?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The COVID-19 pandemic has significantly disrupted healthcare systems at all levels globally, notably affecting routine healthcare services, such as childhood vaccination. This study examined the impact of these disruptions on routine childhood vaccination programmes in Tanzania. We conducted a longitudinal study over four years in five Tanzanian regions: Mwanza, Dar es Salaam, Mtwara, Arusha, and Dodoma. This study analyzed the trends in the use of six essential vaccines: Bacille Calmette-Guérin (BCG), bivalent Oral Polio Vaccine (bOPV), Diphtheria Tetanus Pertussis, Hepatitis-B and Hib (DTP-HepB-Hib), measles-rubella (MR), Pneumococcal Conjugate Vaccine (PCV), and Rota vaccines. We evaluated annual and monthly vaccination trends using time-series and regression analyses. Predictive modeling was performed using an autoregressive integrated moving average (ARIMA) model. A total of 32,602,734 vaccination events were recorded across the regions from 2019 to 2022. Despite declining vaccination rates in 2020, there was a notable rebound in 2021, indicating the resilience of Tanzania’s immunization program. The analysis also highlighted regional differences in vaccination rates when standardized per 1000 people. Seasonal fluctuations were observed in monthly vaccination rates, with BCG showing the most stable trend. Predictive modeling of BCG indicated stable and increasing vaccination coverage by 2023. These findings underscore the robustness of Tanzania’s childhood immunization infrastructure in overcoming the challenges posed by the COVID-19 pandemic, as indicated by the strong recovery of vaccination rates post-2020. We provide valuable insights into the dynamics of vaccination during a global health crisis and highlight the importance of sustained immunization efforts to maintain public health.
KEYWORDS:
- COVID-19
- healthcare disruption
- vaccination
- childhood immunizations
- Tanzania
- vaccination trends
- Bacille Calmette-Guérin (BCG)
- bivalent Oral Polio Vaccine (bOPV)
- measles-rubella (MR)
- Pneumococcal conjugate vaccine (PCV)
- Diphtheria tetanus pertussis-HepB-hib vaccine (DTP-HepB-hib)
- pentavirus vaccine
- rotavirus vaccine
- predictive modeling
- data analytics
Introduction
For several years, childhood vaccination programmes have played a crucial role in public health by controlling numerous communicable diseases. Childhood immunization is one of the most cost-effective and successful strategies for curbing morbidity and mortality associated with preventable diseases.Citation1 However, in 2020, the resurgence of SARS-CoV (coronavirus)-2, which caused COVID-19, disrupted many health services, such as childhood vaccination programmes. Childhood vaccination provides crucial protection against a range of infectious diseases, including measles, mumps, rubella, polio, diphtheria, tetanus, pertussis (DPT), hepatitis B, varicella (chickenpox), and rotavirus.Citation2 It is undeniable that the immunization schedule is crucial for safeguarding infants, children, and adolescents from potentially fatal, preventable infections. The World Health Organization (WHO) and the Centers for Disease Control and Prevention (CDC), among other health organizations, endorse these schedules due to their proven efficacy.Citation2–5 Key vaccines include those for hepatitis B (Hep-B), which are typically administered to newborns at 1–2 months of follow-up and at 6–18 months.Citation2,Citation5 The rotavirus vaccine administered at two, four, or six months, depending on the type of vaccine, is also fundamental. The efficacy of the Haemophilus influenzae type b (Hib) vaccine can vary between three or four doses, depending on the formulation. The recommended immunizations include diphtheria, tetanus, and whole-cell pertussis (DTwP) vaccines, pneumococcal vaccines, meningococcal vaccines, varicella (chickenpox) vaccines, measles-mumps-rubella (MMR) vaccines, and poliovirus vaccines, all at specific age intervals.Citation5
SARS-CoV-2 infection began with an unidentified cluster of new respiratory diseases in Wuhan, China.Citation6,Citation7 The World Health Organization (WHO) declared the global spread of COVID-19 a pandemic on March 11, 2020. The first case of COVID-19 in Tanzania was reported on March 16, 2020.Citation8 During the outbreak, international strategies to prevent the rapid spread and transmission of COVID-19 include social distancing, nonessential business closures, quarantine stay-home orders, temperature measurements for travelers, and restrictions on the transportation of people from one point to another.Citation9 Enforcement of mask and glove use, hand sanitizing, and hand washing efforts were also recommended as they were first implemented in Wuhan and its surrounding areas in China.Citation10 These measures have impacted the reduction in healthcare services provided by patients and the shift in the priorities and resources of the healthcare system toward a pandemic response. A shift in focus from other severe and deadly diseases to COVID-19 has also been observed. Hospitals and other health facilities are sometimes overwhelmed by COVID-19, making access to standard healthcare difficult for patients with acute or chronic illnesses.Citation9 Tanzania instituted preventive measures for the public, albeit for short periods, in early 2020.Citation5 However, even after lifting the measures, individuals observed these according to the WHO standards.
Apart from the COVID-19 pandemic restrictions, the disease has also caused increased health problems, such as stress, insomnia, anxiety, denial, depressive symptoms, anger, and fear. Collective concerns influence daily behaviors, the economy, prevention strategies, and decision-making by policymakers, health organizations, and medical centers, which can weaken strategies for COVID-19 control and result in greater morbidity and mental health problems globally.Citation11,Citation12 In addition, Africa, like the rest of the world, faced an influx of COVID-19 information, misinformation, and disinformation that would impact the vaccination rate. This has resulted in vaccine hesitancy in several countries, including Tanzania.Citation13
Therefore, the restrictions to curb COVID-19, increase in mental distress, and misinformation have adversely affected routine immunization services worldwide, resulting in drawbacks in controlling these diseases.Citation14 It has been reported that, from January to December 2020, 30 million children missed their third DTP dose, and 27.2 million children missed their first measles-containing vaccine (MCV) dose.Citation15 Many countries have increased their budgets to fight COVID-19 with the help of international organizations, such as the World Bank, WHO, and other international monetary cooperatives, leaving other health sectors stuck.Citation16 It is worth noting that even brief lapses in vaccination during emergencies can create pockets of susceptible individuals, potentially leading to outbreaks of infectious diseases such as measles, polio, and pertussis.Citation17,Citation18 According to WHO reports, standard immunization programs have suffered significant disruptions in 68 countries, affecting more than 80 million children, predominantly in developing countries.Citation19 Several other studies have highlighted a decrease in the uptake of childhood vaccinations during the COVID-19 pandemic. This decrease is attributed to fewer parents visiting healthcare centers for many reasons, including misinformation about the pandemic and delays in vaccination schedules. Such a decrease in vaccination may provoke a surge in vaccine-preventable diseases, potentially leading to increased antibiotic use and consequent antimicrobial resistance, thereby affecting morbidity, mortality and healthcare costs.Citation20 While many countries have seen a recovery in vaccination rates, millions of children remain vulnerable to diseases such as measles. Some surges in measles cases have been reported in countries including Tanzania.Citation14,Citation18,Citation21 However, data on the impact of COVID-19 on childhood vaccination trends in Tanzania remain unclear. There is a lack of studies that explored the impact of the COVID-19 pandemic on childhood vaccination and immunization in Tanzania.
This study aimed to evaluate how the COVID-19 pandemic has influenced the patterns of routine childhood vaccination across five regions of Tanzania.
Materials and methods
This study employed a quantitative approach using secondary administrative data from Tanzania’s Health Ministry and other relevant organizations.
Study setting
The United Republic of Tanzania is a union of the Tanzania mainland and the Zanzibar Archipelago Islands, with a confirmed population of 61.2 million (2020 Tanzania National Census), with more than 60% engaging in rural areas. Tanzania’s mainland has 26 regions consisting of districts.
Five regions of the Tanzanian mainland – Mwanza, Dar es Salaam, Mtwara, Arusha, and Dodoma – were selected to represent the country’s central, southern, eastern, and western regions, respectively. Dar es Salaam is Tanzania’s largest commercial capital city, along the eastern coast of the Indian Ocean. At the same time, Dodoma is the governmental capital in the central region of Tanzania.
Data collection
We leveraged the Vaccine Information Management System (VIMS) established in 2019Citation22,Citation23 to extract vaccination records. The VIMS provides comprehensive aggregated digital records across numerous facilities. We extracted data from VIMS for six vaccines in five Tanzanian regions and proceeded with the analysis.
Vaccination dosage standardization
The unit of measurement was the number of children receiving vaccination. To compare the vaccination rate across time and region, the number of children was divided by 1000 using the following formula:
where vaccination is the total number of vaccinations, and the population is the general population in that region.
Vaccine types and recommended vaccination schedule
The six vaccines used for treatment were Bacillus Calmette-Guérin (BCG), a vaccine for tuberculosis (TB) disease; bOPV0, bOPV1, bOPV2, and bOPV3, which are bivalent oral polio vaccine doses of 0, 1, 2, and 3, respectively, were used for immunization against poliomyelitis. In addition, we evaluated the trends of MR1 and MR2, the first and second doses of the measles-rubella vaccine, which is used to protect against both measles and rubella diseases, and PCV1, PCV2, and PCV3, which are Pneumococcal Conjugate Vaccine doses 1, 2, and 3, respectively, which help protect against pneumococcal diseases. Penta1, Penta2, and Penta3: Diphtheria, tetanus, pertussis, hepatitis B, and Haemophilus influenzae type b (Hib) combination vaccine doses of 1, 2, and 3, respectively. Often referred to as the pentavalent vaccine, it protects against these five diseases. Rota1 and Rota2: First and second doses of the rotavirus vaccine protect against rotavirus infections, a common cause of severe diarrhea in young children.
In Tanzania, the schedule is BCG at birth; OPV at birth, 6, 10, and 14 weeks; rotavirus at 6 and 10 weeks; DTwP-Hib-HepB at 6, 10, and 14 weeks; PCV at 6, 10, and 14 weeks; and MR at 9 and 18 months.Citation4
Data analysis
Quantitative data were analyzed using descriptive and inferential statistics to identify patterns and relationships within the data. Vaccination data files were combined, unpivoted, aggregated, reaggregated, and repivoted using Microsoft Power BI. Tables and graphs were plotted to portray trends in childhood vaccination.
The Statistical Package for the Social Sciences (SPSS) software version 26 for Windows was used for the time series to ascertain the annual trend of vaccination via childhood immunization in the selected regions in Tanzania. Time series and regression analyses were performed to determine yearly trends in vaccination.Citation24,Citation25
We performed a time-series analysis using the time-series model to predict the number of BCG vaccinations per 1000 people through December 2023. The dependent variable was the BCG vaccination rate, with the independent variables being year and month. The SPSS Expert Modeler was used to automatically select the optimal model from the autoregressive integrated moving average (ARIMA) and exponential smoothing (EXSMOOTH) models, factoring in potential seasonal variations, and finally selecting the simple seasonal model. We assessed the fitness of the model using the coefficient of determination (RSQUARE) and examined the autocorrelation of the residuals. The analysis included visually comparing the observed data against forecasts and computing 95% confidence intervals (CIs) for the predictions. Statistical significance was set at p < .05.
Ethical compliance
This study used secondary data for analysis, and there was no direct contact with clients, necessitating informed consent. Ethical approval was provided by the Directorate of Research, Publications, and Innovations of Muhimbili University of Health and Allied Sciences (MUHAS) DA 282/298/01/230 on the 9th Feb 2023. The Ministry of Health, Tanzania, granted permission to collect and use vaccination data. Data privacy and confidentiality were assessed throughout the study.
Results
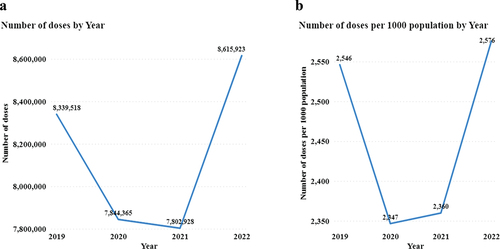
The study revealed 32,602,734 vaccination events involving six vaccines in five regions of Tanzania from 2019 to 2022, with an average of 1,224 children per vaccine in four years (). The number of vaccinations has varied over the four years, from 8,339,518 in 2019 to 7,844,365 in 2020 and 8,615,923 in 2022.
Table 1. Total number and average number of vaccinated children between 2019 and 2022.
The total number declined sharply in 2020 and returned to 2019 levels by 2022 ( and ). A similar trend was observed when the number of vaccinated children was standardized per 1000 inhabitants in the region where vaccination was undertaken (Supplementary Table S1).
Figure 1. Total annual vaccinations presented in number of children vaccinated A) in absolute number and B) per 1000 population.
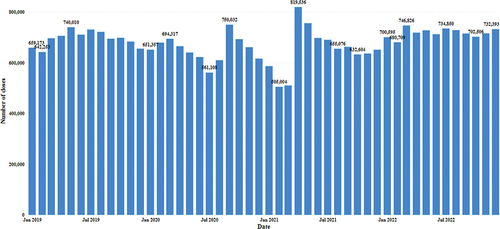
Monthly data showed increasing trends in the later months of 2019. The highest peak for this year occurred in May 2019, with 740,000 vaccinations. A sharp decline was noted, from 561,108 in July 2020 to 505,004 in February 2021. The highest peak occurred in April 2021. In March 2022, 746,826 vaccinations were performed; in December 2022, 732,393 vaccinations were conducted in these five regions ().
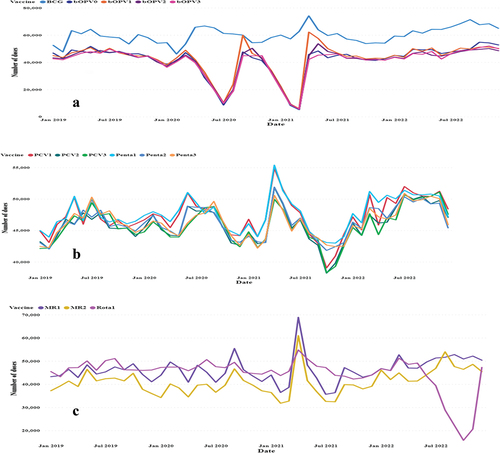
Variations in vaccinations were observed, with some remaining stable over time. Among the six vaccines studied, BCG exhibited the most stable trend. Another low peak for the Rota vaccine was registered in October 2022 () (Supplementary Table S2).
Figure 3. Number of vaccinations per month by type of vaccine between 2019 and 2022. Panel A: BCG and OPV; Panel B: PCV and Penta; and Panel C: MR and Rota.
In terms of the volume and number of children vaccinated, the Dar es Salaam Region had the highest volume of vaccination, followed by Mwanza, Dodoma, Arusha, and Mtwara, in decreasing order (Supplementary Figure S1).
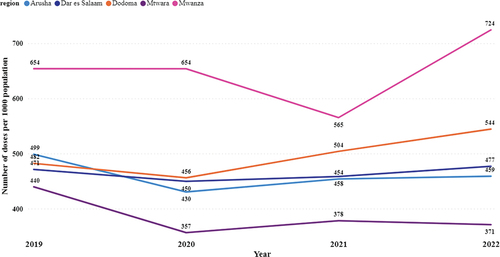
When using a standardized dosage per 1000 people, the use pattern had the highest vaccination rate in Mwanza, Dodoma, Dar es Salaam, Arusha, and Mtwara, in decreasing order () and (Supplementary Figure S2).
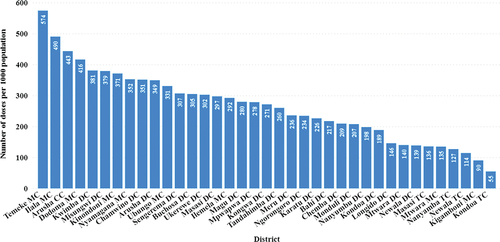
The vaccination rate per 1000 people varies per district, with the highest vaccination rate per 1000 people in Temeke and Ilala Districts in Dar es Salaam, followed by Dodoma, Kwimba, Misungwi, and Kinondoni. The lowest percentages were observed in Kondoa and Kigamboni ().
Figure 5. Cumulative vaccination in each district from 2019 to 2021 as the number of children vaccinated per 1000 people given to children.
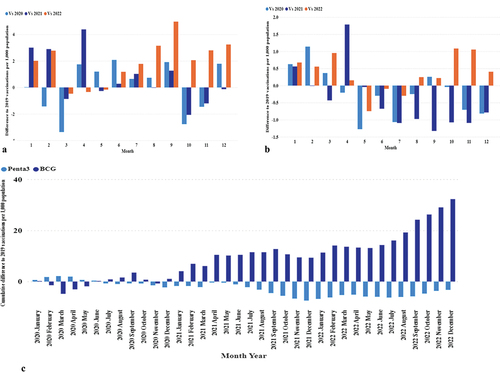
The monthly vaccination rate per 1000 people indicated seasonal fluctuations. BCG had the most stable variations, with peaks and troughs occurring from 2020 to 2023 (). The monthly distribution of the other vaccines varied. The immunization program showed the lowest peaks in vaccination rates for various antigens over specific periods. The lowest peaks occurred during two distinct periods for the bivalent oral polio vaccines (bOPV0, bOPV1, bOPV2, and bOPV3). In July 2020, the incidences of OPV were 2.385 for bOPV0, 2.823 for bOPV1, 2.728 for bOPV2, and 2.735 for bOPV3. A further decline was noted in February 2021, with rates decreasing to 1.409, bOPV0, 1.629 for bOPV1, 1.518 for bOPV2, and 1.561 for bOPV3. Similarly, the measles-rubella vaccines (MR1 and MR2) reached their lowest points in February 2021, with MR1 at 9.941 and MR2 at 8.559. The lowest rates of rotavirus vaccines (Rota1 and Rota2) were observed in October 2022, with Rota1 at 5.004 and Rota2 at 4.746 ().
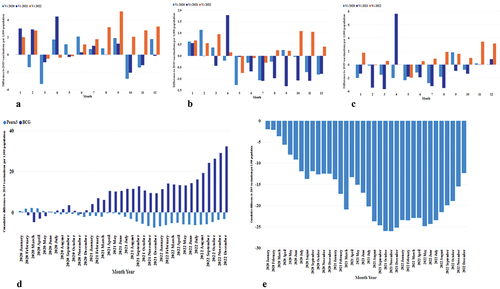
A detailed examination of the vaccination data from 2019 to 2022 (, Panels A-C) reveals distinct patterns of recovery and ongoing challenges. Specifically, the BCG vaccine showed signs of a transient shock in March 2020, suggesting a temporary disruption in vaccination efforts. Conversely, for Penta3 and MR2, the data indicate a sustained shortfall in recovery as of December 2022, as depicted in the cumulative differences (, Panels D-E)
Figure 6. Monthly and cumulative differences in vaccination rates from 2019. Panels A-C: Monthly differences for BCG, Penta3, and MR2. Panels D-E: Cumulative differences for BCG (dark blue) and Penta3 (light blue) (D), and MR2 (E).
Monthly variations in BCG and Penta3 were modeled using a simple seasonal model to predict the data from 2019 to 2023. Data for 12 months from 2023 were predicted because the actual data were unavailable.
The model for 2019 showed that the utilization of BCG was 16.1 vaccinations per 1000 population in January. In contrast, it was 14.8 in February, followed by a gradual increase, peaking at 20.7 vaccinations per 1000 population in May. This trend continued with slight variations, ending the year at an actual rate of 16.5 in December. In 2020, the fluctuation pattern reappeared, with a low rate in February (13.4) and the highest actual rates in the middle of the year, reaching 20.4 in April. The highest peak was observed in May 2021, with 23 vaccinations per 1000 people. January 2022 starts at 18.1, climbs to 20.9 in April, and maintains a high annual value. In 2023, a high and stable coverage was observed, with April having the highest prediction of 22.4. The predictions slightly tapered off as the year progressed but remained above 20 vaccinations per 1000 population (Supplementary Figure S3A) and (Supplementary Table S2).
For Penta3, the time-series model analyzed vaccination trends from January 2019 to December 2023 using a simple seasonal model similar to that employed for BCG. The early months of 2019 began with 12.9 vaccinations per 1000 people in January, showing a relatively stable trend with minor fluctuations throughout the year, peaking at 15.1 in July. In 2020, the actual data exhibited a slight decrease from February to 14.1, with a rebound in April, reaching 14.8. The year 2021 showed a higher peak in May, at 15.6. The year 2022 began with an actual of 13.6 in January, gradually increasing to a peak of 15.1 in July. Forecasts for 2023 revealed a steady and slightly increasing trend in Penta3 vaccine coverage. April was forecasted to have the highest rate of 15.3, while December had a predicted rate of 14.1 vaccinations per 1000 population (Supplementary Figure S3B) and (Supplementary Table S2).
Discussion
Childhood vaccination is essential for protection against infectious diseases, such as measles, mumps, rubella (MMR), Haemophilus influenzae type b (Hib), polio, diphtheria, tetanus, pertussis (DTP), hepatitis B, varicella and rotavirus. Vaccination is recognized as one of the most cost-effective public health strategies for reducing morbidity and mortality from vaccine-preventable diseases. However, the COVID-19 pandemic has caused significant global disruptions in these vital services, undermining efforts to control these diseases over the past two decades.Citation26 In Africa, the impact of COVID-19 on childhood vaccination and immunization is of particular concern because of the lack of data. A decrease in vaccination may lead to increased vaccine-preventable diseases, increased antibiotic use,Citation18,Citation20 and consequent antimicrobial resistance, impacting healthcare costs, morbidity, and mortality.
This study evaluated the influence of the COVID-19 pandemic on childhood vaccination and immunization routines in five administrative regions of Tanzania. The vaccines studied included six critical vaccines, each of which plays a pivotal role in childhood immunization programs worldwide. These include the Bacillus Calmette – Guérin (BCG) vaccine, which is primarily used against tuberculosis, and a series of bivalent oral polio vaccines (bOPV0, bOPV1, bOPV2, and bOPV3) targeting different stages of the poliovirus. Additionally, we examined the efficacy of MR1 and MR2 vaccines, which protect against measles and rubella, as they offer increased immunity through their two-dose regimens. Our study also included pneumococcal conjugate vaccines (PCV1, PCV2, and PCV3), which are vital for preventing infections caused by Streptococcus pneumoniae. Pentavalent vaccines (Penta1, Penta2, and Penta3) combine protection against diphtheria, tetanus, pertussis, hepatitis B, and Haemophilus influenzae type b (Hib). We examined rotavirus vaccines (Rota1 and Rota2), which are key to combating rotavirus-induced severe diarrhea in infants and young children.
This study revealed 32,602,734 vaccination events involving six vaccines, ranging from 8,339,518 in 2019 to 7,844,365 in 2020 and 8,615,923 in 2022. The findings of this study revealed that the COVID-19 pandemic in 2020 disrupted routine childhood immunizations, albeit for a transient period, with recovery occurring again in 2022. The average number of children vaccinated across all vaccines for the four years was 1,224, ranging from an average of 1,093 bOPV3 to 1,623 BCG children, and the total number of children administered each vaccine over the four years ranged from 1,940,424 for bOPV3 to 2,882,923 for BCG.
The vaccination dose trends from 2019 to 2022 revealed significant fluctuations, indicating public health challenges and success. A peak in vaccine administration in May 2019 suggests the need for effective vaccination campaigns. However, a sharp decline by February 2021 highlighted the impact of the COVID-19 pandemic on routine vaccinations. A notable rebound in April 2021 and sustained increases thereafter, peaking in March 2022, demonstrate the resilience and adaptation of the healthcare system to pandemic disruptions.
The significant drop in bOPV vaccinations in July 2020 and February 2021 and simultaneous declines in MR1 and MR2 rates confirmed systemic disruptions in routine immunization efforts due to the COVID-19 pandemic. This trend suggests broad challenges across multiple vaccine types, likely owing to strained healthcare resources or policy changes. This pattern underscores the need for resilience and adaptability in vaccination programmes.Citation27 Proactive measures, including public awareness and strengthening healthcare infrastructure, such as cold chain storage, are essential for maintaining consistent vaccination rates and protecting public health, especially in children vulnerable to preventable diseases. Stable trends were noted for the BCG, PCV, and DTP-HepB-Hib antigens. PCV is a proxy for new vaccines introduced in 2012, whereas DTP-HepB-Hib was rolled out in 2009. The low peaks in the rotavirus antigen may have been due to a change from Rotarix to Rotavac.Citation28 PCV is widely used in Tanzania and sub-Saharan Africa for children with sickle cell disease (SCD).Citation29–31 Antibiotic resistance to antibiotics used to treat pneumococcal infections in these children has increased, making PCV a viable option for improving survival in children with SCD.Citation32,Citation33
Potential reasons for this disruption include spreading the virus during the pandemic, fear of contracting the virus at health facilities, reallocating health resources to combat the pandemic, and changes in government priorities.Citation9,Citation13 The factors identified may help future and current strategies to maintain routine immunization during global health crises, such as implementing mobile vaccination clinics and using telehealth for vaccine education and scheduling.Citation9
Various studies have highlighted the decline in childhood vaccinations during the COVID-19 pandemic. A possible decrease in the vaccination rate was predicted earlier during the COVID-19 pandemic, particularly in low- and middle-income countries (LMICs).Citation34,Citation35 This prediction affected the effectiveness of curative and preventive services at the beginning of the pandemic.Citation36,Citation37 This has been noted in many countries, with Ghana’s Tamale Teaching Hospital showing the greatest decline in the BCG vaccine and the lowest drop in the MR1 vaccine.Citation36 However, MR2 expression was unaffected. In our study, BCG levels remained relatively stable, although seasonal patterns were detected in the time-series analysis. The same decline in Accra-Ghana was observed in the Weija Gbawe municipality.Citation38 These declines may have resulted in a surge in measle cases.Citation18
Declines have also been reported by Gambia.Citation39 Records of the Expanded Program on Immunization (EPI) clinic attendance indicated a monthly decrease of 13.4% in attendance and a 38.3% reduction in vaccination during the pandemic. However, recovery in the number of patients in Gambia was noted within approximately three months, concurrent with the return of vaccination rates to pre-pandemic levels for most vaccines.Citation39
While declines in vaccination are most common in LMICs, where the burden of vaccine-preventable diseases is at the highest level and vaccine coverage is the lowest, declines have also been reported in high-income settings.Citation9,Citation40 A decrease in vaccination rates has also been reported in high-income countries such as England.Citation13,Citation34,Citation41
Our study indicated that vaccination regimens should vary across the five selected regions in Tanzania, first by absolute numbers and then by numbers standardized per 1000 people. The vaccination landscape across various regions and districts is complex and influenced by demographic and logistical variables. The Dar es Salaam region topped the sheer volume of vaccinated children, signaling robust immunization activities, with Mwanza, Dodoma, Arusha, and Mtwara following suit. This hierarchy of vaccine volumes mirrors the population density and healthcare resource allocation in these regions. However, an intriguing shift was observed when population-adjusted vaccination rates were considered. Mwanza outpaces others, followed by Dodoma, indicating a more effective vaccination campaign relative to the size of their populationCitation42,Citation43
Furthermore, a granular look at the district level reveals disparities within the same region. The Temeke and Ilala districts in Dar es Salaam exhibited the highest vaccination rates per 1000 people, suggesting a successful localized response. Conversely, Kondoa and Kigamboni’s lag hinted at potential barriers to vaccine access or acceptance that require targeted interventions. These divergences in vaccination rates at the district level highlight the need for tailored strategies that consider unique demographic and regional challenges. The data insist on a robust approach to vaccination efforts that transcend aggregate figures and address the intricacies of individual communities needs to ensure equitable healthcare delivery and the attainment of herd immunity thresholds.
An East African study examining only BCG was conducted in 2020, including Tanzania,Citation44 and showed a varying range of vaccine coverage. The highest vaccine-specific coverage for BCG was in Rwanda (99.1%), followed by measles in Burundi (93.2%) and polio 3 in Rwanda (97.1%). Pentavalent 3 was in Rwanda (98%), while the lowest vaccine-specific coverage for BCG was in Ethiopia (70.5%), that for measles was in Rwanda (43.6%), that for polio 3 was in Ethiopia (57.7%), and that for pentavalent 3 was in Ethiopia (54.4%).Citation44
The COVID-19 pandemic has significantly impacted Africa, causing widespread fatalities, increasing poverty levels, and creating a heavy dependency on healthcare systems. In addition, the pandemic has interrupted essential health services and stunted progress in combating other lethal diseases such as HIV, TB, and malaria. These interruptions have led to an increase in death rates across the continents. The fear of contracting COVID-19 during healthcare center visits has significantly reduced patient access to these services by 2020. Other impediments to accessing healthcare include disruptions in public transportation and mandated stay-at-home orders.Citation45,Citation46
Similar declines were observed in Latin America, where catch-up vaccination strategies included prioritizing routine vaccinations as per the national immunization schedule. However, declining trends in Latin America were observed before 2020, suggesting multifactorial reasons for declining vaccination rates in Latin America.Citation47
In the context of these global trends, the healthcare system in Jordan experienced a substantial burden during the 2020 COVID-19 pandemic, particularly during the lockdown period from March to April 2020.Citation17 A similar pattern was observed in Alberta, a western Canadian province, where childhood vaccination rates plummeted from 2019 to 2020 following the first confirmed case of COVID-19.
A similar decline was observed in Quebec, Canada,Citation48 during the first months of the pandemic. However, catch-up occurred after that, and vaccination coverage in the affected cohorts was very close to that in 2019 after a few months of follow-upCitation48 and Colombia.Citation11
Following these disruptions, supplemental immunization activities (SIAs) can be conducted to provide additional vaccination opportunities to target groups, usually for specific diseases. One possible solution to address inequalities in vaccination is to transition from nationwide nonselective SIAs to more targeted and selective strategies.Citation15,Citation49
In a systematic review of 26 studies, 21 demonstrated decreased vaccination rates in children during the COVID-19 pandemic. In comparison, three studies reported increased or no significant changes in influenza vaccination rates. The remaining studies from Brazil and Sweden also showed no substantial changes in vaccination rates in children during the pandemic.Citation50
However, some doses experienced a second decline in Alberta, Canada, between September and October 2020. Cumulative coverage analysis showed that the measles-containing vaccine had the most considerable difference in coverage at the end of the follow-up.Citation51
Our analysis of cumulative vaccination differences from the baseline in 2019 compared to the same months in 2020, 2021, and 2022 reveals varied impacts across vaccines. BCG vaccinations experienced a transient shock, quickly recovering due to immediate post-birth administration. In contrast, Penta3 faced sustained challenges with slow recovery to catch up, indicating issues with continued engagement or dropouts. The MR2 vaccine, given at 18 months, saw drop-offs in 2021 and 2022.Citation4 Concurrently, Tanzania’s ramped-up COVID-19 vaccination campaigns further strained immunization services, impacting routine immunizations, which may have shifted healthcare resources and caregivers’ focus away from standard schedules.Citation52
The predictive modeling of BCG and Penta3 vaccination data highlights significant trends and the impact of public health initiatives in Tanzania. The BCG vaccine showed a notable increase in coverage, peaking at 22.4 vaccinations per 1000 people in April 2023, indicating a robust and effective immunization strategy. This trend reflects the successful scale-up of vaccination campaigns and sustained efforts to maintain high immunization rates. For Penta3, the model similarly underscores the resilience and adaptability of the immunization program, with a consistent delivery and gradual increase in vaccination rates, culminating in a predicted high rate in April 2023. These trends highlight the effectiveness of the immunization framework and emphasize the importance of continuing these efforts to provide comprehensive protection against diseases targeted by the Penta3 vaccine. Penta3, which is administered 12 weeks after birth in Tanzania,Citation28 is considered a proxy for the proper utilization of vaccinations in the programme. The trends observed in the Penta3 vaccination rates, as predicted by the time-series model, reflect a narrative of resilience and adaptability in the immunization program, particularly in the face of challenges such as the COVID-19 pandemic. The relative stability of the vaccination rates from 2019, with minor fluctuations, indicates consistent delivery of the Penta3 vaccine. These findings emphasize the critical importance of sustaining such efforts to ensure comprehensive protection against multiple diseases targeted by Penta3.
Although the impact of these predictions on real-world vaccination execution and uptake depends on model fidelity, the observed and predicted increases in vaccination rates reflect the increasing efforts to scale vaccination post-pandemic and demonstrate the resilience of Tanzania’s health systems, as noted in other studies.Citation39,Citation47,Citation51
Limitations of the study
This study had several limitations. First, the findings may not necessarily be applicable to other countries because of Tanzania’s unique social, political, economic, and health factors. Second, the calculation of vaccination rates was based on the total population as an approximate measure, assuming that the overall population figures roughly corresponded to the birth rates and the number of children eligible for vaccination. This method was employed because of the lack of access to precise data on the number of children eligible for each vaccine. For a more precise evaluation, future studies should include age-specific population data of children eligible for specific vaccines. Additionally, the absence of historical data from 2016 to 2018 limited our ability to analyze long-term trends and develop a more robust predictive model. Therefore, we urge readers to approach the standardized vaccination rates presented in this study with caution.
Conclusion
Understanding the impact of the COVID-19 pandemic on routine childhood immunization is essential for developing strategies to ensure the continuity of vital health services during global crises. The findings of this study in Tanzania may provide responses in similar global contexts. The COVID-19 pandemic has underscored the importance of preparedness to maintain essential health services and protect the most vulnerable population from preventable diseases. This study indicates the value of standardized vaccination measures taken during pandemics in predicting recovery from normal vaccination coverage. This approach could potentially influence policy strategies in Tanzania and other regions.
Author contributions
RZS and DJ contributed to the design and implementation of the research, and RZS, HM, and MEM1 analyzed the results and wrote the manuscript. RZS and DK conceived the original study and supervised the project. MEM2, HN, FT, and DPM supervised the collection of data and the analysis and revision of the manuscript. All authors have read and approved the manuscript.
Data access statement
Research data supporting this publication are available in the manuscript and Supplementary Materials.
Supplemental Material
Download PDF (417.1 KB)Acknowledgments
We acknowledge the Immunization and Vaccine Development (IVD) section of the Ministry of Health for providing permission to use the collected data.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2024.2356342
Additional information
Funding
References
- Enyew EB, Tareke AA. Vaccination status and factors associated among children age 12–23 months in Ethiopia, based on 2016 EDHS: logit based multinomial logistic regression analysis. In: Johnson C, editor. PLOS ONE. Vol. 17. 2022. p. e0264004. doi:10.1371/journal.pone.0264004.
- Becker-Dreps S, Butler AM, McGrath LJ, Boggess KA, Weber DJ, Li D, Hudgens MG, Layton JB. Effectiveness of prenatal tetanus, diphtheria, acellular pertussis vaccination in the prevention of infant pertussis in the U.S. Am J Prev Med. 2018;55(2):159–12. doi:10.1016/j.amepre.2018.04.013.
- Centers for Disease Control and Prevention (CDC). Child and adolescent immunization schedule by age. [accessed 2024 Mar 18]. https://www.cdc.gov/vaccines/schedules/hcp/imz/child-adolescent.html.
- World Health Organization. Vaccination schedule for United republic of Tanzania. [accessed 2024 Mar 17]. https://immunizationdata.who.int/pages/schedule-by-country/tza.html?DISEASECODE=DIPHTHERIA&TARGETPOP_GENERAL=.
- Fuchs EL, Starkey JM, Rupp RE, Berenson AB. Prenatal vaccination of mothers and hepatitis B vaccination of their infants. Prev Med. 2019;121:68–73. doi:10.1016/j.ypmed.2019.02.013.
- Morens DM, Breman JG, Calisher CH, Doherty PC, Hahn BH, Keusch GT, Kramer LD, LeDuc JW, Monath TP, Taubenberger JK. et al. The origin of COVID-19 and why it matters. Am J Trop Med Hyg. 2020;103(3):955–9. doi:10.4269/ajtmh.20-0849.
- Zhou P, Yang X, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–3. doi:10.1038/s41586-020-2012-7.
- Tarimo CS, Wu J. The first confirmed case of COVID-19 in Tanzania: recommendations based on lesson learned from China. Trop Med Health. 2020;48(1):25. doi:10.1186/s41182-020-00214-x.
- Cardoso Pinto AM, Shariq S, Ranasinghe L, Sundar Budhathoki S, Skirrow H, Whittaker E, Seddon JA. Reasons for reductions in routine childhood immunisation uptake during the COVID-19 pandemic in low- and middle-income countries: a systematic review. In: Majumdar S, editor. PLOS Global Public Health. Vol. 3, 2023. p. e0001415. doi:10.1371/journal.pgph.0001415.
- Chen Y, Klein SL, Garibaldi BT, Li H, Wu C, Osevala NM, Li T, Margolick JB, Pawelec G, Leng SX, et al. Aging in COVID-19: vulnerability, immunity and intervention. Ageing Res Rev. 2021;65:101205. doi:10.1016/j.arr.2020.101205.
- Moreno-Montoya J, Ballesteros SM, Rojas Sotelo JC, Bocanegra Cervera CL, Barrera-López P, De la Hoz-Valle JA. Impact of the COVID-19 pandemic on routine childhood immunisation in Colombia. Arch Dis Child. 2022;107(3):4–4. doi:10.1136/archdischild-2021-321792.
- Gholami M, Fawad I, Shadan S, Rowaiee R, Ghanem H, Khamis AH, Ho SB. The COVID-19 pandemic and health and care workers: findings from a systematic review and meta-analysis (2020–2021). Int J Public Health. 2023;68:68. doi:10.3389/ijph.2023.1605421.
- Yussuph ZH, Alwy Al-Beity FM, August F, Anaeli A. COVID-19 vaccine hesitancy among pregnant women attending public antenatal clinics in Dar es Salaam, Tanzania. Hum Vaccin Immunother. 2023;19(3):19. doi:10.1080/21645515.2023.2269777.
- Vaidyanathan G. Massive measles outbreak threatens India’s goal to eliminate disease by 2023. Nature. 2022; doi:10.1038/d41586-022-04480-z.
- Spencer N, Markham W, Johnson S, Arpin E, Nathawad R, Gunnlaugsson G, Homaira N, Rubio MLM, Trujillo CJ. The impact of COVID-19 pandemic on inequity in routine childhood vaccination coverage: a systematic review. Vaccines. 2022;10(7):1013. doi:10.3390/vaccines10071013.
- Khetrapal S, Bhatia R. Impact of COVID-19 pandemic on health system & sustainable development goal 3. Indian J Med Res. 2020;151(5):395–9. doi:10.4103/ijmr.IJMR_1920_20.
- Abu-Rish EY, Bustanji Y, Abusal K. Nationwide routine childhood vaccination coverage during the COVID-19 pandemic in Jordan: current situation, reasons, and predictors of vaccination. In: Gonzalez-Lopez T, editor. International Journal of Clinical Practice. Vol. 2022, 2022. p. 1–12. doi:10.1155/2022/7918604.
- Michael F, Mirambo MM, Misinzo G, Minzi O, Beyanga M, Mujuni D, Kalabamu FS, Nyanda EN, Mwanyika-Sando M, Ndiyo D, et al. Trends of measles in Tanzania: A 5-year review of case-based surveillance data, 2018-2022. Int J Infect Dis. 2023;139:176–82. doi:10.1016/j.ijid.2023.12.007.
- Sato APS. Pandemic and vaccine coverage: challenges of returning to schools. Rev Saude Publica. 2020;54:115. doi:10.11606/s1518-8787.2020054003142.
- Sangeda RZ, William SM, Masatu FC, Bitegeko A, Mwalwisi YH, Nkiligi EA. Antibiotic utilisation patterns in Tanzania: a retrospective longitudinal study comparing pre- and post-COVID-19 pandemic using Tanzania medicines and medical devices authority data. medRxiv. 2023; doi:10.1101/2023.11.27.23299060.
- Suvvari TK, Kandi V, Mohapatra RK, Chopra H, Islam M, Dhama K. The re-emergence of measles is posing an imminent global threat owing to decline in its vaccination rates amid COVID-19 pandemic: a special focus on recent outbreak in India – a call for massive vaccination drive to be enhanced at global level. Int J Surg. 2023;109:198–200. doi:10.1097/JS9.0000000000000228.
- Secor AM, Mtenga H, Richard J, Bulula N, Ferriss E, Rathod M, Ryman TK, Werner L, Carnahan E. Added value of electronic immunization registries in low- and middle-income countries: Observational case study in Tanzania. JMIR Public Health Surveill. 2022;8(1):e32455. doi:10.2196/32455.
- Nestory B, Anasel M, Nyandwi JB, Asingizwe D. Vaccine management practices among healthcare workers in Morogoro, Tanzania: a cross-sectional study. J Of Pharm Policy And Pract. 2022;15(1):95. doi:10.1186/s40545-022-00496-y.
- Sangeda RZ, Urassa MI, Buma D, Musiba GN, Chiwanga FS, Chambuso M, Horumpende PG. Seasonality and annual utilization patterns of antibacterials at muhimbili national hospital, Dar es Salaam, Tanzania: a 2015 monthly survey. Front Trop Dis. 2022;2:1–8. doi:10.3389/fitd.2021.768842.
- Sangeda RZ, Saburi HA, Masatu FC, Aiko BG, Mboya EA, Mkumbwa S, Bitegeko A, Mwalwisi YH, Nkiligi EA, Chambuso M, et al. National antibiotics utilization trends for human use in Tanzania from 2010 to 2016 inferred from Tanzania medicines and medical devices authority importation data. Antibiotics. 2021;10(10):1249. doi:10.3390/antibiotics10101249.
- Chippaux J-P. COVID-19 impacts on healthcare access in sub-Saharan Africa: an overview. J Venom Anim Toxins Incl Trop Dis. 2023;29:29. doi:10.1590/1678-9199-jvatitd-2023-0002.
- Ismail SA, Tomoaia-Cotisel A, Noubani A, Fouad FM, Bell S, Borghi J, Blanchet K. Resilience in childhood vaccination: analysing delivery system responses to shocks in Lebanon. BMJ Glob Health. 2023;8(11):e012399. doi:10.1136/bmjgh-2023-012399.
- United Republic of Tanzania. Expanded Programme on Immunization 2010 - 2015 Comprehensive Multi Year Plan. 2011 [accessed 2023 Dec 17]. https://bidinitiative.org/wp-content/uploads/1405554135TanzaniaComprehensivemultiyearplanfor20102015Year2011.pdf.
- Mutagonda RF, Bwire G, Sangeda RZ, Kilonzi M, Mlyuka H, Ndunguru J, Jonathan A, Makani J, Minja IK, Ruggajo P, et al. Nasopharyngeal carriage and antibiogram of pneumococcal and other bacterial pathogens from children with sickle cell disease in Tanzania. Infect Drug Resist. 2022;Volume 15:4407–18. doi:10.2147/IDR.S367873.
- Brown BJ, Madu A, Sangeda RZ, Nkya S, Peprah E, Paintsil V, Mmbando BP, Gyamfi J, Okocha CE, Asala SA, et al. Utilization of pneumococcal vaccine and penicillin prophylaxis in sickle cell disease in three African Countries: Assessment among healthcare providers in sickle in Africa. Hemoglobin. 2021;45(3):163–70. doi:10.1080/03630269.2021.1954943.
- Makani J, Soka D, Rwezaula S, Krag M, Mghamba J, Ramaiya K, Cox SE, Grosse SD. Health policy for sickle cell disease in Africa: experience from Tanzania on interventions to reduce under-five mortality. Trop Med Int Health. 2015;20(2):184–7. doi:10.1111/tmi.12428.
- Emgård M, Msuya SE, Nyombi BM, Mosha D, Gonzales-Siles L, Nordén R, Geravandi S, Mosha V, Blomqvist J, Franzén S, et al. Carriage of penicillin-non-susceptible pneumococci among children in northern Tanzania in the 13-valent pneumococcal vaccine era. Int J Infect Dis. 2019;81:156–66. doi:10.1016/j.ijid.2019.01.035.
- de Miguel S, Pérez-Abeledo M, Ramos B, García L, Arce A, Martínez-Arce R, Yuste J, Sanz JC. Evolution of antimicrobial susceptibility to penicillin in invasive strains of streptococcus pneumoniae during 2007–2021 in Madrid, Spain. Antibiotics. 2023;12(2):289. doi:10.3390/antibiotics12020289.
- Dalton M, Sanderson B, Robinson LJ, Homer CSE, Pomat W, Danchin M, Vaccher S. Impact of COVID-19 on routine childhood immunisations in low- and middle-income countries: a scoping review. In: Babu G, editor. PLOS Global Public Health. Vol. 3. 2023. p. e0002268. doi:10.1371/journal.pgph.0002268.
- Joshi S, Sharma M. A literature survey on vaccine supply chain management amidst COVID-19: literature developments, future directions and open challenges for public health. World. 2022;3(4):876–903. doi:10.3390/world3040049.
- Bimpong KA, Nuertey BD, Seidu AS, Ajinkpang S, Abdul-Mumin A. Decline in uptake of childhood vaccinations in a tertiary hospital in Northern Ghana during the COVID-19 pandemic. In: Angelillo I, editor. BioMed Research International. Vol. 2021, 2021. p. 1–0. doi:10.1155/2021/6995096.
- Roberton T, Carter ED, Chou VB, Stegmuller AR, Jackson BD, Tam Y, Sawadogo-Lewis T, Walker N. Early estimates of the indirect effects of the COVID-19 pandemic on maternal and child mortality in low-income and middle-income countries: a modelling study. Lancet Glob Health. 2020;8(7):901–8. doi:10.1016/S2214-109X(20)30229-1.
- Kissi J, Owusu-Marfo J, Osei E, Dzamvivie K, Akorfa Anku V, Naa Lamiokor Lamptey J. Effects of coronavirus pandemic on expanded program on immunization in weija gbawe municipality (Accra-Ghana). Hum Vaccin Immunother. 2022;18(6):18. doi:10.1080/21645515.2022.2129830.
- Osei I, Sarwar G, Hossain I, Sonko K, Ceesay L, Baldeh B, Secka E, Mackenzie GA. Attendance and vaccination at immunization clinics in rural Gambia before and during the COVID-19 pandemic. Vaccine. 2022;40(44):6367–73. doi:10.1016/j.vaccine.2022.09.031.
- Zar HJ, Dawa J, Fischer GB, Castro-Rodriguez JA. Challenges of COVID-19 in children in low- and middle-income countries. Paediatr Respir Rev. 2020;35:70–4. doi:10.1016/j.prrv.2020.06.016.
- McDonald HI, Tessier E, White JM, Woodruff M, Knowles C, Bates C, Parry J, Walker JL, Scott JA, Smeeth L. et al. Early impact of the coronavirus disease (COVID-19) pandemic and physical distancing measures on routine childhood vaccinations in England, January to April 2020. Eurosurveillance. 2020;25(19):25. doi:10.2807/1560-7917.ES.2020.25.19.2000848.
- National Bureau of Statistics. Report of 2022 Population and housing census, the United Republic of Tanzania [accessed 2023 Dec 10]. https://sensa.nbs.go.tz/publication/matokeo.pdf.
- National Bureau of Statistics. Report of 2012 Population and housing census, the United Republic of Tanzania. 2013 [accessed 2023 Oct 12]. https://www.nbs.go.tz/nbs/takwimu/census2012/Basic_Demographic_and_Socio-Economic_Profile_PopularVersion-KeyFindings_2012_PHC_EnglishVersion.pdf.
- Tesema GA, Tessema ZT, Tamirat KS, Teshale AB. Complete basic childhood vaccination and associated factors among children aged 12–23 months in East Africa: a multilevel analysis of recent demographic and health surveys. BMC Public Health. 2020;20(1):1837. doi:10.1186/s12889-020-09965-y.
- Shapira G, Ahmed T, Drouard SHP, Amor Fernandez P, Kandpal E, Nzelu C, Wesseh CS, Mohamud NA, Smart F, Mwansambo C, et al. Disruptions in maternal and child health service utilization during COVID-19: analysis from eight sub-Saharan African countries. Health Policy And Planning. 2021;36(7):1140–51. doi:10.1093/heapol/czab064.
- Ahmed T, Roberton T, Vergeer P, Hansen PM, Peters MA, Ofosu AA, Mwansambo C, Nzelu C, Wesseh CS, Smart F, et al. Healthcare utilization and maternal and child mortality during the COVID-19 pandemic in 18 low- and middle-income countries: an interrupted time-series analysis with mathematical modeling of administrative data. In: Persson L, editor. PLoS Medicine. Vol. 19, 2022. p. e1004070. doi:10.1371/journal.pmed.1004070.
- Castrejon MM, Leal I, de Jesus Pereira Pinto T, Guzmán-Holst A. The impact of COVID-19 and catch-up strategies on routine childhood vaccine coverage trends in Latin America: a systematic literature review and database analysis. Hum Vaccin Immunother. 2022;18(6):18. doi:10.1080/21645515.2022.2102353.
- Kiely M, Mansour T, Brousseau N, Rafferty E, Paudel YR, Sadarangani M, Svenson LW, Robinson JL, Gagneur A, Driedger SM, et al. COVID-19 pandemic impact on childhood vaccination coverage in Quebec, Canada. Hum Vaccin Immunother. 2022;18(1):18. doi:10.1080/21645515.2021.2007707.
- Yang Y, Kostandova N, Mwansa FD, Nakazwe C, Namukoko H, Sakala C, Bobo P, Masumbu PK, Nachinga B, Ngula D, et al. Challenges addressing inequalities in measles vaccine coverage in Zambia through a Measles–Rubella supplementary immunization activity during the COVID-19 pandemic. Vaccines. 2023;11(3):608. doi:10.3390/vaccines11030608.
- SeyedAlinaghi S, Karimi A, Mojdeganlou H, Alilou S, Mirghaderi SP, Noori T, Shamsabadi A, Dadras O, Vahedi F, Mohammadi P, et al. Impact of COVID ‐19 pandemic on routine vaccination coverage of children and adolescents: a systematic review. Health Sci Rep. 2022;5. doi:10.1002/hsr2.516.
- MacDonald SE, Paudel YR, Kiely M, Rafferty E, Sadarangani M, Robinson JL, Driedger SM, Svenson LW. Impact of the COVID-19 pandemic on vaccine coverage for early childhood vaccines in Alberta, Canada: a population-based retrospective cohort study. BMJ Open. 2022;12(1):e055968. doi:10.1136/bmjopen-2021-055968.
- Mfinanga SG, Gatei W, Tinuga F, Mwengee WMP, Yoti Z, Kapologwe N, Nagu T, Swaminathan M, Makubi A. Tanzania’s COVID-19 vaccination strategy: lessons, learning, and execution. Lancet. 2023;401:1649. doi:10.1016/S0140-6736(23)00723-7.