ABSTRACT
Solid cancer patients, compared to their healthy counterparts, are at a greater risk of contracting and suffering from severe complications and poorer prognosis after COVID-19 infections. They also have different immune responses after doses of COVID-19 vaccination, but limited evidence is available to reveal the effectiveness and help to guide immunization programs for this subpopulation; MEDLINE, Embase, Web of Science, Cochrane Library databases, and clinicaltrials.gov were used to search literature. The pooled seroconversion rate was calculated using a random-effects model and reported with a 95% confidence interval (CI); The review includes 66 studies containing serological responses after COVID-19 vaccination in 13,050 solid cancer patients and 8550 healthy controls. The pooled seropositive rates after the first dose in patients with solid cancer and healthy controls are 55.2% (95% CI 45.9%–64.5% N = 18) and 90.2% (95% CI 80.9%–96.6% N = 13), respectively. The seropositive rates after the second dose in patients with solid cancer and healthy controls are 87.6% (95% CI 84.1%–90.7% N = 50) and 98.9% (95% CI 97.6%-99.7% N = 35), respectively. The seropositive rates after the third dose in patients with solid cancer and healthy controls are 91.4% (95% CI 85.4%–95.9% N = 21) and 99.8% (95% CI 98.1%-100.0% N = 4), respectively. Subgroup analysis finds that study sample size, timing of antibody testing, and vaccine type have influence on the results; Seroconversion rates after COVID-19 vaccination are significantly lower in patients with solid malignancies, especially after the first dose, then shrinking gradually after the following two vaccinations, indicating that subsequent doses or a booster dose should be considered for the effectiveness of this subpopulation.
KEYWORDS:
1. Introduction
Since the COVID-19 pandemic in December 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has infected more than 770 million people worldwide and led to over 6 million deaths to date.Citation1 Vaccination is essential to prevent the spread of COVID-19 and ultimately quell the pandemic.Citation2 While the severe situation prompted an unprecedented pace in vaccine development, limited data are available among patients with active malignancies because of their ineligibility in most studies.
There is evidence suggesting that patients with cancer are at a greater risk of contracting and suffering from severe complications and poorer prognosis due to COVID-19 infections compared to those without,Citation3-7 indicating the need for prompt preventive intervention in this population. The ethics and healthcare of those cancer patients during the pandemic should be considered. As a result, voices came up and actions were taken that cancer patients have been identified as a high-priority subgroup for SARS-CoV-2 vaccinations.Citation8-10
Given the special nature of cancer patients, special attention should be paid to the immune response after receiving single and/or multiple doses of the COVID-19 vaccine. Therefore, combined immunogenicity information is needed to be elicited for this subgroup from the whole population. Those serological responses, such as the seroconversion rate, after doses of COVID-19 vaccination are also intended to provide data support for vaccination clinical development plans in the post-COVID-19 era.
During the COVID-19 pandemic in 2019, the mRNA vaccine technology has played a unique role in controlling the pandemic. The two most remarkable mRNA vaccines, Pfizer-BioNTech’s (BNT162b2) and Moderna’s (mRNA-1273), were the fastest vaccine development case in medical history and have achieved widespread inoculation. Also, another type of vaccine – adenovirus vector-based vaccines like Johnson & Johnson’s (Ad26.COV2.S) and University of Oxford & AstraZeneca’s (AZD1222) have received regulatory approval in many districts. As more approved COVID-19 vaccines become available and more efficacy data from vaccine clinical trials targeting the SARS-CoV-2 virus recently published,Citation11-14 we are seeing more data on the immune response of cancer patients to these vaccines. To shed light on the immunogenicity of those patients with solid cancer, we conducted a systematic review to combine the currently available evidence about the seroconversion rate, as well as the risk ratio to the healthy control after multiple doses of COVID-19 vaccination.
2. Methods
2.1. Data sources and search strategy
A priori-defined protocol was prespecified and registered in the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42023464756). The study was conducted following the Preferred Reporting Items for Systematic Review and Meta-analyses (PRISMA) guideline.Citation15 A systematic and comprehensive search was performed independently by two reviewers in MEDLINE, Embase, Web of Science, Cochrane Library, and the ClinicalTrials.gov databases between January 1, 2020, and October 10, 2023. We limited our search to the English language and did not include preprint publications for the validity of data. Discrepancies of extracted data between reviewers were resolved by consensus or involving a third investigator when necessary.
2.2. Study selection and data extraction
The criteria for the inclusion of studies were predetermined. Prospective cohort or retrospective observational studies as well as randomized clinical trials that included adult patients with a diagnosis of solid cancer after one, two or three doses of COVID-19 vaccine were considered for inclusion. Also, included studies should have reported patients’ antibody response (especially seroconversion rates) with specific exposure time. Case reports/series or cohort studies with an overall population of less than five patients were excluded. Studies with insufficient data on humoral response or only quantitative data without seroconversion rates were also excluded. The final reference list was generated based on their relevance to the scope of this review. This study included 66 studies investigating serological responses to COVID-19 vaccines in patients with solid cancer. We extracted relevant variables such as study design, study population, number of participants, doses of vaccines, methods used for detecting antibodies, and numbers or proportion of seroconversion following primary and booster vaccinations.
Two researchers extracted the data independently. All key extracted data were reviewed and checked at the end of the data extraction phase by the same two researchers.
2.3. Statistical analysis
Statistical analysis was performed using R (version 4.3.1). The pooled seroconversion rate of each outcome was calculated using a random-effects model and reported as the pooled proportion with a 95% confidence interval (CI). The pooled risk ratio (RR) was calculated using the Mantel–Haenszel method with a random-effects model and reported as the pooled RR with 95% CI. Statistical heterogeneity was assessed using the I2 statistics (ranging from 0% to 100%), which measured the degree of inconsistency across studies. Inter-study heterogeneity was assigned as insignificant (I2 = 0–25%), low (I2 = 26–50%), moderate (I2 = 51–75%), and high (I2 >75%).Citation16 Subgroup analysis was performed by sample size of each study (sample size of patients ≤100 and sample size of patients >100), timing of antibody testing (≤4 weeks after vaccination and >4 weeks after vaccination), vaccine type (only using mRNA vaccines and mixed with adenovirus vaccines), study design (prospective and retrospective) and methods to assess antibody titers (Roche, Abbott, ELISA, Liaison, and Not Available). Unless specified otherwise, we considered a two-sided p value of <0.05 to be statistically significant.
3. Results
3.1. Literature search
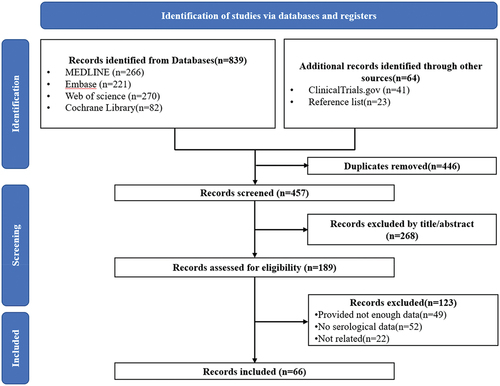
shows the PRISMA flow diagram of this study. The initial search of databases and other sources yielded 903 studies. After the removal of duplicates, a total of 457 unique studies were screened by titles and abstracts. Of these, 268 were excluded, and 189 full texts were screened for eligibility. Eventually, a total of 66 studiesCitation17-82 reported serological data after COVID-19 vaccination in patients with solid cancer were included in this study (Supplementary Table S1).
3.2. Study characteristics
The review included 66 studies containing serological data after COVID-19 vaccination in 13,050 solid cancer patients and 8550 healthy controls. The main characteristics are summarized in Supplementary Table S1. Thirty-nine of them reported serological results in both solid cancer patients and healthy controls. Eighteen studies reported immunogenicity rates following the first dose, 50 of them reported immunogenicity rates after the second dose and 21 of them reported immunogenicity rates after the third (booster) dose.
3.3. Results
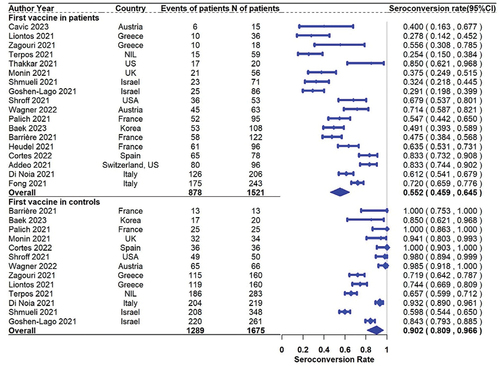
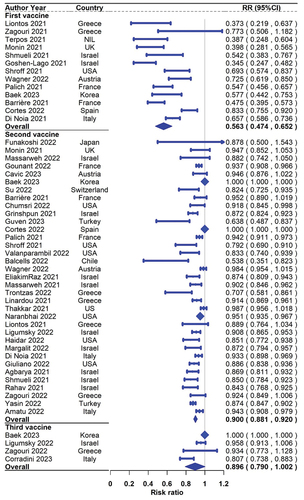
For response rates after the first dose, a total of 18 studies including 1521 solid cancer patients reported seroconversion rates, and 13 of them also reported the results in 1675 healthy controls. The pooled seropositive rates in patients with solid cancer and healthy controls were 55.2% (95% CI 45.9%–64.5% N = 18) and 90.2% (95% CI 80.9%–96.6% N = 13), respectively (). The pooled (95% CI) risk ratio of first-dose seroconversion in patients with solid cancer and healthy controls was 0.563 (0.474–0.652; N = 13, ).
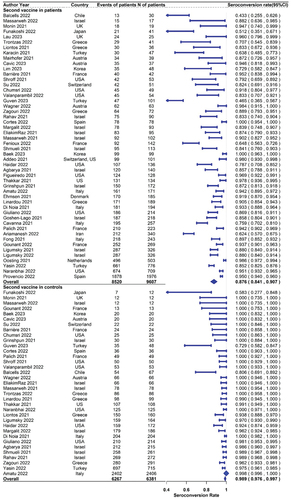
For response rates after the second dose, a total of 50 studies including 9607 solid cancer patients reported seroconversion rates, and 35 of them also reported the results in 6381 healthy controls. The seropositive rates in patients with solid cancer and healthy controls were 87.6% (95% CI 84.1%–90.7% N = 50) and 98.9% (95% CI 97.6%-99.7% N = 35), respectively (). The pooled(95% CI) risk ratio of second-dose seroconversion in patients with solid cancer and healthy controls was 0.900 (0.881–0.920; N = 35, ).
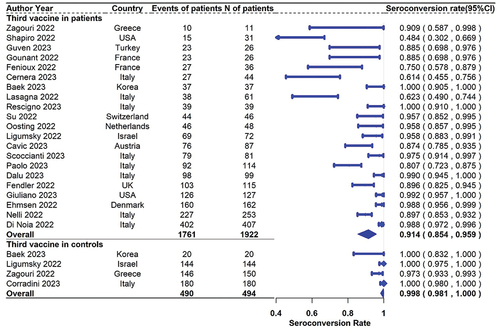
For the response rates after the third (booster) dose, a total of 21 studies including 1922 solid cancer patients reported seroconversion rates, and 4 of them also reported the results in 494 healthy controls. The seropositive rates in patients with solid cancer and healthy controls were 91.4% (95% CI 85.4%–95.9% N = 21) and 99.8% (95% CI 98.1%–100.0% N = 4), respectively (). The pooled (95% CI) risk ratio of third-dose seroconversion in patients with solid cancer and healthy controls was 0.896(0.790–1.002; N = 4, ).
Overall, the pooled analysis suggested poorer response to the COVID‐19 vaccine was observed in solid cancer patients compared with healthy controls after all three doses. Especially, the difference in serologic responses between the two populations was noticeable after the first dose of vaccination, then shrinking gradually after the following two vaccinations.
3.4. Subgroup analysis and publication bias
First, we performed subgroup analyses to determine if results were influenced by sample size, especially by the number of patients recruited in each study. According to whether the number of solid cancer patients included in the study is more than 100, we divided all studies into two subgroups. The results of the subgroup analysis are reported in Supplementary Figures S1-S8. As we concluded from the above analysis, the seroconversion rates in solid cancer patients are also lower compared to healthy controls in both subgroups and so are the risk ratios. Moreover, we can find that the differences in seroconversion rates and risk ratios between solid cancer patients and healthy controls are more evident in smaller-sample-size research after all three doses.
Also, subgroup analyses were taken to determine the extent to which the timing of antibody testing could have influenced the results. According to whether the time between antibody testing and vaccination is more than 4 weeks, we divided all included studies into two subgroups. We can find that the difference between seroconversion rates of solid cancer patients and healthy controls indicated by risk ratios is more evident in studies whose time between antibody testing and vaccination is less than 4 weeks (Supplementary Figures S9–S16).
Moreover, subgroup analysis was applied to evaluate the role of vaccine type on influencing the study findings. All included studies were classified as only using mRNA vaccines and mixed with adenovirus vaccines. There were some differences in effects on seroconversion among only using mRNA vaccines and mixed with adenovirus vaccines, especially after the first vaccination (Supplementary Figures S17-S24). The advantages of mRNA-only vaccines on risk ratios shrunk after the second dose and reversed after the third booster dose, compared to that of the mixed vaccines.
Then, subgroup analyses were carried to evaluate the potential impact of study design – specifically, the distinction between prospective and retrospective cohort – on the study results. No disparities were observed between the results of different study designs (Supplementary Figures S25–S32).
At last, subgroup analyses were conducted to assess the influence of definition of seroconversion on the study outcomes. According to the methods to assess antibody titers, we divided the included studies into five subgroups. No disparities were observed between the results of different study designs (Supplementary Figures S33–S52).
Publication bias assessment was performed qualitatively through visual inspection for funnel plot asymmetry, and quantitatively using Egger’s test.Citation83,Citation84 Supplementary Figures S53–55 show the funnel plot with trim-and-fill imputation of potentially missing studies for patients with solid cancer after three doses. Funnel‐plot analysis showed no publication bias in the second and third doses (p > .1). The heterogeneity of all included research and the correlation between study sizes and seroconversion rates mentioned above may induce the asymmetry in the funnel plot after the first vaccination.Citation85
4. Discussion
Covid-19 vaccination rates among cancer patients remain low,Citation86,Citation87 which indicates dispelled hesitation regarding the vaccine. Studies on vaccination status and attitudes toward COVID-19 vaccines among the cancer population and their family members in various countries have reported the incidences of vaccine hesitancy to be around 18–74%.Citation88–94 The main reason for cancer patients and their family members to refuse the vaccine is doubts about its effectiveness. LiuCitation95 finds that doctor’s advice is significantly associated with decreased vaccine hesitancy. Therefore, our study aims to reveal the real effectiveness of COVID-19 vaccines in the cancer population for both doctors and cancer patients to answer the concerns, guide the immunization program, and provide information for vaccination clinical development plans.
Immune responses vary with different vaccine types and products, as can be found in our subgroup analysis. In subgroup analysis of vaccine type, we found mRNA vaccines more effective than adenovirus vaccines, especially after the first dose, with mixed vaccines more durable after the third booster dose. This is also consistent with Terpos’sCitation96,Citation97 findings in healthy population.
The meta-analysis of 66 studies involving over 13,000 solid cancer patients and 8500 healthy controls reveals that serological response to COVID-19 vaccines within solid cancer patients is reduced in comparison with the healthy population. A single dose of the COVID-19 vaccine yields weaker (55.2% in solid cancer patients and 90.2% in healthy controls) and more heterogeneous (45.9%–64.5% in solid cancer patients and 80.9%–96.6 in healthy controls) serological responses in patients with solid tumors. After two doses of vaccination, serological response rates show a substantial increase (87.6% after the second dose and 91.4% after the third dose) in solid cancer patients but are still lower in comparison with healthy controls (98.9% after the second dose and 99.8% after the third dose). These are also validated in Tran’sCitation98 research.
Vaccine-induced immune responses in solid cancer patients are likely to be impaired by cancer therapies. PeetersCitation99 finds that humoral immune response after COVID-19 vaccination in cancer patients varies between different antineoplastic treatments, which is delayed and diminished mainly in patients receiving chemotherapy. Wankhede’sCitation100 findings imply that cancer immunotherapy may boost the vaccine-induced immune response in solid cancer patients compared with chemotherapy, which corresponds with GoossenCitation101 and Vollaard.Citation102
Similar situations also occur in other kinds of vaccines. In Kotton’sCitation103 and Xu’sCitation104 studies, the use of influenza vaccination in cancer patients results in less robust immune responses. It is nonetheless worthwhile and recommended because many patients develop at least a moderate degree of immune response against disease, even comparable with those in healthy controls. This study has been the largest and most up-to-date systematic review and meta-analysis evaluating immunogenicity following COVID-19 vaccination in patients with solid cancer. The size of the studies included provided sufficient power for quantitative assessment of immunogenicity following both the complete vaccination scheme and the booster dose. Nevertheless, we acknowledge that there are some limitations in our study. First, the limited number of patients included in each study may harm the power to distinguish the difference between serological responses in cancer patients and healthy controls, which is the main source of heterogeneity in our results. Second, this study focused on antibody-mediated responses to COVID-19 vaccines, without considering cellular immunity. Third, several techniques of SARS-CoV-2 antibody testing were used in the included studies without a standard criterion, which may affect the pooled results. Third, many included studies didn’t report seroconversion rates categorized by different types of solid cancers, so we could not get the information about the detailed serological responses of patients with different cancer types. Finally, for the same reason mentioned above, the effects of demographic characteristics and types of anti-cancer treatments were not evaluated in the present study.
5. Conclusions
In this systematic review and meta-analysis of 66 studies containing 13,050 solid cancer patients and 8550 healthy controls, we confirmed that seroconversion rates after doses of COVID-19 vaccination were significantly lower in patients with solid malignancies, especially after the first dose. However, this gap is narrowing with subsequent vaccinations. We suggest that extra interventions for cancer patients, including a subsequent booster vaccine dose, temporary rational suspension of anti-cancer treatment like chemotherapy during COVID-19 vaccination, or customized vaccination plans should be taken into consideration in the future. Also, immunization programs of similar vaccines, such as flu vaccines or vaccines for other respiratory diseases, can be developed or modified for solid cancer patients based on this research.
Author contributions
Conceptualization, HTT and CF.; methodology, search, screening of results and data extraction, HTT, FY, and FR; quality assessment and software, HTT and FR; visualization, HTT and FR; writing – original draft preparation, HTT and FY; writing – review and editing, HTT and FY; supervision, CF. All authors have read and agreed to the published version of the manuscript.
Supplementary Table S1.docx
Download MS Word (40.8 KB)supplementary_rev_cleancopy.docx
Download MS Word (12.8 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2024.2357424
Additional information
Funding
References
- WHO Coronavirus Disease (COVID-19) Dashboard 2021.
- Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020;586(7830):516–11. doi:10.1038/s41586-020-2798-3.
- Kuderer NM, Choueiri TK, Shah DP, Shyr Y, Rubinstein SM, Rivera DR, Shete S, Hsu C-Y, Desai A, de Lima Lopes G, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395(10241):1907–18. doi:10.1016/S0140-6736(20)31187-9.
- Rugge M, Zorzi M, Guzzinati S. SARS-CoV-2 infection in the Italian veneto region: adverse outcomes in patients with cancer. Nat Cancer. 2020;1(8):784–8. doi:10.1038/s43018-020-0104-9.
- Robilotti EV, Babady NE, Mead PA, Rolling T, Perez-Johnston R, Bernardes M, Bogler Y, Caldararo M, Figueroa CJ, Glickman MS. et al. Determinants of COVID-19 disease severity in patients with cancer. Nat Med. 2020;26(8):1218–23. doi:10.1038/s41591-020-0979-0.
- Saini KS, Tagliamento M, Lambertini M, McNally R, Romano M, Leone M, Curigliano G, de Azambuja E. Mortality in patients with cancer and coronavirus disease 2019: a systematic review and pooled analysis of 52 studies. Eur J Cancer. 2020;139:43–50. doi:10.1016/j.ejca.2020.08.011.
- Liang W, Guan W, Chen R, Wang W, Li J, Xu K, Li C, Ai Q, Lu W, Liang H. et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–7. doi:10.1016/S1470-2045(20)30096-6.
- Garassino MC, Vyas M, de Vries EGE, Kanesvaran R, Giuliani R, Peters S. The ESMO call to action on COVID-19 vaccinations and patients with cancer: vaccinate. Monitor Educate Ann Oncol. 2021;32(5):579–81. doi:10.1016/j.annonc.2021.01.068.
- van der Veldt AAM, Oosting SF, Dingemans AMC, Fehrmann RSN, GeurtsvanKessel C, Jalving M, Rimmelzwaan GF, Kvistborg P, Blank CU, Smit EF. et al. COVID-19 vaccination: the VOICE for patients with cancer. Nat Med. 2021;27(4):568–9. doi:10.1038/s41591-021-01240-w.
- Forni G, Mantovani A. COVID-19 vaccines: where we stand and challenges ahead. Cell Death Differ. 2021;28(2):626–39. doi:10.1038/s41418-020-00720-9.
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C. et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603–15. doi:10.1056/NEJMoa2034577.
- Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE. et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi:10.1016/S0140-6736(20)32661-1.
- Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB. et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–16. doi:10.1056/NEJMoa2035389.
- Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, Goepfert PA, Truyers C, Fennema H, Spiessens B. et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. 2021;384(23):2187–201. doi:10.1056/NEJMoa2101544.
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Med. 2009;6(7):e1000097. doi:10.1371/journal.pmed.1000097.
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. doi:10.1136/bmj.327.7414.557.
- Addeo A, Shah PK, Bordry N, Hudson RD, Albracht B, Di Marco M, Kaklamani V, Dietrich P-Y, Taylor BS, Simand P-F. et al. Immunogenicity of SARS-CoV-2 messenger RNA vaccines in patients with cancer. Cancer Cell. 2021;39(8):1091.e2–8.e2. doi:10.1016/j.ccell.2021.06.009.
- Agbarya A, Sarel I, Ziv-Baran T, Agranat S, Schwartz O, Shai A, Nordheimer S, Fenig S, Shechtman Y, Kozlener E. et al. Efficacy of the mRNA-Based BNT162b2 COVID-19 vaccine in patients with solid malignancies treated with anti-neoplastic drugs. Cancers Basel. 2021;13(16):4191. doi:10.3390/cancers13164191.
- Amatu A, Pani A, Patelli G, Gagliardi OM, Loparco M, Piscazzi D, Cassingena A, Tosi F, Ghezzi S, Campisi D. et al. Impaired seroconversion after SARS-CoV-2 mRNA vaccines in patients with solid tumours receiving anticancer treatment. Eur J Cancer. 2022;163:16–25. doi:10.1016/j.ejca.2021.12.006.
- Ariamanesh M, Porouhan P, PeyroShabany B, Fazilat-Panah D, Dehghani M, Nabavifard M, Hatami F, Fereidouni M, Welsh JS, Javadinia SA. Immunogenicity and safety of the inactivated SARS-CoV-2 Vaccine (BBIBP-CorV) in patients with malignancy. Cancer Invest. 2022;40(1):26–34. doi:10.1080/07357907.2021.1992420.
- Baek YJ, Lee Y-J, Park SR, Kim KH, Beom S-H, Lee C-K, Shin SJ, Rha SY, Kim S, Lee KH. et al. Immunogenicity and safety of vaccines against coronavirus disease in actively treated patients with solid tumors: a prospective cohort study. Cancer Res Treat. 2023;55(3):746–57. doi:10.4143/crt.2022.1541.
- Balcells ME, Le Corre N, Durán J, Ceballos ME, Vizcaya C, Mondaca S, Dib M, Rabagliati R, Sarmiento M, Burgos PI. et al. Reduced immune response to inactivated severe acute respiratory syndrome coronavirus 2 vaccine in a cohort of immunocompromised patients in chile. Clin Infect Dis. 2022;75(1):594–602. doi:10.1093/cid/ciac167.
- Barrière J, Chamorey E, Adjtoutah Z, Castelnau O, Mahamat A, Marco S, Petit E, Leysalle A, Raimondi V, Carles M. et al. Impaired immunogenicity of BNT162b2 anti-SARS-CoV-2 vaccine in patients treated for solid tumors. Ann Oncol. 2021;32(8):1053–5. doi:10.1016/j.annonc.2021.04.019.
- Cavanna L, Citterio C, Biasini C, Madaro S, Bacchetta N, Lis A, Cremona G, Muroni M, Bernuzzi P, Lo Cascio G. et al. COVID-19 vaccines in adult cancer patients with solid tumours undergoing active treatment: seropositivity and safety. A prospective observational study in Italy. Eur J Cancer. 2021;157:441–9. doi:10.1016/j.ejca.2021.08.035.
- Cavic G, Almonte AA, Hicks SM, Neeman T, Wang J-W, Brew S, Choi PY, Cockburn I, Gardiner EE, Yip D. et al. Response to COVID-19 vaccination in patients on cancer therapy: analysis in a SARS-CoV-2-naïve population. Asia Pac J Clin Oncol. 2024; doi:10.1111/ajco.14047.
- Cernera G, Gelzo M, De Placido P, Ottaviano M, Pietroluongo E, Raia M, Scalia G, Tortora M, Castaldo G, Formisano P. et al. Immunocytometric analysis of patients with thymic epithelial tumors revealed that COVID-19 vaccine booster strongly enhanced the immune response. Front Immunol. 2023;14:1233056. doi:10.3389/fimmu.2023.1233056.
- Chumsri S, Advani PP, Pai TS, Li Z, Mummareddy A, Acampora M, Reynolds GA, Wylie N, Boyle AW, Lou Y. et al. Humoral responses after SARS-CoV-2 mRNA vaccination and breakthrough infection in cancer patients. Mayo Clin Proc Innov Qual Outcomes. 2022;6(2):120–5. doi:10.1016/j.mayocpiqo.2021.12.004.
- Corradini P, Agrati C, Apolone G, Mantovani A, Giannarelli D, Marasco V, Bordoni V, Sacchi A, Matusali G, Salvarani C. et al. Humoral and T-Cell immune response after 3 doses of messenger RNA severe acute respiratory syndrome coronavirus 2 vaccines in fragile patients: The Italian VAX4FRAIL Study. Clin Infect Dis. 2023;76(3):426–38. doi:10.1093/cid/ciac404.
- Cortés A, Casado JL, Longo F, Serrano JJ, Saavedra C, Velasco H, Martin A, Chamorro J, Rosero D, Fernández M. et al. Limited T cell response to SARS-CoV-2 mRNA vaccine among patients with cancer receiving different cancer treatments. Eur J Cancer. 2022;166:229–39. doi:10.1016/j.ejca.2022.02.017.
- Dalu D, Tarkowski M, Ruggieri L, Cona MS, Gabrieli A, De Francesco D, Fasola C, Ferrario S, Gambaro A, Masedu E. et al. Antibody response to three-dose anti-SARS-CoV-2 mRNA-vaccination in treated solid cancer patients. Int J Cancer. 2023; doi:10.1002/ijc.34817.
- Di Noia V, Pimpinelli F, Renna D, Barberi V, Maccallini MT, Gariazzo L, Pontone M, Monti A, Campo F, Taraborelli E. et al. Immunogenicity and safety of COVID-19 vaccine BNT162b2 for patients with solid cancer: a large cohort prospective study from a single institution. Clin Cancer Res. 2021;27(24):6815–23. doi:10.1158/1078-0432.CCR-21-2439.
- Di Noia V, Pimpinelli F, Renna D, Maccallini MT, Gariazzo L, Riva F, Sperandio E, Giannarelli D, Cognetti F. Potentiation of humoral response to the BNT162b2 vaccine after the third dose in patients with solid cancer. Ann Oncol. 2022;33(5):563–5. doi:10.1016/j.annonc.2022.02.006.
- Ehmsen S, Asmussen A, Jeppesen SS, Nilsson AC, Østerlev S, Kragh A, Frederiksen H, Ditzel HJ. Antibody responses following third mRNA COVID-19 vaccination in patients with cancer and potential timing of a fourth vaccination. Cancer Cell. 2022;40(4):338–9. doi:10.1016/j.ccell.2022.02.011.
- Ehmsen S, Asmussen A, Jeppesen SS, Nilsson AC, Østerlev S, Vestergaard H, Justesen US, Johansen IS, Frederiksen H, Ditzel HJ. Antibody and T cell immune responses following mRNA COVID-19 vaccination in patients with cancer. Cancer Cell. 2021;39(8):1034–6. doi:10.1016/j.ccell.2021.07.016.
- Eliakim-Raz N, Massarweh A, Stemmer A, Stemmer SM. Durability of Response to SARS-CoV-2 BNT162b2 vaccination in patients on active anticancer treatment. JAMA Oncol. 2021;7(11):1716–18. doi:10.1001/jamaoncol.2021.4390.
- Fendler A, Shepherd STC, Au L, Wilkinson KA, Wu M, Schmitt AM, Tippu Z, Farag S, Rogiers A, Harvey R. et al. Immune responses following third COVID-19 vaccination are reduced in patients with hematological malignancies compared to patients with solid cancer. Cancer Cell. 2022;40(2):114–16. doi:10.1016/j.ccell.2021.12.013.
- Fenioux C, Teixeira L, Fourati S, Melica G, Lelievre JD, Gallien S, Zalcman G, Pawlotsky JM, Tournigand C. SARS-CoV-2 antibody response to 2 or 3 doses of the BNT162b2 vaccine in patients treated with anticancer agents. JAMA Oncol. 2022;8(4):612–7. doi:10.1001/jamaoncol.2021.7777.
- Figueiredo JC, Merin NM, Hamid O, Choi SY, Lemos T, Cozen W, Nguyen N, Finster LJ, Foley J, Darrah J. et al. Longitudinal SARS-CoV-2 mRNA vaccine-induced humoral immune responses in patients with cancer. Cancer Res. 2021;81(24):6273–80. doi:10.1158/0008-5472.CAN-21-3554.
- Fong D, Mair MJ, Mitterer M. High levels of anti–SARS-CoV-2 IgG antibodies in previously infected patients with cancer after a single dose of BNT 162b2 vaccine. Eur J Cancer. 2021;154:4–6. doi:10.1016/j.ejca.2021.05.036.
- Funakoshi Y, Yakushijin K, Ohji G, Hojo W, Sakai H, Takai R, Nose T, Ohata S, Nagatani Y, Koyama T. et al. Safety and immunogenicity of the COVID-19 vaccine BNT162b2 in patients undergoing chemotherapy for solid cancer. J Infect Chemother. 2022;28(4):516–20. doi:10.1016/j.jiac.2021.12.021.
- Giuliano A, Kuter B, Pilon‐Thomas S, Whiting J, Mo Q, Leav B, Sirak B, Cubitt C, Dukes C, Isaacs‐Soriano K. et al. Safety and immunogenicity of a third dose of mRNA-1273 vaccine among cancer patients. Cancer Commun (Lond). 2023;43(7):749–64. doi:10.1002/cac2.12453.
- Giuliano AR, Lancet JE, Pilon-Thomas S, Dong N, Jain AG, Tan E, Ball S, Tworoger SS, Siegel EM, Whiting J. et al. Evaluation of antibody response to SARS-CoV-2 mRNA-1273 vaccination in patients with cancer in florida. JAMA Oncol. 2022;8(5):748–54. doi:10.1001/jamaoncol.2022.0001.
- Goshen-Lago T, Waldhorn I, Holland R, Szwarcwort-Cohen M, Reiner-Benaim A, Shachor-Meyouhas Y, Hussein K, Fahoum L, Baruch M, Peer A. et al. Serologic status and toxic effects of the SARS-CoV-2 BNT162b2 vaccine in patients undergoing treatment for cancer. JAMA Oncol. 2021;7(10):1507–13. doi:10.1001/jamaoncol.2021.2675.
- Gounant V, Ferré VM, Soussi G, Charpentier C, Flament H, Fidouh N, Collin G, Namour C, Assoun S, Bizot A. et al. Efficacy of severe acute respiratory syndrome coronavirus-2 vaccine in patients with thoracic cancer: A prospective study supporting a third dose in patients with minimal serologic response after two vaccine doses. J Thorac Oncol. 2022;17(2):239–51. doi:10.1016/j.jtho.2021.10.015.
- Grinshpun A, Rottenberg Y, Ben-Dov IZ, Djian E, Wolf DG, Kadouri L. Serologic response to COVID-19 infection and/or vaccine in cancer patients on active treatment. ESMO Open. 2021;6(6):100283. doi:10.1016/j.esmoop.2021.100283.
- Guven DC, Incesu FGG, Yildirim HC, Erul E, Chalabiyev E, Aktas BY, Yuce D, Arik Z, Kilickap S, Aksoy S. et al. Immunogenicity of two doses of inactive COVID-19 vaccine and third booster dose mRNA vaccine in patients with cancer receiving active systemic therapy. Int J Cancer. 2023;152(4):679–85. doi:10.1002/ijc.34280.
- Haidar G, Agha M, Bilderback A, Lukanski A, Linstrum K, Troyan R, Rothenberger S, McMahon DK, Crandall MD, Sobolewksi MD. et al. Prospective Evaluation of Coronavirus Disease 2019 (COVID-19) vaccine responses across a broad spectrum of immunocompromising conditions: the COVID-19 Vaccination in the Immunocompromised Study (COVICS). Clin Infect Dis. Clin Infect Dis. 2022;75(1):630–44. doi:10.1093/cid/ciac103.
- Heudel P, Favier B, Assaad S, Zrounba P, Blay J-Y. Reduced SARS-CoV-2 infection and death after two doses of COVID-19 vaccines in a series of 1503 cancer patients. Ann Oncol. 2021;32(11):1443–4. doi:10.1016/j.annonc.2021.07.012.
- Karacin C, Eren T, Zeynelgil E, Imamoglu GI, Altinbas M, Karadag I, Basal FB, Bilgetekin I, Sutcuoglu O, Yazici O. et al. Immunogenicity and safety of the CoronaVac vaccine in patients with cancer receiving active systemic therapy. Future Oncol. 2021;17(33):4447–56. doi:10.2217/fon-2021-0597.
- Lasagna A, Bergami F, Lilleri D, Percivalle E, Quaccini M, Serra F, Comolli G, Sarasini A, Sammartino JC, Ferrari A. et al. Six-month humoral and cellular immune response to the third dose of BNT162b2 anti-SARS-CoV-2 vaccine in patients with solid tumors: a longitudinal cohort study with a focus on the variants of concern. ESMO Open. 2022;7(5):100574. doi:10.1016/j.esmoop.2022.100574.
- Lau DK, Aresu M, Planche T, Tran A, Lazaro-Alcausi R, Duncan J, Kidd S, Cromarty S, Begum R, Rana I. et al. SARS-CoV-2 vaccine immunogenicity in patients with gastrointestinal cancer receiving systemic anti-cancer therapy. Oncologist. 2023;28(1):1–8. doi:10.1093/oncolo/oyac230.
- Ligumsky H, Dor H, Etan T, Golomb I, Nikolaevski-Berlin A, Greenberg I, Halperin T, Angel Y, Henig O, Spitzer A. et al. Immunogenicity and safety of BNT162b2 mRNA vaccine booster in actively treated patients with cancer. Lancet Oncol. 2022;23(2):193–5. doi:10.1016/S1470-2045(21)00715-4.
- Ligumsky H, Safadi E, Etan T, Vaknin N, Waller M, Croll A, Nikolaevski-Berlin A, Greenberg I, Halperin T, Wasserman A. et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine among actively treated cancer patients. J Natl Cancer Inst. 2022;114(2):203–9. doi:10.1093/jnci/djab174.
- Lim SH, Choi SH, Ji YS, Kim SH, Kim CK, Yun J, Park SK. Comparison of antibody response to coronavirus disease 2019 vaccination between patients with solid or hematologic cancer patients undergoing chemotherapy. Asia Pac J Clin Oncol. 2023; doi:10.1111/ajco.13959.
- Linardou H, Spanakis N, Koliou G-A, Christopoulou A, Karageorgopoulou S, Alevra N, Vagionas A, Tsoukalas N, Sgourou S, Fountzilas E. et al. Responses to SARS-CoV-2 vaccination in patients with cancer (recover study): a prospective cohort study of the hellenic cooperative oncology group. Cancers Basel. 2021;13(18):4621. doi:10.3390/cancers13184621.
- Liontos M, Terpos E, Markellos C, Zagouri F, Briasoulis A, Katsiana I, Skafida E, Fiste O, Kunadis E, Andrikopoulou A. et al. Immunological response to COVID-19 vaccination in ovarian cancer patients receiving PARP Inhibitors. Vaccines. 2021;9(10):1148. doi:10.3390/vaccines9101148.
- Mairhofer M, Kausche L, Kaltenbrunner S, Ghanem R, Stegemann M, Klein K, Pammer M, Rauscher I, Salzer HJF, Doppler S. et al. Humoral and cellular immune responses in SARS-CoV-2 mRNA-vaccinated patients with cancer. Cancer Cell. 2021;39(9):1171–2. doi:10.1016/j.ccell.2021.08.001.
- Margalit O, Shacham-Shmueli E, Itay A, Berger R, Halperin S, Jurkowicz M, Levin EG, Olmer L, Regev-Yochay G, Lustig Y. et al. Seropositivity and neutralising antibodies at six months after BNT162b2 vaccination in patients with solid tumours. Eur J Cancer. 2022;168:51–5. doi:10.1016/j.ejca.2022.03.013.
- Massarweh A, Eliakim-Raz N, Stemmer A, Levy-Barda A, Yust-Katz S, Zer A, Benouaich-Amiel A, Ben-Zvi H, Moskovits N, Brenner B. et al. Evaluation of seropositivity following BNT162b2 messenger RNA vaccination for SARS-CoV-2 in patients undergoing treatment for cancer. JAMA Oncol. 2021;7(8):1133–40. doi:10.1001/jamaoncol.2021.2155.
- Massarweh A, Tschernichovsky R, Stemmer A, Benouaich-Amiel A, Siegal T, Eliakim-Raz N, Stemmer SM, Yust-Katz S. Immunogenicity of the BNT162b2 mRNA COVID-19 vaccine in patients with primary brain tumors: a prospective cohort study. J Neurooncol. 2022;156(3):483–9. doi:10.1007/s11060-021-03911-7.
- Monin L, Laing AG, Muñoz-Ruiz M, McKenzie DR, Del Molino Del Barrio I, Alaguthurai T, Domingo-Vila C, Hayday TS, Graham C, Seow J. et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22(6):765–78. doi:10.1016/S1470-2045(21)00213-8.
- Naranbhai V, Pernat CA, Gavralidis A, St Denis KJ, Lam EC, Spring LM, Isakoff SJ, Farmer JR, Zubiri L, Hobbs GS. et al. Immunogenicity and Reactogenicity of SARS-CoV-2 vaccines in patients with cancer: The CANVAX cohort study. J Clin Oncol. 2022;40(1):12–23. doi:10.1200/JCO.21.01891.
- Nelli F, Giannarelli D, Fabbri A, Silvestri MA, Berrios JRG, Virtuoso A, Marrucci E, Schirripa M, Mazzotta M, Onorato A. et al. Immunogenicity and early clinical outcome after two or three doses of SARS-CoV-2 mRNA-BNT162b2 vaccine in actively treated cancer patients: results from the prospective observational Vax-On-Third study. Ann Oncol. 2022;33(7):740–2. doi:10.1016/j.annonc.2022.04.002.
- Oosting SF, van der Veldt AAM, Fehrmann RSN, GeurtsvanKessel CH, van Binnendijk RS, Dingemans AMC, Smit EF, Hiltermann TJN, den Hartog G, Jalving M. et al. Immunogenicity after second and third mRNA-1273 vaccination doses in patients receiving chemotherapy, immunotherapy, or both for solid tumours. Lancet Oncol. 2022;23(7):833–5. doi:10.1016/S1470-2045(22)00203-0.
- Oosting SF, van der Veldt AAM, GeurtsvanKessel CH, Fehrmann RSN, van Binnendijk RS, Dingemans AMC, Smit EF, Hiltermann TJN, den Hartog G, Jalving M. et al. mRNA-1273 COVID-19 vaccination in patients receiving chemotherapy, immunotherapy, or chemoimmunotherapy for solid tumours: a prospective, multicentre, non-inferiority trial. Lancet Oncol. 2021;22(12):1681–91. doi:10.1016/S1470-2045(21)00574-X.
- Palich R, Veyri M, Marot S, Vozy A, Gligorov J, Maingon P, Marcelin A-G, Spano J-P. Weak immunogenicity after a single dose of SARS-CoV-2 mRNA vaccine in treated cancer patients. Ann Oncol. 2021;32(8):1051–3. doi:10.1016/j.annonc.2021.04.020.
- Provencio M, Rodríguez-Abreu D, Ortega AL, Serrano G, Aguado C, Franco F, Gutierrez V, López Vivanco G, Guirado M, Benítez G. et al. Seroprevalence and immunological memory against SARS-CoV-2 in lung cancer patients: the SOLID study. Transl Lung Cancer Res. 2022;11(1):53–63. doi:10.21037/tlcr-21-504.
- Rahav G, Lustig Y, Lavee J, Benjamini O, Magen H, Hod T, Shem-Tov N, Shmueli ES, Merkel D, Ben-Ari Z. et al. BNT162b2 mRNA COVID-19 vaccination in immunocompromised patients: A prospective cohort study. EClinicalMedicine. 2021;41:101158. doi:10.1016/j.eclinm.2021.101158.
- Rescigno M, Agrati C, Salvarani C, Giannarelli D, Costantini M, Mantovani A, Massafra R, Zinzani PL, Morrone A, Notari S. et al. Neutralizing antibodies to omicron after the fourth SARS-CoV-2 mRNA vaccine dose in immunocompromised patients highlight the need of additional boosters. Front Immunol. 2023;14:1104124. doi:10.3389/fimmu.2023.1104124.
- Scoccianti S, Delli Paoli C, Infantino M, Paoletti L, Caini S, Meacci F, Russo S, Esposito M, Fondelli S, Grilli Leonulli B. et al. Immunogenicity after two and three doses of mRNA vaccine in patients with cancer treated with exclusive radiotherapy. Int Immunopharmacol. 2023;122:110460. doi:10.1016/j.intimp.2023.110460.
- Shapiro LC, Thakkar A, Campbell ST, Forest SK, Pradhan K, Gonzalez-Lugo JD, Quinn R, Bhagat TD, Choudhary GS, McCort M. et al. Efficacy of booster doses in augmenting waning immune responses to COVID-19 vaccine in patients with cancer. Cancer Cell. 2022;40(1):3–5. doi:10.1016/j.ccell.2021.11.006.
- Shmueli ES, Itay A, Margalit O, Berger R, Halperin S, Jurkowicz M, Levin EG, Levy I, Olmer L, Regev-Yochay G. et al. Efficacy and safety of BNT162b2 vaccination in patients with solid cancer receiving anticancer therapy – a single centre prospective study. Eur J Cancer. 2021;157:124–31. doi:10.1016/j.ejca.2021.08.007.
- Shroff RT, Chalasani P, Wei R, Pennington D, Quirk G, Schoenle MV, Peyton KL, Uhrlaub JL, Ripperger TJ, Jergović M. et al. Immune responses to two and three doses of the BNT162b2 mRNA vaccine in adults with solid tumors. Nat Med. 2021;27(11):2002–11. doi:10.1038/s41591-021-01542-z.
- Su E, Fischer S, Demmer-Steingruber R, Nigg S, Güsewell S, Albrich WC, Rothermundt C, Silzle T, Kahlert CR. Humoral and cellular responses to mRNA-based COVID-19 booster vaccinations in patients with solid neoplasms under active treatment. ESMO Open. 2022;7(5):100587. doi:10.1016/j.esmoop.2022.100587.
- Terpos E, Zagouri F, Liontos M, Sklirou AD, Koutsoukos K, Markellos C, Briasoulis A, Papanagnou E-D, Trougakos IP, Dimopoulos M-A. et al. Low titers of SARS-CoV-2 neutralizing antibodies after first vaccination dose in cancer patients receiving checkpoint inhibitors. J Hematol Oncol. 2021;14(1):86. doi:10.1186/s13045-021-01099-x.
- Thakkar A, Gonzalez-Lugo JD, Goradia N, Gali R, Shapiro LC, Pradhan K, Rahman S, Kim SY, Ko B, Sica RA. et al. Seroconversion rates following COVID-19 vaccination among patients with cancer. Cancer Cell. 2021;39(8):1081.e2–90.e2. doi:10.1016/j.ccell.2021.06.002.
- Trontzas IP, Vathiotis I, Economidou C, Petridou I, Gomatou G, Grammoustianou M, Tsamis I, Syrigos N, Anagnostakis M, Fyta E. et al. Assessment of Seroconversion after SARS-CoV-2 vaccination in patients with lung cancer. Vaccines. 2022;10(4):618. doi:10.3390/vaccines10040618.
- Valanparambil RM, Carlisle J, Linderman SL, Akthar A, Millett RL, Lai L, Chang A, McCook-Veal AA, Switchenko J, Nasti TH. et al. Antibody response to COVID-19 mRNA vaccine in patients with lung cancer after primary immunization and booster: reactivity to the SARS-CoV-2 WT virus and omicron variant. J Clin Oncol. 2022;40(33):3808–16. doi:10.1200/JCO.21.02986.
- Wagner A, Garner-Spitzer E, Schötta A-M, Orola M, Wessely A, Zwazl I, Ohradanova-Repic A, Weseslindtner L, Tajti G, Gebetsberger L. et al. SARS-CoV-2-mRNA booster vaccination reverses non-responsiveness and early antibody waning in immunocompromised patients – a phase four study comparing immune responses in patients with solid cancers, multiple myeloma and inflammatory bowel disease. Front Immunol. 2022;13:889138. doi:10.3389/fimmu.2022.889138.
- Yasin AI, Aydin SG, Sümbül B, Koral L, Şimşek M, Geredeli Ç, Öztürk A, Perkin P, Demirtaş D, Erdemoglu E. et al. Efficacy and safety profile of COVID-19 vaccine in cancer patients: a prospective, multicenter cohort study. Future Oncol. 2022;18(10):1235–44. doi:10.2217/fon-2021-1248.
- Zagouri F, Papatheodoridi A, Liontos M, Briasoulis A, Sklirou AD, Skafida E, Fiste O, Markellos C, Andrikopoulou A, Koutsoukos K. et al. Assessment of postvaccination neutralizing antibodies response against SARS-CoV-2 in cancer patients under treatment with targeted agents. Vaccines. 2022;10(9):1474. doi:10.3390/vaccines10091474.
- Zagouri F, Terpos E, Fiste O, Liontos M, Briasoulis A, Katsiana I, Skafida E, Markellos C, Kunadis E, Andrikopoulou A. et al. SARS-CoV-2 neutralizing antibodies after first vaccination dose in breast cancer patients receiving CDK4/6 inhibitors. Breast. 2021;60:58–61. doi:10.1016/j.breast.2021.08.017.
- Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54(10):1046–55. doi:10.1016/S0895-4356(01)00377-8.
- Terrin N, Schmid CH, Lau J. In an empirical evaluation of the funnel plot, researchers could not visually identify publication bias. J Clin Epidemiol. 2005;58(9):894–901. doi:10.1016/j.jclinepi.2005.01.006.
- Sterne JA, Sutton AJ, Ioannidis JPA, Terrin N, Jones DR, Lau J, Carpenter J, Rucker G, Harbord RM, Schmid CH. et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343(jul22 1):d4002. doi:10.1136/bmj.d4002.
- Brodziak A, Sigorski D, Osmola M, Wilk M, Gawlik-Urban A, Kiszka J, Machulska-Ciuraj K, Sobczuk P. Attitudes of patients with cancer towards vaccinations-results of online survey with special focus on the vaccination against COVID-19. Vaccines (Basel). 2021 Apr 21;9(5):411. doi:10.3390/vaccines9050411.
- Tagliamento M, Agostinetto E, Bruzzone M, Ceppi M, Saini KS, de Azambuja E, Punie K, Westphalen CB, Morgan G, Pronzato P. et al. Mortality in adult patients with solid or hematological malignancies and SARS-CoV-2 infection with a specific focus on lung and breast cancers: A systematic review and meta-analysis. Crit Rev Oncol Hematol. 2021;163:103365. doi:10.1016/j.critrevonc.2021.103365.
- Peng X, Gao P, Wang Q, Wu H-G, Yan Y-L, Xia Y, Wang J-Y, Lu F, Pan H, Yang Y. et al. Prevalence and Impact Factors of COVID-19 vaccination hesitancy among breast cancer survivors: A multicenter cross-sectional study in China. Front Med. 2021;8:741204. doi:10.3389/fmed.2021.741204.
- Villarreal-Garza C, Vaca-Cartagena BF, Becerril-Gaitan A, Ferrigno AS, Mesa-Chavez F, Platas A, Platas A. Attitudes and factors associated with COVID-19 vaccine hesitancy among patients with breast cancer. JAMA Oncol. 2021;7(8):1242–4. doi:10.1001/jamaoncol.2021.1962.
- Hong J, Xu X-W, Yang J, Zheng J, Dai S-M, Zhou J, Zhang Q-M, Ruan Y, Ling C-Q. Knowledge about, attitude and acceptance towards, and predictors of intention to receive the COVID-19 vaccine among cancer patients in Eastern China: a cross-sectional survey. J Integr Med. 2022;20(1):34–44. doi:10.1016/j.joim.2021.10.004.
- Chun JY, Kim SI, Park EY, Park S-Y, Koh S-J, Cha Y, Yoo HJ, Joung JY, Yoon HM, Eom BW. et al. Cancer patients’ willingness to take COVID-19 Vaccination: a nationwide multicenter survey in korea. Cancers Basel. 2021;13(15):3883. doi:10.3390/cancers13153883.
- Overheu O, Lendowski S, Quast DR, Marheinecke CS, Kourti E, Lugnier C, Andreica I, Kiltz U, Pfaender S, Reinacher-Schick A. et al. Attitude towards and perception of individual safety after SARS-CoV-2 vaccination among German cancer patients. J Cancer Res Clin Oncol. 2023;149(5):1985–92. doi:10.1007/s00432-022-04099-7.
- He Y, Ding Y, Cao B, Huang Y, Wang X. COVID-19 vaccine development from the perspective of cancer patients. Hum Vaccin Immunother. 2021;17(10):3281–7. doi:10.1080/21645515.2021.1943988.
- Suri TM, Ghosh T, Singhal S, Arunachalam M, Alwani H, Mohan A. COVID-19 vaccine uptake among patients undergoing treatment for lung cancer: a cross-sectional study in India. Lung India. 2023;40(3):294–6. doi:10.4103/lungindia.lungindia_539_22.
- Liu W, Wu Y, Yang R, Chen R, Huang Y, Zhao X, Xie M, Li Q, Wang Q, Chen J. COVID-19 vaccination status and hesitancy among breast cancer patients after two years of pandemic: a cross-sectional survey. Vaccines. 2022;10(9):1530. doi:10.3390/vaccines10091530.
- Terpos EA-O, Karalis V, Ntanasis-Stathopoulos I, Evangelakou Z, Gavriatopoulou M, Manola MS, Malandrakis P, Gianniou DD, Kastritis E, Trougakos IP, Dimopoulos MA. et al. Comparison of Neutralizing antibody responses at 6 months post vaccination with BNT162b2 and AZD1222 LID. Print. 2022;338(2):2227–9059. doi:10.3390/biomedicines10020338[ doi.
- Terpos EA-O, Trougakos IP, Karalis V, Ntanasis-Stathopoulos I, Sklirou AD, Bagratuni T, Papanagnou ED, Patseas D, Gumeni S, Malandrakis P, et al. Comparison of neutralizing antibody responses against SARS-CoV-2 in healthy volunteers who received the BNT162b2 mRNA or the AZD1222 vaccine: should the second AZD1222 vaccine dose be given earlier? Am J Hematol. 2021;96(9):E321–E324. doi:10.1002/ajh.26248.
- Tran S, Truong TH, Narendran A. Evaluation of COVID-19 vaccine response in patients with cancer: an interim analysis. Eur J Cancer. 2021;159:259–74. doi:10.1016/j.ejca.2021.10.013.
- Peeters M, Verbruggen L, Teuwen L, Vanhoutte G, Vande Kerckhove S, Peeters B, Raats S, Van der Massen I, De Keersmaecker S, Debie Y. et al. Reduced humoral immune response after BNT162b2 coronavirus disease 2019 messenger RNA vaccination in cancer patients under antineoplastic treatment. ESMO Open. 2021;6(5):100274. doi:10.1016/j.esmoop.2021.100274.
- Wankhede D, Grover S, Hofman P. Determinants of humoral immune response to SARS-CoV-2 vaccines in solid cancer patients: a systematic review and meta-analysis. Vaccine. 2023;41(11):1791–8. doi:10.1016/j.vaccine.2023.01.072.
- Goossen GM, Kremer LC, van de Wetering MD. Influenza vaccination in children being treated with chemotherapy for cancer. Cochrane Database Syst Rev. 2013;2013(8):Cd006484. doi:10.1002/14651858.CD006484.pub3.
- Vollaard A, Schreuder I, Slok-Raijmakers L, Opstelten W, Rimmelzwaan G, Gelderblom H. Influenza vaccination in adult patients with solid tumours treated with chemotherapy. Eur J Cancer. 2017;76:134–43. doi:10.1016/j.ejca.2017.02.012.
- Kotton CN, Poznansky MC. Vaccination of oncology patients: an effective tool and an opportunity not to be missed. Oncologist. 2012;17(1):1–2. doi:10.1634/theoncologist.2011-0383.
- Xu Y, Methuku N, Coimbatore P, Fitzgerald T, Huang Y, Xiao Y-Y, Pagala M, Gupta S, Solomon W, Rubin P. et al. Immunogenicity of an inactivated monovalent 2009 influenza a (H1N1) vaccine in patients who have cancer. Oncologist. 2012;17(1):125–34. doi:10.1634/theoncologist.2011-0220.