inpatient system of Xiangya Hospital of Central South University. These records contained information such as demographic characteristics, preexisting conditions, prescription and drug dispensing records, laboratory test results, and dates of discharge or death. To enrich the data, we linked the health records with anonymized vaccination records provided by the Department of Immunization, Center for Disease Control and Prevention of Hunan Province. This linkage was done using unique identification numbers, specifically the China Identity Card number. As previously reported, our study considered a composite outcome of disease progression, including noninvasive respiratory support, initiation of endotracheal intubation, intensive care unit admission, and all-cause death.Citation11
Statistical analysis
Continuous variables were shown as median and interquartile range. Categorical variables were presented as counts and proportions, and compared by Chi-square test or Fisher’s exact test. The univariate and multivariable Cox regression model was used to estimate hazard ratio (HR) with 95% confidence interval (CI) for the association of possible factors with composite disease progression outcome of COVID-19, and adjusted for potential confounders. All statistical analyses were performed with SPSS version 26.0 (IBM). Statistical charts were generated using Excel 2016 (Microsoft). For all the statistical analyses, a p value < 0.05 was considered significant.
Results
Baseline characteristics of the patients of COVID-19
During the study period (05 December 2022 to 31 January 2023), data were reported for 210 hospitalized COVID-19 patients fully vaccinated with ZF2001 vaccine from Xiangya hospital of Central South University. The demographic and clinical characteristics of these patients on admission are shown in . Of these 210 patients, the median age was 66.5 years (IQR, 56–73), and 140 (66.7%) were male. Prior to hospital admission their most common symptoms were fever (61.4%), dry cough (75.2%) and expectoration (62.9%). Twenty five percent of the patients had underlying diseases, including hypertension (40.5%), coronary disease (16.2%), diabetes mellitus (25.2%) and chronic obstructive pulmonary disease (4.3%).
Table 1. Clinical characteristics of hospitalized patients with COVID-19 after fully ZF2001 vaccination.
We further outlined the medication, oxygen support, and follow-up in . 108 patients (51.4%) received antiviral therapy; 79 patients (37.6%) were administrated antibiotics; and 46 patients (21.9%) were treated with steroids. Most patients had oxygen support, mainly nasal cannula (80.0%) and a minority with mask oxygen, high-flow oxygen and invasive mechanical ventilation (4.8%, 4.8%, 2.4%, respectively). The majority of COVID-19 patients had a good prognosis with, 95.7% patients were discharged from the hospital. Only 12.4% patients had composite outcome and 4.3% patients died in this cohort as of January 31, 2023.
Besides white blood cell (WBC), lymphocyte count, and lactate dehydrogenase (LDH), laboratory findings of the patients included in the study were within the normal range. The median WBC count was 5.5 (IQR, 4.5–7.3) × 109/L, of which 77.2% had increased count, while 7.8% had normal count and 15.0% had decreased count. The median lymphocyte count was 1.0 (IQR, 0.7–1.4) × 109/L, of which 108 patients (52.4%) had low lymphocyte count. The median blood level of LDH was 213 (IQR, 173–263) U/L, of which 34.1% had high LDH level. The minority of COVID-19 patients showed laboratory index abnormalities, including elevated levels of serum creatinine (Scr) (9.6%), blood urea nitrogen (BUN) (11.1%), troponin (9.8%), D-dimer (17.9%), and procalcitonin (PCT) (18.7%).
Risk factors for the composite outcome in COVID-19 inpatients with fully ZF2001 vaccination
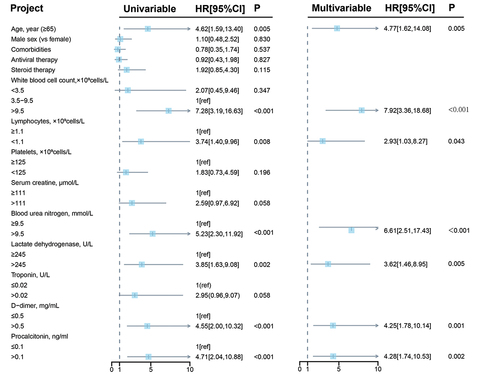
We further assessed the possible risk factors associated with composite outcomes in these patients ( and Supplementary Table S1). In the univariate Cox regression analysis, seven variables were associated with significantly higher risks of composite outcomes, including older than 65 years, increased WBC count, decreased lymphocyte, higher levels of BUN, LDH, D-dimer and PCT. After adjusting for age, sex, comorbidities, antiviral therapy and steroid therapy, the multivariable adjusted Cox proportional hazard regression model also showed consistent conclusions (older than 65 years, HR = 4.77, 95% CI: 1.62, 14.08; p = .005; increased WBC count, HR = 7.92, 95% CI: 3.36, 18.68; p < .001; decreased lymphocytes, HR = 2.93, 95% CI: 1.03, 8.27; p = .043; higher levels of BUN, HR = 6.61, 95% CI: 2.51, 17.43; p < .001; LDH, HR = 3.62, 95% CI: 1.46, 8.95; p = .005; D-dimer, HR = 4.25, 95% CI: 1.78, 10.14; p = .001; PCT, HR = 4.28, 95% CI: 1.74, 10.53; p = .002). These results suggested that these factors were independent risk factor for disease progression outcome in COVID-19 patients.
Figure 1. Cox regression analysis of risk factors associated with composite outcome among COVID-19 inpatients fully vaccinated with ZF2001. Multivariable cox regression was performed, covariates included sex, age, comorbidities, antiviral therapy, steroid therapy. HR: hazard ratio; CI: confidence interval.
Discussion
In response to the worldwide SARS-CoV-2 outbreak and the ensuing COVID-19 pandemic, antiSARS-Cov-2 vaccines have been made widely available.Citation12 The ZF2001 vaccine, which has undergone phase 1 and 2 clinical trials, has been demonstrated to be safe in adultsCitation3 Moreover, it has shown effectiveness against symptomatic and severe-to-critical COVID-19 for a minimum of 6 months after full vaccination in a large cohort of adults.Citation4 However, there is still a lack of real-world data on breakthrough COVID-19 cases in individuals who have received the ZF2001 vaccine, especially during the period when the Omicron variant of SARS-CoV-2 was dominant. To address this gap, our retrospective cohort study identified a total of 210 COVID-19 patients who had been fully vaccinated with ZF2001 and subsequently experienced breakthrough infections requiring hospitalization. We analyzed the clinical characteristics of these patients and identified seven variables that were significantly associated with a higher risk of adverse outcomes. These variables included age over 65, elevated WBC, decreased lymphocyte count, elevated levels of BUN, LDH, troponin, D-dimer, and PCT.
The clinical presentation of the hospitalized patients in our study is consistent with that of other COVID-19 patients. Most of them exhibited common symptoms at the onset of the disease, such as fever, dry cough, expectoration, poor appetite, rapid breathing, fatigue, nasal congestion, muscle pain, and headache, which aligns with previous reports.Citation13 However, it is important to note that these symptoms do not indicate vaccine failure in preventing COVID-19. Many of these patients had other significant medical reasons for hospital admission that could be related to SARS-CoV-2 infection, such as underlying chronic obstructive pulmonary disease (COPD), cardiovascular disease, or exacerbation of preexisting health conditions. Additionally, it is worth mentioning that these patients were identified after hospitalization and were not compared with uninfected controls.Citation14 It’s worth noting that the median age of these patients was 66.5 years in this study, suggesting that old age remains a risk factor for COVID-19 patients who received the vaccine. Moreover, we identified that older than 65 years were associated with significantly higher risks of composite outcomes, similarly with the data reported by the US Center for Disease Control and Prevention that significantly higher rates of hospitalizations, ICU admissions, and deaths secondary to COVID-19 among older adults (>65 years) than any younger age groups.Citation15 Therefore, elderly individuals, even if they have received the vaccine, require special attention, particularly those with preexisting health conditions.Citation16 Our study also revealed a high prevalence of underlying diseases among hospitalized patients, including hypertension, coronary disease, diabetes mellitus, and COPD. The increased prevalence of comorbidities may be attributed to lower vaccine effectiveness in patients with underlying health conditions, the risk of exacerbation of comorbidities following breakthrough infections, or both. Generally, the clinical profile of patients in our study includes advanced age and the presence of comorbidities, which aligns with another study conducted in Israel, where 152 fully vaccinated COVID-19 patients were hospitalized and exhibited similar clinical characteristics.Citation17
COVID-19 is a systemic infection with a significant impact on the hematopoietic system and hemostasis. In our cohort, 77.2% patients had increased WBC count, 52.4% patients had lymphocyte count and 34.1% patients had high LDH blood level. In addition, part of COVID-19 patients showed laboratory index abnormalities, including elevated levels of Scr, BUN, troponin, D-dimer, and PCT. However, these abnormalities may be due to underlying diseases in these patients, or may be caused by COVID-19 itself. Since most laboratory findings of patients in our study are normal, we considered that these patients might have developed resistance to SARS-CoV-2. Accordingly, 95.7% patients were discharged from the hospital after receiving standard treatment. Similarly, a randomized, double-blind, placebo-controlled (RCT), phase 3 trial showed that the three-dose ZF2001 regimen had a vaccine efficacy against COVID-19 of any severity of 81.4% in the short-term follow-up and 75.7% in the long-term follow-up.Citation4 Although our study did not conduct a long-term follow-up, the rate of improvement in our cohort was higher than that reported in the short-term follow-up of the RCT study. However, this difference may be attributed to the small sample size in our study.
Identified prognostic factors can help clinicians and policy makers in tailoring management strategies for patients with COVID-19 infectious disease while researchers can utilize our findings to develop multivariable prognostic models that could eventually facilitate decision-making and improve patient important outcomes. Numerous studies have demonstrated ability of many possible factors to risk-stratify patients, including high blood PCT, myocardial injury markers, high blood WBC, low blood platelet count, Scr increase, high blood D-dimer, high blood LDH, decrease in lymphocyte count, decrease in blood albumin, high blood neutrophil count, high BUN and so on.Citation18 However, potent predictive markers of critical condition development and mortality in COVID-19 still remain unknown with the fact that the currently approved vaccines have been extremely effective in preventing COVID-19.Citation19 In the present study, we found that older than 65 years, increased WBC count, decreased lymphocyte, higher blood levels of BUN, LDH, D-dimer and PCT were associated with significantly higher risks of composite outcome, suggesting that these factors might be used as predictive factors for disease progression outcome in COVID-19 patients. Therefore, clinicians should pay more attention to prevent adverse prognosis if the above indicators are abnormal in COVID-19 patients vaccinated with ZF2001,
This study has inherent limitations mainly due to its retrospective, single-center design, so we included a very small proportion of patients with laboratory-confirmed SARS-CoV-2 infection and fully vaccinated with ZF2001. Berkson bias might be introduced since asymptomatic patients and those with mild symptoms were less likely to be enrolled. In addition, as with any retrospective cohort study that is based on data from clinical and administrative databases, a possible limitation may be related to the quality of the data. Finally, our study failed to evaluate the effectiveness of ZF2001 on COVID-19 patients due to lack of a control group of unvaccinated COVID-19 patients within the same study period.
Conclusions
In conclusion, this study represents the first real-world analysis of hospitalized COVID-19 patients who had received full vaccination with the ZF2001 vaccine during the pandemic in China. Despite the high effectiveness of the vaccine, fully vaccinated individuals can still experience severe SARS-CoV-2 infection, necessitating hospitalization. However, our findings support the notion that the ZF2001 vaccine may provide significant clinical benefits for COVID-19 patients. Additionally, older age (>65 years), elevated WBC, decreased lymphocyte count, and higher blood levels of BUN, LDH, D-dimer, and PCT might serve as predictive factors for disease progression and outcomes in COVID-19 patients.
Availability of data and materials
Further information and requests for data and materials will be fulfilled by the lead contact, Furong Zeng ([email protected]).
Contributions
Conception and design: Furong Zeng. Acquisition of data: Liping Jin and Yating Dian. Interpretation of data, statistical analysis and manuscript writing: Furong Zeng, Liping Jin and Chaofei Chao. Revision of manuscript and administrative, technical, or material support: Liping Jin, Guangtong Deng, Furong Zeng, Yating Dian, Yuming Sun and Chaofei Chao.
Consent to participate
Written informed consent was obtained from all the participants.
Ethics approval
HGUA-ISABIAL Ethics Committee approved the study (expedient no. 200145).
Supplementary Materials.docx
Download MS Word (15.3 KB)Acknowledgments
We sincerely thank all hospital staff for their efforts in gathering the information used in this study. Thank the patients who participated in this study, and their families, and the medical, nursing and research staff at the study center.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2024.2355683
Additional information
Funding
References
- Zhang XR, Li ZJ, Fu Q, Wang JD, Huang QM, Song WQ, Xu XY, Li ZH, Mao C. The coverage of SARS-CoV-2 vaccination and the willingness to receive the SARS-CoV-2 variant vaccine among employees in China. BMC Public Health. 2023;23(1):542. doi:10.1186/s12889-023-15294-7.
- Dai L, Zheng T, Xu K, Han Y, Xu L, Huang E, An Y, Cheng Y, Li S, Liu M. et al. A universal design of betacoronavirus vaccines against COVID-19, MERS, and SARS. Cell. 2020;182(3):722–33.e11. doi:10.1016/j.cell.2020.06.035.
- Yang S, Li Y, Dai L, Wang J, He P, Li C, Fang X, Wang C, Zhao X, Huang E. et al. Safety and immunogenicity of a recombinant tandem-repeat dimeric RBD-based protein subunit vaccine (ZF2001) against COVID-19 in adults: two randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Infect Dis. 2021;21(8):1107–6. doi:10.1016/s1473-3099(21)00127-4.
- Dai L, Gao L, Tao L, Hadinegoro SR, Erkin M, Ying Z, He P, Girsang RT, Vergara H, Akram J. et al. Efficacy and safety of the RBD-dimer–based covid-19 vaccine ZF2001 in adults. N Engl J Med. 2022;386(22):2097–111. doi:10.1056/NEJMoa2202261.
- An Y, Li S, Jin X, Han JB, Xu K, Xu S, Han Y, Liu C, Zheng T, Liu M. et al. A tandem-repeat dimeric RBD protein-based COVID-19 vaccine zf2001 protects mice and nonhuman primates. Emerg Microbes Infect. 2022;11(1):1058–71. doi:10.1080/22221751.2022.2056524.
- Author. Approved vaccines. https://covid19.trackvaccines.org/vaccines/approved.
- Dian Y, Meng Y, Sun Y, Deng G, Zeng F. Azvudine versus paxlovid for oral treatment of COVID-19 in Chinese patients with pre-existing comorbidities. J Infect. 2023;87(2):24–7. doi:10.1016/j.jinf.2023.05.012.
- Zhao D, He Y, Dian Y, Meng Y, Zeng F, Deng G. Elevated troponin levels predict the reduced efficacy of paxlovid in COVID-19 patients. J Infect. 2023;87(2):148–50. doi:10.1016/j.jinf.2023.03.026.
- Sun Y, Jin L, Dian Y, Shen M, Zeng F, Chen X, Deng G. Oral azvudine for hospitalised patients with COVID-19 and pre-existing conditions: a retrospective cohort study. EClinicalMedicine. 2023;59:101981. doi:10.1016/j.eclinm.2023.101981.
- Deng G, Li D, Sun Y, Jin L, Zhou Q, Xiao C, Wu Q, Sun H, Dian Y, Zeng F. et al. Real-world effectiveness of azvudine versus nirmatrelvir–ritonavir in hospitalized patients with COVID-19: A retrospective cohort study. J Med Virol. 2023;95(4):e28756. doi:10.1002/jmv.28756.
- Zhao D, He Y, Dian Y, Meng Y, Zeng F, Deng G. Elevated troponin levels predict the reduced efficacy of paxlovid in COVID-19 patients. J Infect. 2023;87(2):148–50. doi:10.1016/j.jinf.2023.03.026.
- Francis AI, Ghany S, Gilkes T, Umakanthan S. Review of COVID-19 vaccine subtypes, efficacy and geographical distributions. Postgrad Med J. 2022;98(1159):389–94. doi:10.1136/postgradmedj-2021-140654.
- Ochani R, Asad A, Yasmin F, Shaikh S, Khalid H, Batra S, Sohail MR, Mahmood SF, Ochani R, Hussham Arshad M. et al. COVID-19 pandemic: from origins to outcomes. A comprehensive review of viral pathogenesis, clinical manifestations, diagnostic evaluation, and management. Infez Med. 2021;29(1):20–36.
- Chen Y, Klein SL, Garibaldi BT, Li H, Wu C, Osevala NM, Li T, Margolick JB, Pawelec G, Leng SX. Aging in COVID-19: vulnerability, immunity and intervention. Ageing Res Rev. 2021;65:101205. doi:10.1016/j.arr.2020.101205.
- Bialek S, Boundy E, Bowen V, Chow N, Cohn A, Dowling N, Ellington S, Gierke R, Hall A, MacNeil J. et al. Severe outcomes among patients with coronavirus disease 2019 (COVID-19) — United States, February 12–March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):343–6. doi:10.15585/mmwr.mm6912e2.
- Ejaz H, Alsrhani A, Zafar A, Javed H, Junaid K, Abdalla AE, Abosalif KOA, Ahmed Z, Younas S. COVID-19 and comorbidities: deleterious impact on infected patients. J Infect Public Health. 2020;13(12):1833–9. doi:10.1016/j.jiph.2020.07.014.
- Brosh-Nissimov T, Orenbuch-Harroch E, Chowers M, Elbaz M, Nesher L, Stein M, Maor Y, Cohen R, Hussein K, Weinberger M. et al. BNT162b2 vaccine breakthrough: clinical characteristics of 152 fully vaccinated hospitalized COVID-19 patients in Israel. Clin Microbiol Infect. 2021;27(11):1652–7. doi:10.1016/j.cmi.2021.06.036.
- Izcovich A, Ragusa MA, Tortosa F, Lavena Marzio MA, Agnoletti C, Bengolea A, Ceirano A, Espinosa F, Saavedra E, Sanguine V. et al. Prognostic factors for severity and mortality in patients infected with COVID-19: a systematic review. PLoS One. 2020;15(11):e0241955. doi:10.1371/journal.pone.0241955.
- Tregoning JS, Flight KE, Higham SL, Wang Z, Pierce BF. Progress of the COVID-19 vaccine effort: viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat Rev Immunol. 2021;21(10):626–36. doi:10.1038/s41577-021-00592-1.
