ABSTRACT
The 4-component meningococcal serogroup B (MenB) vaccine, 4CMenB, the first broadly protective, protein-based MenB vaccine to be licensed, is now registered in more than 50 countries worldwide. Real-world evidence (RWE) from the last decade confirms its effectiveness and impact, with infant immunization programs showing vaccine effectiveness of 71–95% against invasive MenB disease and cross-protection against non-B serogroups, including a 69% decrease in serogroup W cases in 4CMenB-eligible cohorts in England. RWE from different countries also demonstrates the potential for additional moderate protection against gonorrhea in adolescents. The real-world safety profile of 4CMenB is consistent with prelicensure reports. Use of the endogenous complement human serum bactericidal antibody (enc-hSBA) assay against 110 MenB strains may enable assessment of the immunological effectiveness of multicomponent MenB vaccines in clinical trial settings. Equitable access to 4CMenB vaccination is required to better protect all age groups, including older adults, and vulnerable groups through comprehensive immunization policies.
Plain Language Summary
Invasive meningococcal disease, caused by the bacterium Neisseria meningitidis(meningococcus), is rare but often devastating and can be deadly. Effective vaccines are available, including vaccines against meningococcal serogroup B disease. In 2013, the 4-component meningococcal serogroup B vaccine, 4CMenB, became the first broadly protective, protein-based vaccine against serogroup B to be licensed, with the second (bivalent vaccine, MenB-FHbp) licensed the following year. 4CMenB is now registered in more than 50 countries, in the majority, for infants and all age groups. In the US, it is approved for individuals aged 10–25 years. Evidence from immunization programs in the last decade, comparing vaccinated and unvaccinated individuals and the same population before and after vaccination, confirms the effectiveness and positive impact of 4CMenB against serogroup B disease. This also demonstrates that 4CMenB can provide protection against invasive diseases caused by other meningococcal serogroups. Furthermore, N. meningitidis is closely related to the bacterium that causes gonorrhea, N. gonorrhoeae, and emerging real-world evidence suggests that 4CMenB provides additional moderate protection against gonococcal disease. The safety of 4CMenB when given to large numbers of infants, children, adolescents, and adults is consistent with the 4CMenB safety profile reported before licensure.
For the future, it would be beneficial to address differences among national guidelines for the recommended administration of 4CMenB, particularly where there is supportive epidemiological evidence but no equitable access to vaccination. New assays for assessing the potential effectiveness of meningococcal serogroup B vaccines in clinical trials are also required because serogroup B strains circulating in the population are extremely diverse across different countries.
Introduction
Invasive meningococcal disease (IMD) is unpredictable and life-threatening, and is associated with long-term physical, neurological, psychological, and behavioral complications.Citation1,Citation2 Most cases are caused by six meningococcal serogroups (A, B, C, W, Y, and X) and effective vaccines are available to prevent IMD caused by the five most common serogroups: vaccines that target the polysaccharide capsule of meningococcal serogroups A, C, W, and Y (MenACWY), and protein-based vaccines against meningococcal serogroup B (MenB).Citation3 Additionally, a pentavalent MenABCWY vaccine was licensed recentlyCitation4 and another is in phase 3 clinical development,Citation5 with a Biologics License Application accepted for filing. The development of a MenACWYX vaccine is also at an advanced stage.Citation6
Protein-based MenB vaccines were developed because the MenB polysaccharide is poorly immunogenic and has the potential to induce autoimmune antibodies.Citation7 The first protein-based MenB vaccines, VA-MENGOC-BC (Finlay Institute; Cuba), MenBvac (Norwegian Institute of Public Health; Norway), and MeNZB (Novartis; New Zealand), were based on outer membrane vesicles (OMV) and ‘tailor-made’ for specific strains, but these lacked broad protective activity against diverse, heterologous MenB strains.Citation8 The first broad-spectrum protein-based MenB vaccine, 4CMenB (Bexsero, GSK), was licensed in 2013 after over 20 years of research and development, for individuals aged 2 months or older.Citation9–11 This was followed the next year by the licensure of the bivalent vaccine, MenB-FHbp (Trumenba, Pfizer) for individuals aged 10–25 years.Citation12 4CMenB includes three recombinant protein antigens, Neisseria adhesin A (NadA), neisserial heparin-binding antigen (NHBA), and factor H binding protein (fHbp), plus detergent-extracted OMV from an epidemic strain collected in New Zealand, containing Porin A (PorA P1.4) as the main immunodominant antigen.Citation13,Citation14
In this narrative review, we chart the 10-year journey since 4CMenB licensure in Europe, including recommendations for its administration in different countries worldwide and accumulating real-world evidence (RWE) on its impact and effectiveness against IMD and its safety profile. Emerging evidence on the potential for protection against non-MenB disease and Neisseria gonorrhoeae is also discussed, as well as the need for improved access to MenB vaccination. Finally, we examine methods that have been used to evaluate multicomponent MenB vaccines before the availability of RWE, and describe a new assay (the endogenous complement human serum bactericidal antibody assay, enc-hSBA assay) for the assessment of the immunological effectiveness of new and existing multicomponent MenB vaccines in clinical trial settings.
A set of audio slides and a graphical abstract linked to this review article can be found at https://www.tandfonline.com/doi/full/10.1080/21645515.2024.2357924.
Evolution of 4CMenB national approvals and recommendations
As IMD is unpredictable and uncommon,Citation3,Citation15-17 the efficacy of meningococcal vaccines cannot be determined with sufficient statistical power via conventional placebo-controlled clinical endpoint trials. The initial licensure of MenB vaccines therefore relied on demonstrations of safety and immunogenicity, derived by human serum bactericidal antibody (hSBA) assay on a small number of vaccine antigen-specific indicator MenB strains.Citation18 These data, combined with Meningococcal Antigen Typing System (MATS) estimations of vaccine coverage,Citation19 provided the basis for the licensure of 4CMenB in the European Union (EU) in 2013 as a three-dose series plus booster dose (3 + 1) schedule for individuals from the age of 2 months (). In 2020, the EU recommendations were updated to include a two-dose series plus booster dose (2 + 1) schedule from the age of 2 months,Citation11 following demonstrations of similar immunogenicity, antibody persistence, and safety profiles with either a 2 + 1 or 3 + 1 vaccination scheduleCitation20,Citation21 and no evidence for a difference in MenB strain coverage.Citation22
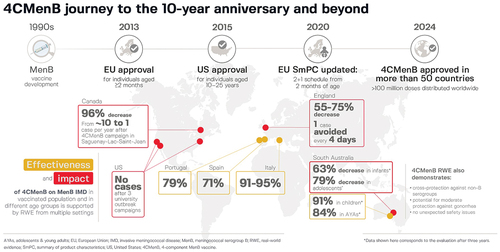
Figure 1. Road map leading to the regulatory approval and distribution of 4CMenB in more than 50 countries worldwideCitation9–11 and to the inclusion of 4CMenB in national and regional immunization programs since initial registration.
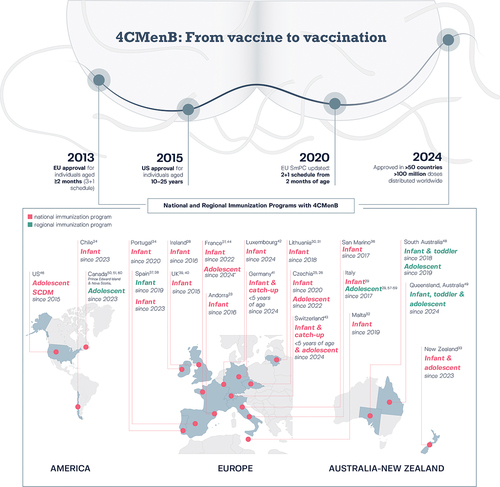
National and regional MenB vaccination policies are generally driven by the local epidemiology of MenB disease and local recommending bodies. 4CMenB is registered in more than 50 countries worldwide and, to date, 4CMenB is included in the national infant immunization program of 16 (): Andorra,Citation23 Chile,Citation24 Czechia,Citation25,Citation26 France,Citation27 Ireland,Citation28 Italy,Citation29 Lithuania,Citation30,Citation31 Malta,Citation32 New Zealand,Citation33 Portugal,Citation34,Citation35 San Marino,Citation36 Spain,Citation37,Citation38 the United Kingdom (UK),Citation39,Citation40 and, most recently, Germany,Citation41 Luxembourg,Citation42 and Switzerland.Citation43 4CMenB is also included in a national immunization program for adolescents (aged 14–15 years) in CzechiaCitation25,Citation26 and Switzerland (aged 11–15 years, with catch-up until age 20 years),Citation43 and adolescents/young adults (aged 13–25 years) in close-living situations in New Zealand.Citation33 4CMenB has been recommended recently for vaccination of adolescents/young adults (aged 15–24 years) in France, who wish to be vaccinated.Citation44 In the United States (US), the Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices recommends 4CMenB, given as two doses at least 1 month apart, for adolescents and young adults aged 16–23 years on the basis of shared clinical decision-making.Citation45,Citation46 In 2018–2019, a regional immunization program for children younger than 4 years and adolescents (15–20 years) was introduced in South Australia,Citation47 and this program is ongoing for infants and adolescents aged 15–16 years.Citation48 In 2024, this was joined by a funded MenB immunization program in Queensland, Australia, for infants, children aged 13–23 months (catch-up vaccination), and adolescents aged 15–19 years.Citation49 In Italy, in addition to the national infant program, a regional adolescent immunization program is recommended and funded for adolescents aged 11–18 years based on the decision of individual regions.Citation29 In Canada, a regional adolescent immunization program was introduced in 2023 in the provinces of Prince Edward Island and Nova Scotia.Citation50,Citation51
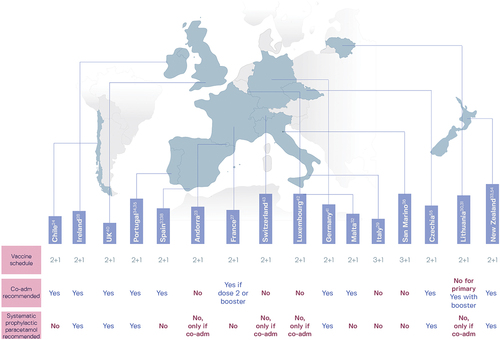
National guidelines vary for the coadministration of 4CMenB with routine childhood vaccines and for antipyretic prophylaxisCitation52,Citation53 (). Of the countries that include 4CMenB in their national infant immunization program, 10 recommend coadministration (for at least one primary dose) with routine childhood vaccines and six recommend antipyretic prophylaxis with paracetamol ().Citation23,Citation24,Citation27-38,Citation40-43,Citation54,Citation55 Few countries recommend immunization against five meningococcal serogroups in the same age group. Most of the 16 countries with national infant 4CMenB immunization programs recommend MenACWY vaccine administration to adolescents or young adults, but only four (Czechia, France, Chile, and Malta) recommend MenACWY vaccination of young children.Citation24,Citation25,Citation32,Citation44,Citation56 Both MenB and MenACWY vaccination of adolescents is recommended in Czechia,Citation25,Citation26 Switzerland,Citation43 France,Citation44 the US,Citation45 South Australia and Queensland, Australia,Citation48,Citation49 three regions of Italy (Puglia, Calabria, and Sicily; in Sicily, MenB vaccination up to age 12 years),Citation57–59 and Prince Edward Island and Nova Scotia in Canada.Citation51,Citation60 Concomitant administration of 4CMenB with MenACWY enables broad IMD protection against five meningococcal serogroups; no immunological interference is detected when both vaccines are administered to infants, adolescents, or adults at the same vaccination visit, and there is no notable increase in reactogenicity compared to separate administration of the vaccines.Citation61–63
Figure 2. Recommendations in 16 national immunization programs for the coadministration (co-adm) of routine childhood vaccines with 4CMenB and the use of prophylactic paracetamol (known as acetaminophen in the United States).Citation52,Citation53
Real world effectiveness and impact against MenB disease
Post-licensure data that have accumulated over the last 10 years provide evidence of the real-world effectiveness and impact of 4CMenB against MenB disease. Recent reviews of these data are available;Citation53,Citation64-66 a summary is provided here, along with the latest RWE.
Real-world effectiveness data are available from four European countries (the UK, Italy, Spain, and Portugal), the US, Canada, and South Australia (). In September 2015, the UK became the first country to include 4CMenB in a national infant immunization programCitation39 and outcomes data are available from up to 5 years of implementation of two-dose priming in infancy and a booster dose at 12 months of age.Citation65,Citation67–69 Evaluation of the effect of vaccination on MenB disease incidence during the first 3 years of the program (September 2015 to August 2018) in England showed a 75% decrease in incidence among the age groups that were fully eligible for vaccination, with 63 observed cases versus 253 expected cases (incidence rate ratio [IRR] 0.25; 95% confidence interval [CI]: 0.19–0.36), and vaccine protection lasting for at least 2 years after the 2 + 1 regimen.Citation67 Over 3 years, there were 169 MenB disease cases in the vaccine eligible cohorts, and it was estimated that a further 277 cases (95% CI: 236–323) were prevented, representing one case avoided every 4 days. Further reductions in MenB disease in children younger than 5 years in England were also reported in the fourth year of the immunization program.Citation65 Another analysis of MenB disease cases in England between September 2015 and March 2021 also found a sharp decline among vaccine-eligible children younger than 5 years, from the pre-vaccine average of 328 cases per year (2010–2015) to 148 cases per year after 4CMenB implementation, a significant decline of 55% (p < .0001).Citation69
Figure 3. Real-world evidence of the effectiveness, impact, and safety of 4CMenB.

Evidence of persistent protection following 4CMenB vaccination is also available from Canada, where a mass vaccination campaign was launched in 2014 to control a long-lasting MenB disease outbreak in the Saguenay-Lac-Saint-Jean (SLSJ) region of Quebec.Citation70 By the end of the campaign in 2014, 83% of residents aged between 2 months and 20 years (approximately 59,500 individuals) had been vaccinated. In the four-year post-campaign period, there was a 96% decrease in incidence of MenB disease in the target age group, from 11.4/100,000 person-years in 2006–2014 to 0.4/100,000 person-years in 2014–2018 (p < .0001). No MenB disease cases were reported in the target age group between May 2014 and the end of 2016.Citation71 The MenB disease rate in 2014–2018 was 0.51/100,000 person-years in vaccinated persons (one case) and 2.45/100,000 in unvaccinated persons (one case), resulting in vaccine effectiveness of 79%, with a large 95% CI (−231% to 99%).Citation70 Outbreak control data are also reported from the US, where 4CMenB was used to control MenB disease outbreaks among college students, two in 2013–2014Citation72 and one in 2016.Citation73 Following two vaccine doses administered at least 1 month apart, no additional cases of MenB-related disease were reported among individuals vaccinated during the three immunization campaigns.
In Italy, when 4CMenB was introduced in the national infant immunization program in 2017 as a 3 + 1 schedule starting at 2 months of age,Citation29 two regions had already introduced publicly-funded infant immunization programs: Tuscany, where vaccination started in 2014 using a 3 + 1 schedule from 2 months of age, and Veneto, where vaccination began in 2015 with a 2 + 1 schedule from 7 months of age.Citation74 Vaccine effectiveness against MenB disease was 93.6% (95% CI: 55.4–99.1) in Tuscany in 2014–2018 and 91.0% (59.9–97.9) in Veneto in 2015–2018. The overall impact of 4CMenB vaccination, estimated from pre- and post-vaccine incidence rates of MenB disease in all children aged 0–5 years, was 68% in Tuscany and 31% in Veneto, suggesting a greater impact of vaccination when the immunization program was started in younger infants.Citation74 More recently, 4CMenB vaccine effectiveness against MenB disease in fully immunized children in three Italian regions (Tuscany, Veneto, and Piedmont) was found to be 94.9% (95% CI: 83.1–98.4) by a screening method and 91.7% (24.4–98.6) in six regions (Tuscany, Veneto, Piedmont, Apulia, Liguria, and Sicily) by a case-control method.Citation75 Almost 20% of the cases in unvaccinated children were among infants too young to be vaccinated, suggesting earlier vaccination may prevent more MenB disease cases.
The results of case-control studies in Portugal and Spain, made possible through the widespread use of the vaccine in the private market following scientific society recommendations, provide further evidence of the real-world effectiveness of 4CMenB.Citation76,Citation77 In Portugal, analysis of MenB cases presenting in 2014–2019 at 31 pediatric services showed that vaccination with 4CMenB was significantly less likely among children and adolescents who developed MenB disease than in matched controls, with an odds ratio of 0.21 (95% CI: 0.08–0.55), corresponding to vaccine effectiveness of 79%.Citation76 In Spain, a nationwide matched case-control study of the effectiveness of 4CMenB in children younger than 5 years found the effectiveness of complete vaccination (at least two 4CMenB doses) was 71% (95% CI: 45–85) against MenB disease.Citation77
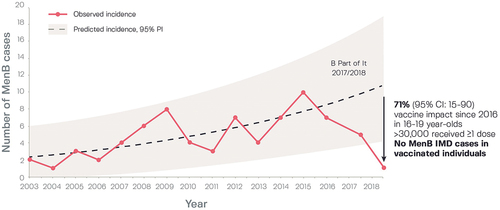
Various analyses have been conducted of the success of the infant, child, and adolescent 4CMenB vaccination program in South Australia, where the number of MenB disease cases had increased by an average of 10% per year from 2003 to 2016, peaking at 10 cases in 2015.Citation78 An observational time series analysis assessed vaccine impact among adolescents aged 16–19 years in 237 senior schools randomized to the 4CMenB immunization program in 2017 (intervention) or 2018 (control).Citation78 The number of MenB disease cases fell to five in 2017–2018 and one in 2018–2019 (), showing persistent protection, and comparing the 14-year pre-vaccine period with the 2-year post-vaccine period (2017–2019) showed an overall 71% (95% CI: 15–90, p = .02) reduction in number of MenB disease cases in adolescents. Another analysis reported no cases of MenB disease in the trial population in 2017–2018 and afterward in 2019, as compared with 12 cases among students aged 15–18 years in the pre-vaccine period, 2015–2016.Citation79 Evaluation 2 years after implementation of the program showed 100% vaccine effectiveness against MenB disease in adolescents and young adults, since no cases were reported after implementation, and vaccine effectiveness in children of 94.2% (95% CI: 36.6–99.5) using a screening method and 94.7% (40.3–99.5) by case-control method.Citation80 Moreover, 3 years after implementation, reductions in MenB disease incidence were 63.1% (95% CI: 29.0–80.9) in infants and 78.5% (33.0–93.1) in adolescents, and vaccine effectiveness against MenB disease was 90.7% (6.9–99.1) for the childhood program and 83.5% (0–98.2) for the adolescent program.Citation81
Figure 4. Observed and projected serogroup B invasive disease cases in South Australia before (2003–2016) and after 4CMenB vaccination of students aged 16–19 years as part of the ‘B part of It’ program.Citation78
Real world safety of 4CMenB in different settings
In the clinical development studies, involving more than 6000 participants, 4CMenB had an acceptable safety and tolerability profile in infants, toddlers, adolescents, and adults.Citation82 The most common local and systemic adverse events (AEs) in infants and children were injection site reactions, fever, and irritability, while in adolescents and adults, malaise, and headache were most frequently reported.
The safety observations in infants were generally similar to those reported following other routine infant vaccines, such as combined diphtheria-tetanus-acellular pertussis (DTaP), inactivated poliomyelitis (IPV), Haemophilus influenzae type b (Hib), hepatitis B (HBV), and pneumococcal conjugate (PCV) vaccines.Citation83 Coadministration of 4CMenB with routine childhood vaccines was however more reactogenic than routine vaccines administered alone.Citation82,Citation83 In one study of 1885 infants, fever was observed after 26–41% of 4CMenB doses when administered alone, 23–36% after routine vaccines given alone, and 51–61% after 4CMenB and routine vaccines administered together.Citation84 The incidence of fever following 4CMenB was low in adolescents and adults.Citation82,Citation83 Antipyretic prophylaxis with paracetamol can improve the tolerability of 4CMenB. A randomized controlled trial of infants given 4CMenB with routine vaccines (DTaP-HBV-IPV/Hib and PCV) reported lower rates of fever (defined as ≥ 39.0°C) and solicited local AEs in infants given three doses of prophylactic paracetamol (one dose at the time of vaccination and two doses at intervals of 4–6 h) compared with those receiving the same vaccinations without paracetamol, without affecting the immunogenicity of any of the 4CMenB or routine vaccine antigens.Citation85 Antipyretic prophylaxis and advising parents on the appropriate management of fever after vaccination also substantially reduced reports of fever requiring medical attention.Citation85–87
A decade of active and passive safety reporting in different age groups in conjunction with 4CMenB immunization campaigns or outbreak control provides additional valuable safety experience (). This includes further evidence on the effectiveness of antipyretic prophylaxis from the 4CMenB vaccination campaign in Quebec (SLSJ region),Citation88 with the finding that at least two doses of paracetamol reduced the odds of fever after each 4CMenB dose by more than half (odds ratio 0.28 to 0.40) among children younger than 2 years old. In the UK, a retrospective case note review of hospitalized preterm infants showed implementation of prophylactic paracetamol reduced the risk of fever and AEs to levels seen before the introduction of 4CMenB into the national immunization program.Citation89
Other surveillance studies describe the characteristics of AEs reported following mass immunization. Suspected AEs from the 21-month period after 4CMenB implementation in the UK infant immunization program were examined in a prospective surveillance study.Citation90 During this time, approximately 1.29 million children aged 2–18 months were vaccinated and around 3 million doses of 4CMenB were administered. Of 902 reports of suspected AEs received via the UK Yellow Card Scheme, 41% were related to local reactions and 40% to fever, with no significant safety concerns and no reduction in compliance with doses of other routine vaccinations. Further examination of UK safety data after 4CMenB vaccination of 107,231 children aged 1–18 months in 2015–2018 detected few cases of specific safety outcomes (seizures, febrile seizures, and Kawasaki disease), although there was an increased risk of seizures (adjusted IRR 1.43; 95% CI: 1.02–2.02) and febrile seizures (1.72; 1.08–2.75).Citation91 Since 93% of 4CMenB doses were administered on the same day as other routine vaccines in the national immunization schedule, it was not possible to attribute this finding to a specific vaccine.
Post-marketing surveillance data for Italy in 2017 (2161 suspected AEs following immunization [AEFI]) and Germany in 2013 to 2016 (1960 AEFI) indicated no safety concerns with 4CMenB and a safety profile consistent with that described in the vaccine’s summary of product characteristics.Citation92,Citation93 In South Australia, the safety profile of 4CMenB was examined as part of the immunization program in adolescents in relation to AEs reported via the South Australian Vaccine Safety Surveillance System, a spontaneous reporting system for AEFI.Citation94,Citation95 Following 58,637 doses of 4CMenB administered to 30,522 students (median age, 16 years), 193 AEFI were reported in 187 students, giving an AEFI reporting rate of 0.33% (95% CI: 0.28–0.38), or 329 AEFI per 100,000 doses. Almost all students (166 of 169) who were contactable for AEFI follow-up reported resolution of the AE. The most common AEFI were injection site reactions, headache, and nausea.Citation94 The rate of AEFI reporting was therefore low in adolescents, but was higher than the rate reported nationally in individuals aged 7–17 years via passive reporting following 4CMenB use in the private market; in 2020, following 40,888 4CMenB doses, there were 39 AEFI, translating into an AEFI reporting rate of 95.4 per 100,000 doses in Australia.Citation96 Overall, 4CMenB was well tolerated with no unexpected safety issues.
4CMenB safety surveillance data gathered in conjunction with MenB disease outbreak control in France, the US, and CanadaCitation88,Citation97,Citation98 are in line with AEFI reporting in funded or private immunization programs. No new safety concerns were reported and AEFI were consistent with those reported in the 4CMenB clinical trials before licensure. In Canada, as part of the immunization campaign of individuals aged 2 months to 20 years in Quebec (SLSJ region), an active surveillance questionnaire was issued to vaccinated individuals or their parents 7 days after each vaccine dose and 6 months after the last dose.Citation99 Of 154 reported serious AEs that were eligible for further analysis, the most frequent were respiratory problems (34%) and other infections (25%) that were considered as expected for the age group. Three cases of nephrotic syndrome were diagnosed in the 6-month period after the second 4CMenB dose and an additional case was identified by hospitalization database review in children aged 2–5 years. This potential signal was evaluated in the larger cohort of vaccinated children in England, which found no evidence of an increased risk of nephrotic syndrome,Citation100 suggesting the cases observed in Quebec may have occurred by chance.Citation64 Another study of a national healthcare database in England also found no increased risk of Kawasaki disease associated with 4CMenB administration in infants or toddlers.Citation101
Safety data are available from the US from 4CMenB vaccination campaigns that began before 4CMenB licensure, under a CDC sponsored Investigational New Drug protocol, to control two MenB outbreaks on college campuses.Citation97 Following vaccination of 15,236 individuals and administration of 28,229 4CMenB doses, the rate of reported serious AEs was 3.3 per 1000 vaccinated individuals, and a causal relationship with 4CMenB was reported for two serious AEs (rhabdomyolysis and anaphylaxis; both individuals recovered fully). Overall, the study found an acceptable safety profile for the continued use of 4CMenB.
Protection beyond MenB disease: non-B serogroups and N. gonorrhoeae
Antigenic components of 4CMenB can also be expressed in non-B meningococcal strains and in other pathogenic Neisseria species, including N. gonorrhoeae strains. Furthermore, 4CMenB has been shown to elicit immune responses against both non-B strains and gonococcal strains, demonstrating the potential for protection beyond MenB disease.Citation102
The hSBA assay of serogroups C, W, and Y invasive disease isolates from England and Wales, France, Germany, and Brazil showed that, of 147 non-B isolates, 109 were killed (hSBA titer ≥ 4) by sera from infants immunized with 4CMenB, resulting in overall coverage of 74%, with 64% of MenC, 80% of MenW, and 94% of MenY isolates killed, versus only 14% of isolates killed by pooled pre-immune sera.Citation103 Testing this non-B isolate panel with adolescent post-vaccination sera also showed 4CMenB cross-reactivity, with 62% of 147 isolates killed; 55% of MenC, 74% of MenW, and 66% of MenY isolates versus 7.5% of isolates killed by pre-immune sera.Citation104 Moreover, a study of MenX isolates found evidence of 4CMenB coverage for nine isolates from several countries in Africa but not for two unrelated MenX isolates from France.Citation105
The first RWE of 4CMenB protection against MenW disease came from England following implementation of the 4CMenB infant immunization program and an emergency MenACWY program for adolescents, which started in 2015 to control an outbreak of MenW disease.Citation106 MenW cases were compared in the 4 years before and 4 years after implementation of both vaccines, using Poisson modeling to estimate direct protection offered by the infant 4CMenB program and the indirect impact of the adolescent MenACWY program in children eligible for 4CMenB but not MenACWY. This showed 69% (adjusted IRR 0.31; 95% CI: 0.20–0.67) fewer MenW cases than predicted in children eligible for 4CMenB, regardless of vaccination status, with 4CMenB directly preventing 98 cases (95% CI: 34–201). Further RWE of an effect against non-B serogroups comes from an analysis in Spain, in which 243 (79.4%) of 306 IMD cases in children younger than 5 years were MenB, 20 (6.6%) were MenW, five (1.6%) were MenC, and seven (2.3%) were MenY, with the remainder caused by non-groupable meningococcal strains or the serogroup could not be identified.Citation77 Vaccine effectiveness of two 4CMenB doses was 76% (95% CI: 57–87) against IMD caused by any serogroup and 92% (28–99) against non-MenB disease, demonstrating effectiveness against serogroup B and non-serogroup B meningococci.
The potential for 4CMenB effectiveness against gonorrhea was indicated by the presence in N. gonorrhoeae isolates of a highly conserved gene for the surface-exposed antigen NHBA, the finding that three NHBA variants, which account for 82% of gonococcal strains, each share 67% identity to NHBA peptide 2 included in 4CMenB, and the recognition of gonococcal NHBA by antibodies induced by 4CMenB.Citation107,Citation108 Accessory proteins may also contribute to this potential, given the high level of sequence identity between 4CMenB OMV proteins and N. gonorrhoeae homologs, and recognition of gonococcal OMV proteins by 4CMenB-induced antibodies.Citation108 Furthermore, results from retrospective analyses conducted in New Zealand on individuals immunized with the MenB OMV vaccine, MeNZB, showed 31% vaccine effectiveness against gonorrheaCitation109 and 24% vaccine effectiveness against hospitalization caused by gonorrhea.Citation110 These observations have particular importance given the long-term consequences of gonococcal infection, especially when left undiagnosed due to asymptomatic infection, which occurs more frequently in women than in men.Citation111 Moreover, there is no effective vaccine against gonorrhea and antibiotic-resistant gonococcal strains present a global threat.Citation112
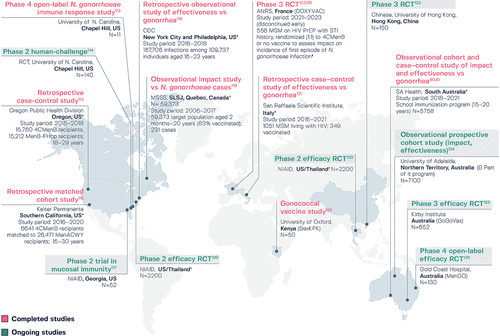
Various studies to investigate the potential for 4CMenB protection against N. gonorrhoeae infection are ongoing or have been completed,Citation80,Citation81,Citation113–127 as summarized in . Encouraging results have been reported in populations vaccinated with 4CMenB in Quebec (SLSJ region),Citation119 the US (New York City, Philadelphia, Southern California, and Oregon),Citation115,Citation116,Citation118 South Australia,Citation80,Citation81 and ItalyCitation121 (). An estimated risk reduction for N. gonorrhoeae infection of 59% (95% CI: −22 to 84; p = .1) was reported in conjunction with the 4CMenB immunization campaign in Quebec, with a reported decrease in cases among 14–20 year-olds and an increase in older, unvaccinated individuals.Citation119 A retrospective observational study of surveillance and immunization registry records for individuals aged 16–23 years in New York City and Philadelphia found that two-dose 4CMenB had 40% (95% CI: 23–53) effectiveness against gonorrhea compared to no vaccination and after adjusting for gender, race, and jurisdiction.Citation118 Furthermore, when the methods in this study were replicated in a second study of the effectiveness of MenB-FHbp, a non-OMV vaccine, against gonorrhea, vaccine effectiveness was 3%, suggesting the results of the original study were unlikely to be confounded by healthy vaccinee bias.Citation128 In Southern California, a study of 6641 4CMenB recipients matched to 26,471 MenACWY recipients found that gonorrhea rates were 46% lower among recipients of 4CMenB versus MenACWY, while chlamydia rates were similar.Citation116 Moreover, a recent case-control analysis examined gonorrhea prevalence following MenB vaccination campaigns with 4CMenB and MenB-FHbp prompted by university outbreaks in Oregon in 2015 and 2016.Citation115 Comparison of 15,760 recipients of 4CMenB with 15,212 recipients of non-OMV-based MenB-FHbp showed a lower gonorrhea prevalence in the 4CMenB group (24 versus 44 cases), with 47% (95% CI: 13–68) effectiveness with 4CMenB among those aged 18–29 years and 59% (95% CI: 20–79) effectiveness for those aged 18–19 years. Another case-control analysis, conducted in South Australia, reported 33.2% (95% CI: 15.9–47.0) effectiveness of two-dose 4CMenB against gonorrhea in adolescents and young adults, using age-matched individuals with chlamydia infection as controls, during 3 years of program implementation.Citation80,Citation81 In Italy, an unmatched case-control study of 1051 men who have sex with men (MSM) living with HIV, reported an adjusted effectiveness estimate with 4CMenB of 44% (95% CI: 9–65; p = .02) against gonorrhea, with a median follow-up of 3.8 years.Citation121 Additionally, results were presented recently from the first phase 3 randomized study to evaluate 4CMenB against gonorrhea in MSM.Citation127,Citation129 The DOXYVAC trial in France assessed 4CMenB and doxycycline post-exposure prophylaxis in MSM on HIV pre-exposure prophylaxis. The patients had a median age of 40 years, median 10 sexual partners in the previous 3 months, and 98% had a history of sexually-transmitted infection in the last 12 months.Citation127 The incidence of a first episode of gonorrhea was 58.3 and 77.1 per 100 person-years in the 4CMenB and no vaccine arms, respectively. Overall, the results were inconclusive, with vaccine efficacy against gonorrhea infection not meeting statistical significance (22%; 95% CI: −1 to 40; p = .06), although some benefits could not be ruled out.Citation129 The study had been discontinued due to the Data and Safety Monitoring Board’s recommendation to stop enrollment, based on interim analysis results that were later modified post audit. Two additional studies are completed, with final results pending: the phase 4 N. gonorrhoeae immune response study in North Carolina, US,Citation113 and the BexKPK gonococcal vaccine study in Kenya.Citation122
Figure 5. Ongoing and completed studies on the potential for 4CMenB to protect against Neisseria gonorrhoeae infection.
According to two modeling studies, modest vaccine effectiveness against gonorrhea could result in a reduction in gonorrhea prevalence that has a meaningful public health impact.Citation130,Citation131 A heterosexual N. gonorrhoeae transmission model showed that a vaccine with an efficacy of 30% or greater and 2-year duration of protection could reduce gonorrhea prevalence in the US by more than 10%,Citation131 fitting with the 10% goal set by the US Department of Health and Human Services for reducing gonorrhea rates in male adolescents and young men.Citation132 The potential public health impact of adolescent 4CMenB vaccination on N. gonorrhoeae infection in England found that a national adolescent immunization program for a vaccine with 31% efficacy, 6 years’ duration of protection, and 85% uptake could avert 50,000 gonococcal infections over 10 years, equivalent to 10% of heterosexual infections.Citation130 Vaccine impact was predicted to increase over time with catch-up and booster vaccination.
The need for improved access to MenB vaccination among all age and at-risk groups
Epidemiological data, mostly generated in Europe and North America, show the incidence of IMD is highest in children younger than 5 years, with a second peak in adolescents and young adults, and a third in older adults.Citation133,Citation134 For 4CMenB, the most common age-based recommendation is for infant immunization.Citation53 As highlighted earlier, adolescent 4CMenB immunization is recommended in few countries (). In Czechia, the adolescent recommendation is for those aged 14–15 years,Citation25,Citation26 in Switzerland, 11–15 year-olds (with catch-up until age 20 years),Citation43 in France, 15–24 year-olds who wish to be vaccinated,Citation44 while in New Zealand, it is for adolescents/young adults in close-living situations.Citation33,Citation53 South Australia and Queensland, Australia, have immunization programs for infants and adolescents,Citation47–49 and a regional adolescent program exists in Italy alongside the national infant immunization program.Citation29 Canada has a regional adolescent immunization program,Citation50,Citation51 and in the US, 4CMenB is recommended at age 16–23 years, on the basis of shared clinical decision-making.Citation45,Citation46
Immunization of adolescents and young adults is supported by RWE of the effectiveness of adolescent vaccination from the South Australia 4CMenB immunization program, which showed a sharp decline in MenB disease cases in the region () and no cases in 4CMenB-vaccinated adolescents,Citation78 and from outbreak control data from US colleges,Citation72,Citation73 where no additional cases of MenB disease were found among immunized individuals. Furthermore, immune responses persist after 4CMenB vaccination in both early childhood and adolescence. Studies show seroprotective hSBA titers are maintained against at least one vaccine antigen for 24 to 36 months by 76–100% of children after priming in infancy, and by 84–100% of children after priming in the second year of life.Citation135 Among adolescents or young adults, antibody persistence extended 7.5 years after primary vaccination in a variety of settings,Citation135 so 4CMenB vaccination at 16–18 years of age may protect adolescents and young adults during a peak period for IMD.Citation136 Booster vaccination induces robust anamnestic responses in all age groups.Citation135 This suggests prior vaccination may provide residual antibody protection in outbreak settings, with a single booster dose administered to adolescents or young adults likely to provide protective immunity faster than a two-dose series given to vaccine-naïve individuals. The future immunization strategy for this age group should therefore include both vaccine-naïve individuals and boosting those primed in infancy.
There is also a strong argument for considering the inclusion of older adults in MenB immunization programs. Data from Europe and North America show older adults generally have higher case fatality rates with IMD than younger adults, adolescents, and children, which is probably linked to underlying comorbidities.Citation137 IMD in this age group is associated with atypical symptoms that may be misdiagnosed, and with a high healthcare burden and economic cost.Citation56 In addition, the incidence of IMD in older adults appears to be increasing, which may be due to a combination of waning immunity, immune senescence, and the success of meningococcal immunization programs in younger age groups.Citation56,Citation137
Other populations at increased risk of IMD include individuals with immune system disorders, such as functional or anatomic asplenia and complement deficiency, and those living with HIV.Citation138–140 MSM in outbreak settings and MSM with HIV are also reported to be at increased risk of IMD.Citation138–142 The risk of IMD in populations with immune system disorders is considerably higher than for healthy persons; for example, up to 10,000-fold higher for individuals with complement deficiencies and up to 23-fold higher for those living with HIV.Citation45,Citation143 One study conducted in England found the relative risk of IMD in people living with HIV was 4.5 that in people without HIV, and the risk of MenB disease in individuals living with HIV was higher in the 16–24 years age group than in older age groups.Citation143 Despite this, meningococcal vaccination coverage in these at-risk populations is low. Data available from the US show uptake rates of MenACWY and MenB vaccination of only 4.6% and 2.2%, respectively, among patients with complement deficiencies,Citation144 and 28.1% and 9.7%, respectively, among those with asplenia.Citation145 Another US study found that, among people living with HIV, 16.3% received a MenACWY vaccine dose in the two years after diagnosis in 2019–2022.Citation146 In Australia, MenB vaccination coverage for people medically at risk of IMD was 12.1% in 2019,Citation147 and in Norway, two doses of MenACWY vaccine and MenB vaccine were received by, respectively, 4.2% and 8.0% of splenectomized patients up to 2020.Citation148
Individuals at high risk of IMD because of underlying medical conditions are usually included in national or regional recommendations for 4CMenB vaccination. The immunogenicity and safety of 4CMenB has been demonstrated in several studies of special populations including children and adolescents with complement deficiencies, asplenia, or splenic dysfunction,Citation149,Citation150 adults vaccinated from 6 months after allogeneic hematopoietic cell transplantation,Citation151 and individuals living with HIV.Citation152,Citation153 The safety profile of 4CMenB in individuals aged 2–17 years with underlying conditions was comparable to that in healthy controls.Citation149 In this group, immune responses tended to be lower in complement-deficient individuals with terminal chain complement deficiency or those on eculizumab treatment;Citation149 several case reports of vaccine failures also suggest a reduced ability of meningococcal immunization to protect against IMD in eculizumab-treated immunocompromised patients.Citation45,Citation149,Citation154 In the study of people living with HIV, participants received two doses a month apart of 4CMenB co-administered with the licensed MenACWY-CRM vaccine (MenACWY conjugated to nontoxic mutant of diphtheria toxin; Menveo, GSK).Citation152 One month after two vaccine doses, at least 98% of the participants achieved protective antibody titers for each MenB indicator strain and at least 94% achieved protective titers against serogroups A, C, W, and Y, and the safety profile of vaccine co-administration was acceptable.
In countries or regions where epidemiological evidence indicates a need for improved control of MenB disease, better MenB vaccination coverage is warranted in all age groups, including adolescents/young adults and older adults, those with underlying medical conditions, and people living with HIV. This would be consistent with the road map introduced by the World Health Organization (WHO) for defeating meningitis by 2030, specifically reduction of vaccine-preventable bacterial meningitis cases by 50% and deaths by 70%, with equal access to locally relevant meningococcal disease vaccination programs for all those at risk, driven by the national or regional epidemiology of IMD and with adequate funding in place.Citation155 Non-funded access to MenB vaccination promotes a socioeconomic disparity between those who have the ability to pay for the vaccine and those who do not but are at risk of IMD because of age, low socioeconomic status, or underlying medical condition.Citation156,Citation157 In 2022, France became the first country to introduce 4CMenB in the national immunization program for equity reasons.Citation27,Citation156,Citation158 Similarly, the stated aim of the Queensland MenB Vaccination Program, initiated in 2024, is to “remove financial barriers to vaccination, improve vaccine uptake, and improve the protection that MenB vaccination provides against meningococcal disease.”Citation49
Evaluating multicomponent MenB vaccines before the RWE era
The true population effectiveness of MenB vaccines can only be confirmed through RWE of clinical outcomes, as demonstrated for 4CMenB. Before vaccine licensure, and before RWE is generated, reliable and accurate in vitro methods are required for assessing the performance of multicomponent MenB vaccines in clinical trial settings.
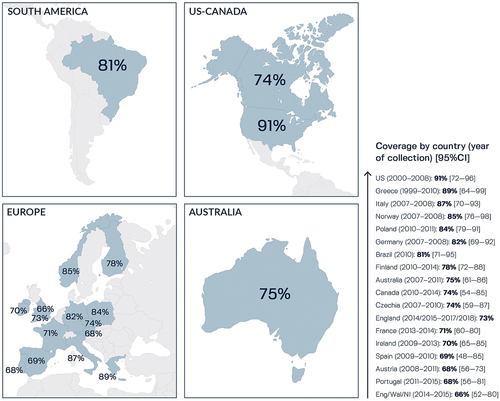
The traditional hSBA assay measures antigen-specific responses to vaccination but this method is not useful for predicting MenB vaccine strain coverage at a population level due to broad diversity in different MenB vaccine antigens in circulating strains,Citation159–161 requiring many MenB strains to be tested. In this scenario, the amount of serum needed from vaccinated individuals is likely to be challenging, especially for young children. Methods were therefore developed for predicting 4CMenB strain coverage on genetically diverse MenB strains, including MATS,Citation162,Citation163 which correlates with killing in the hSBA assay, and the genotyping tool, genetic MATS (gMATS), which is based on the correlation between antigen genotypes and MATS outcome.Citation164 MATS point estimates generated using panels of epidemiologically-representative MenB strains predicted 4CMenB strain coverage of between 66% and 91% in various countries ().Citation68,Citation164-176 gMATS, which can be performed using genome sequencing data from cultivable and non-cultivable clinical isolates, predicted 58–89% 4CMenB coverage of MenB strain panels from 11 European countries, Canada, US, and Australia.Citation164,Citation169,Citation176-179
Figure 6. Potential coverage of 4CMenB against MenB strains circulating worldwide, as predicted by Meningococcal Antigen Typing System (MATS).
MATS and gMATS provide conservative predictions of 4CMenB strain coverage, as indicated by MenB strains that were not predicted to be covered by MATS or gMATS but were found to be killed by hSBA assay using sera from vaccinated individuals.Citation159,Citation180–182 This is likely to be because, like the traditional hSBA assay, MATS, and gMATS do not account for a potential synergistic immunogenic effect of antibodies induced by two or more vaccine components, i.e. simultaneous binding of antibodies to multiple antigens leading to bactericidal killing, or the contribution to protection of OMV components other than PorA.Citation183 Also, gMATS cannot provide data for MenB isolates harboring new antigen-encoding alleles.
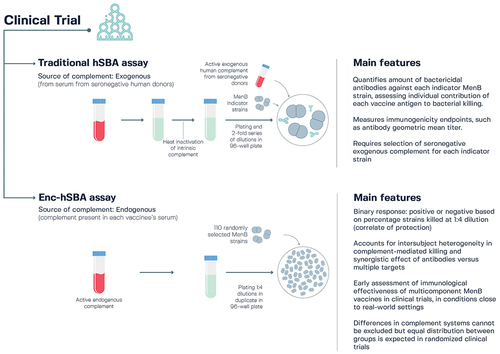
More recently, the enc-hSBA assay has been developed to complement immunogenicity results obtained with the traditional hSBA assay, enabling a more complete assessment of the performance of 4CMenB in clinical trials. The enc-hSBA assay uses endogenous complement present in each vaccinated person’s serum to provide a measure of the killing activity of vaccine-specific antibodies present in sera at a single dilution against each strain tested,Citation184,Citation185 while the traditional hSBA assay uses an exogenous complement source from seronegative donors and measures vaccine-induced responses against four antigen-specific indicator strains (). The enc-hSBA assay accounts for intersubject heterogeneity in complement-mediated bactericidal killing of MenB strains and the synergistic effects of antibodies raised by multiple MenB vaccine antigens against circulating strains with diverse genetic features.Citation184
Figure 7. Characteristics of the traditional human serum bactericidal antibody (hSBA) assay and the endogenous complement hSBA (enc-hSBA) in relation to the assessment of meningococcal serogroup B (MenB) vaccines.Citation159,Citation184–186.
The enc-hSBA assay was qualified using a panel of 110 invasive MenB strains and validated using four 4CMenB vaccine antigen-specific indicator strains.Citation19,Citation184 The 110 strains are representative of global strain diversity, with an antigen genotype repertoire representing approximately 87–97% of strains in Europe, the US, Canada, and Australia, and 89% of MenB strains overall.Citation186 The enc-hSBA assay thereby enables the assessment of immunological vaccine effectiveness, defined as the ability of a multicomponent vaccine to induce a bactericidal immune response against a broad panel of MenB strains carrying more than one antigen, in the context of a clinical trial, in conditions that mirror real-world settings. Since the enc-hSBA assay uses the individual’s own complement, bactericidal activity might be impaired for people with certain underlying conditions or those undergoing treatment that impacts the complement system.Citation184 However, as the enc-hSBA assay is used in a clinical trial context, such individuals are likely to be balanced between randomized groups. Any limitations are also counteracted by the ability to test a large panel of MenB strains, and its highly standardized and robust methodology.Citation184
The enc-hSBA assay has demonstrated utility in clinical trial settings.Citation185,Citation187 In a phase 3 trial of 4CMenB and the investigational MenABCWY vaccine (NCT04502693), the immunological vaccine effectiveness of both vaccines was assessed in adolescents and young adults.Citation188 Results from this study show immunological vaccine effectiveness of 79% and 82% following two 4CMenB doses given, respectively, 2 months and 6 months apart (calculated from the ratio between the percentages of samples lacking bactericidal activity against the 110-strain panel in 4CMenB-immunized participants versus those who received MenACWY-CRM).Citation187 This is in line with the 71% reduction in MenB disease cases observed post-licensure in adolescents in conjunction with the South Australia 4CMenB immunization program.Citation78
A decade of progress and future prospects
Information from surveillance systems shows that MenB is a predominant cause of IMD in Europe, North America, North Africa, Australasia, and other Asia – Pacific countries.Citation3,Citation15–17 The introduction 10 years ago of 4CMenB, the first protein-based vaccine with broad effectiveness against diverse MenB strains, was therefore an important advance in the control of IMD. Large-scale use of 4CMenB immunization in the last decade in different countries, settings, and populations is providing a wealth of RWE on the impact and effectiveness of 4CMenB on IMD case numbers in the risk groups of young children and adolescents/young adults, and a safety profile consistent with prelicensure clinical study results. Moreover, its protective effects go beyond MenB disease, with coverage predicted against non-B serogroups and RWE of effectiveness against MenW disease from the UK.Citation106 N. gonorrhoeae shares homology with the components of 4CMenB vaccine (NHBA and other OMV minor proteins), and real-world data from Canada, the US, South Australia, and ItalyCitation80,Citation81,Citation115,Citation116,Citation118,Citation119,Citation121 demonstrate the potential for moderate protection with 4CMenB against gonococcal infection. This includes the first comparison of 4CMenB and non-OMV-based MenB-FHbp,Citation115 which shows data aligned with growing evidence of some protection against gonorrhea with 4CMenB and the role of OMV in this effect; findings that are of great importance considering the emergence of multidrug-resistant gonococcal strains. This has been recognized in the recommendation issued recently by the New York State Department of Health AIDS Institute, which concluded that early evidence was sufficient to recommend 4CMenB to prevent gonorrhea in at-risk adults, and a similar recommendation issued by the UK Joint Committee on Vaccination and Immunization.Citation189,Citation190 The inconclusive results from the first randomized clinical trial to assess the effect of 4CMenB against gonorrhea, which was discontinued before planned completion,Citation127,Citation129 are inconsistent with RWE from large-scale studies. This may be due to differences in the population under assessment, such as adolescents and young adultsCitation80,Citation81,Citation115,Citation116,Citation118,Citation119 versus MSM on HIV pre-exposure prophylaxis in the DOXYVAC trial.Citation127 The trial results are also inconsistent with those from a case-control study of over 1000 MSM living with HIV.Citation121 Further data are awaited from other ongoing randomized controlled trials assessing the effectiveness of 4CMenB vaccination on gonorrhea.
Over the last 10 years, the number of countries that have introduced 4CMenB infant immunization programs has grown, as has the number of regional adolescent immunization programs in countries with or without infant recommendations. However, there is room for improvement to achieve the WHO goal of providing equal access to vaccines for all those at risk of meningitis in alignment with epidemiological evidence.Citation155 There is diversity among countries in age-based recommendations for meningococcal vaccination, even among neighboring countries with similar economic resources and epidemiological risk,Citation191 and few countries recommend adolescent 4CMenB vaccination despite the at-risk status of this age group and RWE of 4CMenB effectiveness from South Australia.Citation78 Also, older adults are not yet included in the recommendations, even though this age group has the highest case fatality rate and their inclusion could reduce the burden of meningococcal disease on healthcare systems, which appears to be on the increase.Citation56,Citation137 Depending on the regional or national epidemiology of IMD and disease burden, and healthcare priorities, immunization programs should, where possible, consider additional age-based risk groups, with an immunization strategy that is comprehensive for the general population and encompasses boosting those primed in infancy and vaccinating vaccine-naïve individuals. Moreover, countries should emphasize recommendations in populations at higher risk of IMD because of underlying medical conditions, such as complement deficiencies, asplenia, or splenic dysfunction, or because of treatments that inhibit complement activation, as well as people living with HIV. There is evidence of poor vaccination coverage in these groups so increased efforts are needed to address the lack of adherence to recommendations, such as educating healthcare providers, tailoring this education to specific patient populations, and clear recommendations to patients and parents through disease-awareness initiatives.Citation192,Citation193
Over 100 million 4CMenB doses have been administered so far and 4CMenB is confirmed to have an acceptable tolerability profile, with no safety concerns, including when co-administered with routine infant vaccines. Co-administration of MenB vaccination with MenACWY vaccination is recommended in adolescents in a growing number of countries,Citation56,Citation191 and would provide protection against the five most important meningococcal serogroups, supporting equity and safer travel. The addition of 4CMenB vaccination to adolescent MenACWY immunization programs, and for other groups at higher risk of gonorrhea infection, may also have an important long-term population impact on N. gonorrhoeae infections.Citation130 There is, however, a need for better awareness of the need for two vaccines against IMD among healthcare providers, parents, and patients.Citation192
Ongoing international studies and safety monitoring will continue to improve understanding of the benefits and risks of 4CMenB in immunization programs, outbreaks, and in specific populations. This includes continuing randomized controlled trials and observational studies in different countries on the effect of 4CMenB against gonococcal infections. Also, recent studies did not show any significant reduction of the acquisition of MenB carriage in adolescents following MenB vaccination,Citation83,Citation194,Citation195 highlighting the need to protect those at risk of IMD. However, the effect of 4CMenB against meningococcal carriage in this age group is being investigated further.Citation196,Citation197
The future may include the licensure of MenB-containing combination vaccines, which requires comprehensive evaluation of vaccine performance before licensure, in clinical trials. The enc-hSBA assay has been developed to assess the ability to induce a bactericidal immune response in conditions close to real-world settings via use of endogenous complement and an epidemiologically-relevant panel of 110 diverse MenB strains representative of the meningococcal genetic landscape.Citation184 This enables an assessment of the immunological effectiveness of multicomponent MenB vaccines in randomized clinical trial settings, complementing immunogenicity data generated using the traditional hSBA assay.
In conclusion, real-world experience gained over the last decade confirms the impact, effectiveness, and safety profile of 4CMenB against invasive MenB disease and shows cross-protection against some non-B serogroups and N. gonorrhoeae infections. More countries should reinforce recommendations for vaccination and consider a broader 4CMenB immunization policy. This includes 4CMenB vaccination of older adults, and adolescents and young adults, preferably co-administered with MenACWY to protect against the five most common invasive meningococcal serogroups and may potentially provide moderate protection against gonococcal infection. Equal access to 4CMenB vaccination is warranted to better protect all age groups and vulnerable groups, given the wealth of supporting data gained from 10 years of administration to various populations across the world.
Authors’ contribution
All authors were involved in the analysis and interpretation of the relevant literature and the development of the manuscript. All authors gave final approval before submission.
Trademark statement
Bexsero and Menveo are trademarks owned by or licensed to GSK. MeNZB is a trademark of Novartis. Trumenba is a trademark of Pfizer. VA-MENGOC-BC is a trademark of Finlay Institute. MenBvac is a trademark of Norwegian Institute of Public Health.
Audioslides.pdf
Download PDF (4.9 MB)Acknowledgments
The authors thank Business & Decision Life Sciences Medical Communication Service Center for editorial assistance and manuscript coordination, on behalf of GSK. Joanne Knowles (independent medical writer, on behalf of GSK) provided medical writing support.
Disclosure statement
VA, AM, DT, SB, and LS are employed by and hold financial equities in GSK.
RR and MP are former employees of GSK. MP is Professor at Imperial College.
FMT received honoraria from Sanofi, MSD, Moderna, GSK, Biofabri, AstraZeneca, Novavax, Janssen, and Pfizer. He also received financial support for travel and attending meetings from Pfizer, MSD, GSK, and Sanofi. He is a member of ETAGE – WHO Europe, coordinator of Spanish Pediatric Critical Trials Network, and coordinator of WHO collaborating center for vaccine safety of Santiago de Compostela. As Principal Investigator in randomized controlled trials of Ablynx, Abbot, Seqirus, Sanofi, MSD, Merck, Pfizer, Roche, Regeneron, Janssen, Medimmune, Novavax, Novartis, and GSK, he received financial support through his institution.
MKT declares that the Institut Pasteur received work contracts funded by GSK, Pfizer, and Sanofi. He also reports the patent NZ630133A “Vaccines for serogroup X meningococcus” issued with GSK.
TN declares that the University of Melbourne and Murdoch Children’s Research Institute received research grants for clinical trials from Iliad, Dynavax, Sanofi, Moderna, and CSL Seqirus. He also received personal payments for consultant fees from AstraZeneca, Pfizer, CSL Seqirus, and MSD; for participation on a Data Safety Monitoring Board or Advisory Board from Moderna, Clover, Novavax, Serum Institute of India, Technovalia, and Moderna; and support for attending meetings from MSD, GSK, and AstraZeneca. He also declares leadership or fiduciary role in another board, society, committee, or advocacy group for the mRNA Victoria Scientific Ad Board (unpaid), and for the Advances in mRNA Science Advisory Board (personal payment. Independent body but funded by an untied grant from Moderna).
RB performs contract research on behalf of UKHSA for GSK, Pfizer, and Sanofi Pasteur.
The authors declare no other financial and non-financial relationships and activities.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2024.2357924
Additional information
Funding
References
- Shen J, Begum N, Ruiz-Garcia Y, Martinon-Torres F, Bekkat-Berkani R, Meszaros K. Range of invasive meningococcal disease sequelae and health economic application - a systematic and clinical review. BMC Public Health. 2022;22(1):1078. doi:10.1186/s12889-022-13342-2.
- Deghmane AE, Taha S, Taha MK. Global epidemiology and changing clinical presentations of invasive meningococcal disease: a narrative review. Infect Dis (Lond). 2022;54(1):1–21. doi:10.1080/23744235.2021.1971289.
- Parikh S, Campbell H, Bettinger JA, Harrison LH, Marshall HS, Martinon-Torres F, Safadi MA, Shao Z, Zhu B, von Gottberg A. et al. The everchanging epidemiology of meningococcal disease worldwide and the potential for prevention through vaccination. J Infect. 2020;81:483–98. doi:10.1016/j.jinf.2020.05.079.
- U.S. Food & Drug Administration. PENBRAYA. 2023 [accessed 2024 Mar 15]. https://www.fda.gov/vaccines-blood-biologics/vaccines/penbraya.
- Bekkat-Berkani R, Fragapane E, Preiss S, Rappuoli R, Sohn WY, Soumahoro L, Vadivelu K. Public health perspective of a pentavalent meningococcal vaccine combining antigens of MenACWY-CRM and 4CMenB. J Infect. 2022;85(5):481–91. doi:10.1016/j.jinf.2022.09.001.
- Haidara FC, Umesi A, Sow SO, Ochoge M, Diallo F, Imam A, Traore Y, Affleck L, Doumbia MF, Daffeh B. et al. Meningococcal ACWYX conjugate vaccine in 2-to-29-year-olds in Mali and Gambia. N Engl J Med. 2023;388(21):1942–55. doi:10.1056/NEJMoa2214924.
- Feavers IM, Maiden MCJ. Recent progress in the prevention of serogroup B meningococcal disease. Clin Vaccine Immunol. 2017;24(5):e00566. doi:10.1128/cvi.00566-16.
- Holst J, Oster P, Arnold R, Tatley MV, Næss LM, Aaberge IS, Galloway Y, McNicholas A, O’Hallahan J, Rosenqvist E. et al. Vaccines against meningococcal serogroup B disease containing outer membrane vesicles (OMV): lessons from past programs and implications for the future. Hum Vaccin Immunother. 2013;9(6):1241–53. doi:10.4161/hv.24129.
- De Gregorio E, Rappuoli R. From empiricism to rational design: a personal perspective of the evolution of vaccine development. Nat Rev Immunol. 2014;14(7):505–14. doi:10.1038/nri3694.
- GSK. Bexsero prescribing information (U.S.A). 2022 [accessed 2024 Mar 15]. https://gskpro.com/content/dam/global/hcpportal/en_US/Prescribing_Information/Bexsero/pdf/BEXSERO.PDF.
- European Medicines Agency. Annex 1. Summary of product characteristics (Bexsero). 2020 [accessed 2024 Mar 15]. https://www.ema.europa.eu/en/documents/product-information/bexsero-epar-product-information_en.pdf.
- Perez JL, Absalon J, Beeslaar J, Balmer P, Jansen KU, Jones TR, Harris S, York LJ, Jiang Q, Radley D. et al. From research to licensure and beyond: clinical development of MenB-FHbp, a broadly protective meningococcal B vaccine. Expert Rev Vaccines. 2018;17(6):461–77. doi:10.1080/14760584.2018.1483726.
- Serruto D, Bottomley MJ, Ram S, Giuliani MM, Rappuoli R. The new multicomponent vaccine against meningococcal serogroup B, 4CMenB: immunological, functional and structural characterization of the antigens. Vaccine. 2012;30(Suppl 2):B87–97. doi:10.1016/j.vaccine.2012.01.033.
- Rappuoli R, Pizza M, Masignani V, Vadivelu K. Meningococcal B vaccine (4CMenB): the journey from research to real world experience. Expert Rev Vaccines. 2018;17(12):1111–21. doi:10.1080/14760584.2018.1547637.
- Aye AMM, Bai X, Borrow R, Bory S, Carlos J, Caugant DA, Chiou CS, Dai VTT, Dinleyici EC, Ghimire P. et al. Meningococcal disease surveillance in the Asia-Pacific region (2020): the global meningococcal initiative. J Infect. 2020;81(5):698–711. doi:10.1016/j.jinf.2020.07.025.
- Asturias EJ, Bai X, Bettinger JA, Borrow R, Castillo DN, Caugant DA, Chacon GC, Dinleyici EC, Aviles GE, Garcia L. et al. Meningococcal disease in North America: updates from the global meningococcal initiative. J Infect. 2022;85(6):611–22. doi:10.1016/j.jinf.2022.10.022.
- Dogu AG, Oordt-Speets AM, van Kessel-de Bruijn F, Ceyhan M, Amiche A. Systematic review of invasive meningococcal disease epidemiology in the Eastern Mediterranean and North Africa region. BMC Infect Dis. 2021;21(1):1088. doi:10.1186/s12879-021-06781-6.
- Borrow R, Carlone GM, Rosenstein N, Blake M, Feavers I, Martin D, Zollinger W, Robbins J, Aaberge I, Granoff DM. et al. Neisseria meningitidis group B correlates of protection and assay standardization. International meeting report Emory University, Atlanta, Georgia, United States, 16–17 March 2005. Vaccine. 2006;24(24):5093–107. doi:10.1016/j.vaccine.2006.03.091.
- O’Ryan M, Stoddard J, Toneatto D, Wassil J, Dull PM. A multi-component meningococcal serogroup B vaccine (4CMenB): the clinical development program. Drugs. 2014;74(1):15–30. doi:10.1007/s40265-013-0155-7.
- Martinón-Torres F, Carmona Martinez A, Simkó R, Infante Marquez P, Arimany JL, Gimenez-Sanchez F, Couceiro Gianzo JA, Kovács É, Rojo P, Wang H. et al. Antibody persistence and booster responses 24–36 months after different 4CMenB vaccination schedules in infants and children: a randomised trial. J Infect. 2018;76(3):258–69. doi:10.1016/j.jinf.2017.12.005.
- Martinón-Torres F, Safadi MAP, Martinez AC, Marquez PI, Torres JCT, Weckx LY, Moreira EDJ, Mensi I, Calabresi M, Toneatto D. Reduced schedules of 4CMenB vaccine in infants and catch-up series in children: immunogenicity and safety results from a randomised open-label phase 3b trial. Vaccine. 2017;35(28):3548–57. doi:10.1016/j.vaccine.2017.05.023.
- Biolchi A, Tomei S, Santini L, Welsch JA, Toneatto D, Gaitatzis N, Bai X, Borrow R, Giuliani MM, Mori E. et al. Evaluation of strain coverage of the multicomponent meningococcal serogroup B vaccine (4CMenB) administered in infants according to different immunisation schedules. Hum Vaccin Immunother. 2019;15(3):725–31. doi:10.1080/21645515.2018.1537756.
- Govern d’Andorra. Recomanacions sobre l’administració de la vacunació enfront del meningococ B (Bexsero). 2016 [accessed 2024 Mar 15]. https://www.salut.ad/images/stories/Salut/pdfs/temes_salut/Recomanacions_Vacuna_Bexero.pdf.
- Chile Ministerio de Salud. National immunization program. 2024 [accessed 2024 Mar 15]. https://www.minsal.cl/programa-nacional-de-inmunizaciones/.
- European Centre for Disease Prevention and Control. Czechia: recommended vaccinations. 2024 [accessed 2024 Mar 15]. https://vaccine-schedule.ecdc.europa.eu/Scheduler/ByCountry?SelectedCountryId=201&IncludeChildAgeGroup=true&IncludeChildAgeGroup=false&IncludeAdultAgeGroup=true&IncludeAdultAgeGroup=false.
- MUDr Jaromír Vomáčka. Vaccination against infectious diseases in the Czech Republic. 2023 [accessed 2024 Mar 15]. http://www.mudrvomacka.cz/index.php?option=com_content&view=article&id=8&Itemid=152&lang=en.
- Ministère de la Santé et de la Prévention. Le calendrier des vaccinations. 2023 [accessed 2024 Mar 15]. https://sante.gouv.fr/prevention-en-sante/preserver-sa-sante/vaccination/calendrier-vaccinal.
- Health Service Executive (Ireland). Immunisation schedule 2023. 2023 [accessed 2024 Mar 15]. https://www.hse.ie/eng/health/immunisation/pubinfo/currentschedule.html.
- Ministero della Salute (Italy). Piano Nazionale Prevenzione Vaccinale PNPV 2023–2025. 2023 [accessed 2024 Mar 15]. https://www.quotidianosanita.it/allegati/allegato1679488094.pdf.
- Ministry of Health for the Republic of Lithuania. Recommendation for revised vaccination against meningococcal type B infection. 2018 [accessed 2024 Mar 15]. https://nvsc.lrv.lt/uploads/nvsc/documents/files/Rekomendacijos%20skiepijant%20nuo%20B%20tipo%20MI(2).pdf.
- Ministry of Health for the Republic of Lithuania. m. Birželio 12 d. Įsakymo nr. V-757 “dėl lietuvos respublikos vaikų profilaktinių skiepijimų kalendoriaus patvirtinimo“pakeitimo. 2018 [accessed 2024 Mar 15]. https://e-seimas.lrs.lt/portal/legalAct/lt/TAD/f4a925d0f50f11e79a1bc86190c2f01a.
- Ministry for Health (Malta). National immunisation schedule. 2023 [accessed 2024 Mar 15]. https://healthservices.gov.mt/en/phc/pchyhi/Pages/National-Immunisation-Schedule.aspx.
- Health New Zealand – Te Whatu Ora. New Zealand immunisation schedule. 2023 [accessed 2024 Mar 15]. https://www.tewhatuora.govt.nz/for-the-health-sector/vaccine-information/new-zealand-immunisation-schedule/.
- Gabinete do Secretário de Estado da Saúde (Portugal). Aprova o novo esquema vacinal do Programa Nacional de Vacinação (PNV), revo-gando, com exceção do seu n.º 6, o Despacho n.º 10441/2016, de 9 de agosto. 2019 [accessed 2024 Mar 15]. https://files.dre.pt/2s/2019/12/250000000/0003000031.pdf.
- SIP-SPP Vaccine Commission (Portugal). Recommendations on vaccines outside the national vaccination program. 2020 [accessed 2024 Mar 15]. https://www.spp.pt/UserFiles/file/Seccao_Infecciologia/recomendacoes%20vacinas_sip_final_28set_2.pdf.
- Istituto per la Sicurezza Sociale (San Marino). Recommended vaccinations. 2023 [accessed 2024 Mar 15]. https://www.iss.sm/on-line/home/vaccini-e-vaccinazioni/vaccinazioni-raccomandate.html.
- Asociación Española de Pediatría. Vacuna meningococo B. 2023 [accessed 2024 Mar 15]. https://vacunasaep.org/familias/vacunas-una-a-una/vacuna-meningococo-b.
- Consejo Interterritorial del Sistema Nacional de Salud. Calendario común de vacunación a lo largo de toda la vida. Calendario recomendado año 2024. 2024 [accessed 2024 Mar 15]. https://www.sanidad.gob.es/areas/promocionPrevencion/vacunaciones/calendario/docs/CalendarioVacunacion_Todalavida.pdf.
- Ladhani SN, Campbell H, Parikh SR, Saliba V, Borrow R, Ramsay M. The introduction of the meningococcal B (MenB) vaccine (Bexsero®) into the national infant immunisation programme - New challenges for public health. J Infect. 2015;71(6):611–4. doi:10.1016/j.jinf.2015.09.035.
- UK Health Security Agency. The routine immunisation schedule. 2023 [accessed 2024 Mar 15]. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1055877/UKHSA-12155-routine-complete-immunisation-schedule_Feb2022.pdf.
- Epidemiologisches Bulletin. STIKO: Standardimpfung von Säuglingen gegen Meningokokken der Serogruppe B. 2024 [accessed 2024 Mar 15]. https://www.rki.de/DE/Content/Infekt/EpidBull/Archiv/2024/Ausgaben/03_24.pdf?__blob=publicationFile.
- Higher Council of Infectious Diseases. Méningite et infections invasives à méningocoques. 2023 [accessed 2024 Mar 15]. https://sante.public.lu/fr/espace-professionnel/recommandations/conseil-maladies-infectieuses/meningite.html.
- Office fédéral de la santé publique OFSP. Plan de vaccination suisse. 2024 [accessed 2024 Mar 15]. https://www.bag.admin.ch/bag/fr/home/gesund-leben/gesundheitsfoerderung-und-praevention/impfungen-prophylaxe/schweizerischer-impfplan.html.
- Haute Autorité de Santé. Stratégie de vaccination contre les infections invasives à méningocoques. Révision de la stratégie contre les sérogroupes ACWY et B. 2024 [accessed 2024 Mar 15]. https://www.has-sante.fr/upload/docs/application/pdf/2024-03/eco_sp_424_recovac_meningo.pdf#page=8.
- Mbaeyi SA, Bozio CH, Duffy J, Rubin LG, Hariri S, Stephens DS, MacNeil JR. Meningococcal vaccination: recommendations of the advisory committee on immunization practices, United States, 2020. MMWR Recomm Rep. 2020;69(9):1–41. doi:10.15585/mmwr.rr6909a1.
- Centers for Disease Control and Prevention. Meningococcal vaccine recommendations. 2023 [accessed 2024 Mar 15]. https://www.cdc.gov/vaccines/vpd/mening/hcp/recommendations.html.
- Marshall HS, Lally N, Flood L, Phillips P. First statewide meningococcal B vaccine program in infants, children and adolescents: evidence for implementation in South Australia. Med J Aust. 2020;212(2):89–93. doi:10.5694/mja2.50481.
- Government of South Australia. Meningococcal B immunisation program. 2023 [accessed 2024 Mar 15]. https://www.sahealth.sa.gov.au/wps/wcm/connect/public+content/sa+health+internet/conditions/immunisation/immunisation+programs/meningococcal+b+immunisation+program.
- Queensland Government. Immunisation schedule Queensland. 2024 [accessed 2024 Mar 15]. https://www.health.qld.gov.au/clinical-practice/guidelines-procedures/diseases-infection/immunisation/schedule.
- Provincial government of Prince Edward Island. Free meningitis vaccine available to post-secondary students living in residence. 2023 [accessed 2024 Mar 15]. https://www.princeedwardisland.ca/en/news/free-meningitis-vaccine-available-to-post-secondary-students-living-in-residence.
- Provincial government of Nova Scotia. More Nova Scotians eligible for meningococcal B vaccine. 2023 [accessed 2024 Mar 15]. https://novascotia.ca/news/release/?id=20230525003.
- Abitbol V, Sohn WY, Horn M, Safadi MAP. Safety and immunogenicity of co-administered meningococcal serogroup B (4CMenB) vaccine: A literature review. Hum Vaccin Immunother. 2023;19(2):2245705. doi:10.1080/21645515.2023.2245705.
- Sohn WY, Tahrat H, Novy P, Bekkat-Berkani R. Real-world implementation of 4-component meningococcal serogroup B vaccine (4CMenB): implications for clinical practices. Expert Rev Vaccines. 2022;21(3):325–35. doi:10.1080/14760584.2022.2021881.
- Immunisation Advisory Centre (New Zealand). MenB Bexsero quick facts. 2023 [accessed 2024 Mar 15]. https://www.immune.org.nz/factsheets/menb-bexsero.
- České vakcinologické společnosti ČLS JEP (Czechia). Doporučení České vakcinologické společnosti ČLS JEP pro očkování proti invazivním meningokokovým onemocněním. 2023 [accessed 2024 Mar 15]. https://www.vakcinace.eu/storage/files/3/doporuceni_a_stanoviska/2023/doporuceniockovaniprotiimofinal_6_3_2023.pdf.
- Taha MK, Bekkat-Berkani R, Abitbol V. Changing patterns of invasive meningococcal disease and future immunization strategies. Hum Vaccin Immunother. 2023;19(1):2186111. doi:10.1080/21645515.2023.2186111.
- Region of Puglia. Calendario vaccinale per la vita della regione Puglia - ed. 2021. 2021 [accessed 2024 Mar 15]. https://www.sanita.puglia.it/documents/20182/285275922/DGR+1589_2021+Nuovo+calendario+vaccinale+ed.2021.pdf/186500a3-134e-4dae-9d5c-c6e139280bcd.
- Region of Calabria. DCA 43/2015 Calendario vaccinale regionale. 2022 [accessed 2024 Mar 15]. https://www.regione.calabria.it/website/portaltemplates/view/view_provvedimenti.cfm?63132.
- Region of Sicilia. Il calendario vaccinale della Regione Sicilia. 2017 [accessed 2024 Mar 15]. https://www.vaccinarsinsicilia.org/vaccinazioni-sicilia/calendario-vaccinale.
- Provincial government of Prince Edward Island. Meningococcal group A, B, C, Y and W-135 vaccines. 2023 [accessed 2024 Mar 15]. https://www.princeedwardisland.ca/en/information/health-and-wellness/meningococcal-group-a-b-c-y-and-w-135-vaccines.
- Findlow J, Bai X, Findlow H, Newton E, Kaczmarski E, Miller E, Borrow R. Safety and immunogenicity of a four-component meningococcal group B vaccine (4CMenB) and a quadrivalent meningococcal group ACWY conjugate vaccine administered concomitantly in healthy laboratory workers. Vaccine. 2015;33(29):3322–30. doi:10.1016/j.vaccine.2015.05.027.
- Macias Parra M, Gentile A, Vazquez Narvaez JA, Capdevila A, Minguez A, Carrascal M, Willemsen A, Bhusal C, Toneatto D. Immunogenicity and safety of the 4CMenB and MenACWY-CRM meningococcal vaccines administered concomitantly in infants: a phase 3b, randomized controlled trial. Vaccine. 2018;36(50):7609–17. doi:10.1016/j.vaccine.2018.10.096.
- Beran J, Dražan D, Enweonye I, Bhusal C, Toneatto D. Immunogenicity and safety of investigational MenABCWY vaccine and of 4CMenB and MenACWY vaccines administered concomitantly or alone: A phase 2 randomized study of adolescents and young adults. mSphere. 2021;6(6):e0055321. doi:10.1128/mSphere.00553-21.
- Martinón-Torres F, Banzhoff A, Azzari C, De Wals P, Marlow R, Marshall H, Pizza M, Rappuoli R, Bekkat-Berkani R. Recent advances in meningococcal B disease prevention: real-world evidence from 4CMenB vaccination. J Infect. 2021;83(1):17–26. doi:10.1016/j.jinf.2021.04.031.
- Isitt C, Cosgrove CA, Ramsay ME, Ladhani SN. Success of 4CMenB in preventing meningococcal disease: evidence from real-world experience. Arch Dis Child. 2020;105(8):784–90. doi:10.1136/archdischild-2019-318047.
- Cinconze E, Rosillon D, Rappuoli R, Vadivelu K, Bekkat-Berkani R, Abbing-Karahagopian V. Challenges in synthesis of real-world vaccine effects on meningococcal serogroup B disease for 4CMenB vaccine post-licensure effectiveness studies: a systematic review. Vaccine. 2023;41(30):4347–58. doi:10.1016/j.vaccine.2023.05.025.
- Ladhani SN, Andrews N, Parikh SR, Campbell H, White J, Edelstein M, Bai X, Lucidarme J, Borrow R, Ramsay ME. Vaccination of infants with meningococcal group B vaccine (4CMenB) in England. N Engl J Med. 2020;382(4):309–17. doi:10.1056/NEJMoa1901229.
- Lucidarme J, Bai X, Lekshmi A, Clark SA, Willerton L, Ribeiro S, Campbell H, Serino L, De Paola R, Holland A. et al. Invasive serogroup B meningococci in England following three years of 4CMenB vaccination - first real-world data. J Infect. 2022;84(2):136–44. doi:10.1016/j.jinf.2021.11.015.
- Mensah AA, Campbell H, Clark SA, Ribeiro S, Lucidarme J, Bai X, Borrow R, Ladhani SN. Outcomes of meningococcal serogroup B disease in children after implementation of routine infant 4CMenB vaccination in England: an active, prospective, national surveillance study. Lancet Child Adolesc Health. 2023;7(3):190–8. doi:10.1016/s2352-4642(22)00379-0.
- Deceuninck G, Lefebvre B, Tsang R, Betala-Belinga JF, De Serres G, De Wals P. Impact of a mass vaccination campaign against Serogroup B meningococcal disease in the Saguenay-Lac-Saint-Jean region of Quebec four years after its launch. Vaccine. 2019;37(31):4243–5. doi:10.1016/j.vaccine.2019.06.021.
- De Wals P, Deceuninck G, Lefebvre B, Tsang R, Law D, De Serres G, Gilca V, Gilca R, Boulianne N. Impact of an immunization campaign to control an increased incidence of serogroup B meningococcal disease in one region of Quebec, Canada. Clin Infect Dis. 2017;64(9):1263–7. doi:10.1093/cid/cix154.
- Banzhoff A. Multicomponent meningococcal B vaccination (4CMenB) of adolescents and college students in the United States. Ther Adv Vaccines. 2017;5(1):3–14. doi:10.1177/2051013616681365.
- Biswas HH, Han GS, Wendorf K, Winter K, Zipprich J, Perti T, Martinez L, Arellano A, Kyle JL, Zhang P. et al. Notes from the field: Outbreak of serogroup B meningococcal disease at a university - California, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(20):520–1. doi:10.15585/mmwr.mm6520a3.
- Azzari C, Moriondo M, Nieddu F, Guarnieri V, Lodi L, Canessa C, Indolfi G, Giovannini M, Napoletano G, Russo F. et al. Effectiveness and impact of the 4CMenB vaccine against group B meningococcal disease in two Italian regions using different vaccination schedules: A five-year retrospective observational study (2014-2018). Vaccines (Basel). 2020;8(3):469. doi:10.3390/vaccines8030469.
- Lodi L, Barbati F, Amicizia D, Baldo V, Barbui AM, Bondi A, Costantino C, Da Dalt L, Ferrara L, Fortunato F. et al. Four-component recombinant protein-based vaccine effectiveness against serogroup B meningococcal disease in Italy. JAMA Netw Open. 2023;6(8):e2329678. doi:10.1001/jamanetworkopen.2023.29678.
- Rodrigues FMP, Marlow R, Simões MJ, Danon L, Ladhani S, Finn A. Association of use of a meningococcus group B vaccine with group B invasive meningococcal disease among children in Portugal. JAMA. 2020;324(21):2187–94. doi:10.1001/jama.2020.20449.
- Castilla J, García Cenoz M, Abad R, Sánchez-Cambronero L, Lorusso N, Izquierdo C, Cañellas Llabrés S, Roig J, Malvar A, González Carril F. et al. Effectiveness of a meningococcal group B vaccine (4CMenB) in children. N Engl J Med. 2023;388(5):427–38. doi:10.1056/NEJMoa2206433.
- McMillan M, Wang B, Koehler AP, Sullivan TR, Marshall HS. Impact of meningococcal B vaccine on invasive meningococcal disease in adolescents. Clin Infect Dis. 2021;73(1):233–7. doi:10.1093/cid/ciaa1636.
- Marshall HS, McMillan M, Koehler AP, Lawrence A, Sullivan TR, MacLennan JM, Maiden MCJ, Ladhani SN, Ramsay ME, Trotter C. et al. Meningococcal B vaccine and meningococcal carriage in adolescents in Australia. N Engl J Med. 2020;382(4):318–27. doi:10.1056/NEJMoa1900236.
- Wang B, Giles L, Andraweera P, McMillan M, Almond S, Beazley R, Mitchell J, Lally N, Ahoure M, Denehy E. et al. Effectiveness and impact of the 4CMenB vaccine against invasive serogroup B meningococcal disease and gonorrhoea in an infant, child, and adolescent programme: an observational cohort and case-control study. Lancet Infect Dis. 2022;22(7):1011–20. doi:10.1016/s1473-3099(21)00754-4.
- Wang B, Giles L, Andraweera P, McMillan M, Almond S, Beazley R, Mitchell J, Ahoure M, Denehy E, Flood L. et al. 4CMenB sustained vaccine effectiveness against invasive meningococcal B disease and gonorrhoea at three years post programme implementation. J Infect. 2023;87(2):95–102. doi:10.1016/j.jinf.2023.05.021.
- Marshall GS, Abbing-Karahagopian V, Marshall HS, Cenci S, Conway JH, Occhipinti E, Bekkat-Berkani R, Banzhoff A, Sohn WY. A comprehensive review of clinical and real-world safety data for the four-component serogroup B meningococcal vaccine (4CMenB). Expert Rev Vaccines. 2023;22(1):530–44. doi:10.1080/14760584.2023.2222015.
- Deghmane AE, Taha MK. Product review on the IMD serogroup B vaccine Bexsero®. Hum Vaccin Immunother. 2022;18(1):e2020043. doi:10.1080/21645515.2021.2020043.
- Gossger N, Snape MD, Yu LM, Finn A, Bona G, Esposito S, Principi N, Diez-Domingo J, Sokal E, Becker B. et al. Immunogenicity and tolerability of recombinant serogroup B meningococcal vaccine administered with or without routine infant vaccinations according to different immunization schedules: a randomized controlled trial. JAMA. 2012;307(6):573–82. doi:10.1001/jama.2012.85.
- Prymula R, Esposito S, Zuccotti GV, Xie F, Toneatto D, Kohl I, Dull PM. A phase 2 randomized controlled trial of a multicomponent meningococcal serogroup B vaccine (I). Hum Vaccin Immunother. 2014;10(7):1993–2004. doi:10.4161/hv.28666.
- Vesikari T, Esposito S, Prymula R, Ypma E, Kohl I, Toneatto D, Dull P, Kimura A. Immunogenicity and safety of an investigational multicomponent, recombinant, meningococcal serogroup B vaccine (4CMenB) administered concomitantly with routine infant and child vaccinations: results of two randomised trials. Lancet. 2013;381(9869):825–35. doi:10.1016/s0140-6736(12)61961-8.
- Ladhani SN, Riordan A. The yin and yang of fever after meningococcal B vaccination. Arch Dis Child. 2017;102(10):881–2. doi:10.1136/archdischild-2017-313419.
- De Serres G, Billard MN, Gariépy MC, Rouleau I, Toth E, Landry M, Boulianne N, Gagné H, Gilca V, Deceuninck G. et al. Short-term safety of 4CMenB vaccine during a mass meningococcal B vaccination campaign in Quebec, Canada. Vaccine. 2018;36(52):8039–46. doi:10.1016/j.vaccine.2018.10.095.
- Dubus M, Ladhani S, Vasu V. Prophylactic paracetamol after meningococcal B vaccination reduces postvaccination fever and septic screens in hospitalized preterm infants. Pediatr Infect Dis J. 2020;39(1):78–80. doi:10.1097/inf.0000000000002507.
- Bryan P, Seabroke S, Wong J, Donegan K, Webb E, Goldsmith C, Vipond C, Feavers I. Safety of multicomponent meningococcal group B vaccine (4CMenB) in routine infant immunisation in the UK: a prospective surveillance study. Lancet Child Adolesc Health. 2018;2(6):395–403. doi:10.1016/s2352-4642(18)30103-2.
- Hall GC, Douglas I, Heath PT, Prabhakar P, Rosillon D, Khan J, Abbing-Karahagopian V. Post-licensure observational safety study after meningococcal B vaccine 4CMenB (Bexsero) vaccination within the routine UK immunisation program. Vaccine. 2021;39(24):3296–303. doi:10.1016/j.vaccine.2021.02.065.
- Mentzer D, Oberle D, Keller-Stanislawski B. Adverse events following immunisation with a meningococcal serogroup B vaccine: report from post-marketing surveillance, Germany, 2013 to 2016. Euro Surveill. 2018;23(17):17–00468. doi:10.2807/1560-7917.es.2018.23.17.17-00468.
- Agenzia Italiana del Farmaco. Rapporto vaccini 2017. 2018 [accessed 2024 Mar 15]. https://www.aifa.gov.it/sites/default/files/Rapp_Vaccini_2017_0.pdf.
- Marshall HS, Koehler AP, Wang B, A’Houre M, Gold M, Quinn H, Crawford N, Pratt N, Sullivan TR, Macartney K. Safety of meningococcal B vaccine (4CMenB) in adolescents in Australia. Vaccine. 2020;38(37):5914–22. doi:10.1016/j.vaccine.2020.07.009.
- Australian Government Therapeutic Goods Administration. Bexsero meningococcal B vaccine. Final update - monitoring finds no new or unexpected safety issues. 2016 [accessed 2024 Mar 15]. https://www.tga.gov.au/news/safety-alerts/bexsero-meningococcal-b-vaccine.
- Dey A, Wang H, Quinn H, Pillsbury A, Hickie M, Deng L, Wood N, Beard F, Macartney K. Surveillance of adverse events following immunisation in Australia annual report, 2020. Commun Dis Intell (2018). 2022;46. doi:10.33321/cdi.2022.46.47.
- Watson PS, Turner DP. Clinical experience with the meningococcal B vaccine, Bexsero®: prospects for reducing the burden of meningococcal serogroup B disease. Vaccine. 2016;34(7):875–80. doi:10.1016/j.vaccine.2015.11.057.
- Thabuis A, Tararbit K, Taha MK, Dejour-Salamanca D, Ronin V, Parent du Chatelet I, Spaccaferri G. Community outbreak of serogroup B invasive meningococcal disease in Beaujolais, France, February to June 2016: from alert to targeted vaccination. Euro Surveill. 2018;23(28):1700590. doi:10.2807/1560-7917.es.2018.23.28.1700590.
- De Serres G, Billard MN, Gariépy MC, Roy MC, Boucher FD, Gagné H, Belley S, Toth E, Landry M, Skowronski DM. Nephrotic syndrome following four-component meningococcal B vaccination: epidemiologic investigation of a surveillance signal. Vaccine. 2019;37(35):4996–5002. doi:10.1016/j.vaccine.2019.07.017.
- Andrews N, Stowe J, Miller E. Nephrotic syndrome in infants and toddlers before and after introduction of the meningococcal B vaccine programme in England: an ecological study. Vaccine. 2020;38(31):4816–9. doi:10.1016/j.vaccine.2020.05.055.
- Stowe J, Andrews NJ, Turner PJ, Miller E. The risk of Kawasaki disease after pneumococcal conjugate & meningococcal B vaccine in England: a self-controlled case-series analysis. Vaccine. 2020;38(32):4935–9. doi:10.1016/j.vaccine.2020.05.089.
- Ruiz García Y, Sohn WY, Seib KL, Taha MK, Vázquez JA, de Lemos APS, Vadivelu K, Pizza M, Rappuoli R, Bekkat-Berkani R. Looking beyond meningococcal B with the 4CMenB vaccine: the Neisseria effect. NPJ Vaccines. 2021;6(1):130. doi:10.1038/s41541-021-00388-3.
- Biolchi A, De Angelis G, Moschioni M, Tomei S, Brunelli B, Giuliani M, Bambini S, Borrow R, Claus H, Gorla MCO. et al. Multicomponent meningococcal serogroup B vaccination elicits cross-reactive immunity in infants against genetically diverse serogroup C, W and Y invasive disease isolates. Vaccine. 2020;38(47):7542–50. doi:10.1016/j.vaccine.2020.09.050.
- Biolchi A, Tomei S, Brunelli B, Giuliani M, Bambini S, Borrow R, Claus H, Gorla MCO, Hong E, Lemos APS. et al. 4CMenB immunization induces serum bactericidal antibodies against non-serogroup B meningococcal strains in adolescents. Infect Dis Ther. 2021;10(1):307–16. doi:10.1007/s40121-020-00370-x.
- Hong E, Giuliani MM, Deghmane AE, Comanducci M, Brunelli B, Dull P, Pizza M, Taha MK. Could the multicomponent meningococcal serogroup B vaccine (4CMenB) control Neisseria meningitidis capsular group X outbreaks in Africa? Vaccine. 2013;31(7):1113–16. doi:10.1016/j.vaccine.2012.12.022.
- Ladhani SN, Campbell H, Andrews N, Parikh SR, White J, Edelstein M, Clark SA, Lucidarme J, Borrow R, Ramsay ME. First real-world evidence of meningococcal group B vaccine, 4CMenB, protection against meningococcal group W disease: Prospective enhanced national surveillance, England. Clin Infect Dis. 2021;73(7):1661–8. doi:10.1093/cid/ciaa1244.
- Semchenko EA, Day CJ, Seib KL. The Neisseria gonorrhoeae vaccine candidate NHBA elicits antibodies that are bactericidal, opsonophagocytic and that reduce gonococcal adherence to epithelial cells. Vaccines. 2020;8(2):219. doi:10.3390/vaccines8020219.
- Semchenko EA, Tan A, Borrow R, Seib KL. The serogroup B meningococcal vaccine Bexsero elicits antibodies to Neisseria gonorrhoeae. Clin Infect Dis. 2019;69(7):1101–11. doi:10.1093/cid/ciy1061.
- Petousis-Harris H, Paynter J, Morgan J, Saxton P, McArdle B, Goodyear-Smith F, Black S. Effectiveness of a group B outer membrane vesicle meningococcal vaccine against gonorrhoea in New Zealand: a retrospective case-control study. Lancet. 2017;390(10102):1603–10. doi:10.1016/s0140-6736(17)31449-6.
- Paynter J, Goodyear-Smith F, Morgan J, Saxton P, Black S, Petousis-Harris H. Effectiveness of a group B outer membrane vesicle meningococcal vaccine in preventing hospitalization from gonorrhea in New Zealand: a retrospective cohort study. Vaccines (Basel). 2019;7(1):5. doi:10.3390/vaccines7010005.
- Tuddenham S, Hamill MM, Ghanem KG. Diagnosis and treatment of sexually transmitted infections: a review. JAMA. 2022;327(2):161–72. doi:10.1001/jama.2021.23487.
- Frost I, Sati H, Garcia-Vello P, Hasso-Agopsowicz M, Lienhardt C, Gigante V, Beyer P. The role of bacterial vaccines in the fight against antimicrobial resistance: an analysis of the preclinical and clinical development pipeline. Lancet Microbe. 2023;4(2):e113–25. doi:10.1016/s2666-5247(22)00303-2.
- ClinicalTrials.gov. Study to assess gonorrhoeae immune responses induced by a N. Meningitidis vaccine (4cmenB). 2021 [accessed 2024 Mar 15]. https://www.clinicaltrials.gov/study/NCT04094883?term=NCT04094883&rank=1.
- ClinicalTrials.gov. Efficacy of immunization with 4C-MenB in preventing experimental urethral infection with Neisseria gonorrhoeae. 2023 [accessed 2024 Mar 15]. https://www.clinicaltrials.gov/study/NCT05294588?term=NCT05294588&rank=1.
- Robison SG, Leman RF. Association of group B meningococcal vaccine receipt with reduced gonorrhea incidence among university students. JAMA Netw Open. 2023;6(8):e2331742. doi:10.1001/jamanetworkopen.2023.31742.
- Bruxvoort KJ, Lewnard JA, Chen LH, Tseng HF, Chang J, Veltman J, Marrazzo J, Qian L. Prevention of Neisseria gonorrhoeae with meningococcal B vaccine: A matched cohort study in southern California. Clin Infect Dis. 2023;76(3):1341–9. doi:10.1093/cid/ciac436.
- ClinicalTrials.gov. Mucosal immunity against Neisseria gonorrhoeae after 4CMenB vaccination. 2023 [accessed 2024 Mar 15]. https://www.clinicaltrials.gov/study/NCT04722003?term=NCT04722003&rank=1.
- Abara WE, Bernstein KT, Lewis FMT, Schillinger JA, Feemster K, Pathela P, Hariri S, Islam A, Eberhart M, Cheng I. et al. Effectiveness of a serogroup B outer membrane vesicle meningococcal vaccine against gonorrhoea: a retrospective observational study. Lancet Infect Dis. 2022;22(7):1021–9. doi:10.1016/s1473-3099(21)00812-4.
- Longtin J, Dion R, Simard M, Betala Belinga J-F, Longtin Y, Lefebvre B, Labbé A-C, Deceuninck G, De Wals P. Possible impact of wide-scale vaccination against serogroup B Neisseria meningitidis on gonorrhea incidence rates in one region of Quebec, Canada. Open Forum Infect Dis. 2017;4(Suppl 1):S734–5. doi:10.1093/ofid/ofx180.002.
- ClinicalTrials.gov. Safety and efficacy study of meningococcal group B vaccine rMenB+OMV NZ (Bexsero) to prevent gonococcal infection. 2024 [accessed 2024 Mar 15]. https://www.clinicaltrials.gov/study/NCT04350138?term=NCT04350138&rank=1.
- Raccagni AR, Galli L, Spagnuolo V, Bruzzesi E, Muccini C, Bossolasco S, Ranzenigo M, Gianotti N, Lolatto R, Castagna A. et al. Meningococcus B vaccination effectiveness against Neisseria gonorrhoeae infection in people living with HIV: A case-control study. Sex Transm Dis. 2023;50(5):247–51. doi:10.1097/olq.0000000000001771.
- ClinicalTrials.gov. Gonococcal vaccine study in key populations in Kenya (BexKPK). 2022 [accessed 2024 Mar 15]. https://www.clinicaltrials.gov/study/NCT04297436?term=NCT04297436&rank=1.
- ClinicalTrials.gov. Efficacy trial on meningococcal B vaccine for preventing gonorrhea infections. 2023 [accessed 2024 Mar 15]. https://www.clinicaltrials.gov/study/NCT05766904?term=NCT05766904&rank=1.
- ClinicalTrials.gov. Immunisation for adolescents against serious communicable diseases (B Part of it NT). 2023 [accessed 2024 Mar 15]. https://www.clinicaltrials.gov/study/NCT04398849?term=NCT04398849&rank=1.
- ClinicalTrials.gov. Efficacy study of 4CMenB (Bexsero®) to prevent gonorrhoea infection in gay and bisexual men (GoGovax). 2023 [accessed 2024 Mar 15]. https://www.clinicaltrials.gov/study/NCT04415424?term=NCT04415424&rank=1.
- Australian New Zealand Clinical Trials Registry. MenGO: Does the licensed meningococcal vaccine Bexsero® provide cross- protection against gonorrhoea in gay and bisexual men? 2023 [accessed 2024 Mar 15]. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12619001478101.
- Molina J-M, Berçot B, Assoumou L, Algarte-Genin M, Rubenstein E, Pialoux G, Katlama C, Surgers L, Bebear C, Dupin N. et al. Final results of ANRS 174 DOXYVAC: A randomized trial to prevent STI in MSM on PrEP. Conference on Retroviruses and Opportunistic Infections Abstract book (Abstract 124); 2024 Mar 3-6; Denver, Colorado, USA https://www.croiconference.org/abstract/final-results-of-anrs-174-doxyvac-a-randomized-trial-to-prevent-sti-in-msm-on-prep/.
- Abara WE, Bernstein KT, Lewis FMT, Pathela P, Islam A, Eberhart M, Cheng I, Ternier A, Sanderson Slutsker J, Madera R. et al. Healthy vaccinee bias and MenB-FHbp vaccine effectiveness against gonorrhea. Sex Transm Dis. 2023;50(6):e8–10. doi:10.1097/olq.0000000000001793.
- Molina J-M, Berçot B, Assoumou L, Algarte-Genin M, Rubenstein E, Pialoux G, Katlama C, Surgers L, Bebear C, Dupin N. et al. Final results of ANRS 174 DOXYVAC: A randomized trial to prevent STIs in MSM on PrEP. Slides presented at Conference on Retroviruses and Opportunistic Infections; 2024 Mar 3–6 [accessed 2024 Mar 15]; Denver, Colorado, USA. https://www.natap.org/2024/CROI/croi_33.htm.
- Looker KJ, Booton R, Begum N, Beck E, Shen J, Turner KME, Christensen H. The potential public health impact of adolescent 4CMenB vaccination on Neisseria gonorrhoeae infection in England: a modelling study. BMC Public Health. 2023;23(1):1. doi:10.1186/s12889-022-14670-z.
- Carey KA, Newman LM, Spicknall IH. Estimating the population level impact of a gonococcal vaccine candidate: predictions from a simple mathematical model. Vaccine. 2022;40(50):7176–81. doi:10.1016/j.vaccine.2022.10.031.
- U.S. Department of Health and Human Services. Reduce gonorrhea rates in male adolescents and young men — STI‑02. 2020 [accessed 2024 Mar 15]. https://health.gov/healthypeople/objectives-and-data/browse-objectives/sexually-transmitted-infections/reduce-gonorrhea-rates-male-adolescents-and-young-men-sti-02.
- European Centre for Disease Prevention and Control. Invasive meningococcal disease. Annual epidemiological report for 2021. 2023 [accessed 2024 Mar 15]. https://www.ecdc.europa.eu/sites/default/files/documents/MEN%20AER%202021.pdf.
- Centers for Disease Control and Prevention. Meningococcal disease Surveillance and Trends. 2024 [accessed 2024 Mar 15]. https://www.cdc.gov/meningococcal/php/surveillance/?CDC_AAref_Val=https://www.cdc.gov/meningococcal/surveillance/index.html.
- Martinón-Torres F, Nolan T, Toneatto D, Banzhoff A. Persistence of the immune response after 4CMenB vaccination, and the response to an additional booster dose in infants, children, adolescents, and young adults. Hum Vaccin Immunother. 2019;15(12):2940–51. doi:10.1080/21645515.2019.1627159.
- Watson PS, Novy P, Bekkat-Berkani R, Strubbe F, Banzhoff A. Optimizing the timing of 4CMenB vaccination in adolescents and young adults based on immune persistence and booster response data. Expert Rev Vaccines. 2019;18(4):343–52. doi:10.1080/14760584.2019.1580579.
- Guedes S, Bertrand-Gerentes I, Evans K, Coste F, Oster P. Invasive meningococcal disease in older adults in North America and Europe: is this the time for action? A review of the literature. BMC Public Health. 2022;22(1):380. doi:10.1186/s12889-022-12795-9.
- Acevedo R, Bai X, Borrow R, Caugant DA, Carlos J, Ceyhan M, Christensen H, Climent Y, De Wals P, Dinleyici EC. et al. The global Meningococcal Initiative meeting on prevention of meningococcal disease worldwide: epidemiology, surveillance, hypervirulent strains, antibiotic resistance and high-risk populations. Expert Rev Vaccines. 2019;18(1):15–30. doi:10.1080/14760584.2019.1557520.
- Burman C, Findlow J, Marshall HS, Safadi MAP. National and regional differences in meningococcal vaccine recommendations for individuals at an increased risk of meningococcal disease. Expert Rev Vaccines. 2023;22(1):839–48. doi:10.1080/14760584.2023.2245467.
- Chang L, Lim BCW, Flaherty GT, Torresi J. Travel vaccination recommendations and infection risk in HIV-positive travellers. J Travel Med. 2019;26(6):taz034. doi:10.1093/jtm/taz034.
- Bozio CH, Blain A, MacNeil J, Retchless A, Weil LM, Wang X, Jenkins LT, Rodriguez-Rivera LD, Jarashow C, Ngo V. et al. Meningococcal disease surveillance in men who have sex with men - United States, 2015-2016. MMWR Morb Mortal Wkly Rep. 2018;67(38):1060–3. doi:10.15585/mmwr.mm6738a4.
- Folaranmi TA, Kretz CB, Kamiya H, MacNeil JR, Whaley MJ, Blain A, Antwi M, Dorsinville M, Pacilli M, Smith S. et al. Increased risk for meningococcal disease among men who have sex with men in the United States, 2012–2015. Clin Infect Dis. 2017;65(5):756–63. doi:10.1093/cid/cix438.
- Simmons RD, Kirwan P, Beebeejaun K, Riordan A, Borrow R, Ramsay ME, Delpech V, Lattimore S, Ladhani S. Risk of invasive meningococcal disease in children and adults with HIV in England: a population-based cohort study. BMC Med. 2015;13(1):297. doi:10.1186/s12916-015-0538-6.
- Marshall GS, Ghaswalla PK, Bengtson LGS, Buikema AR, Bancroft T, Koep E, Novy P, Hogea CS. Low meningococcal vaccination rates among patients with newly diagnosed complement component deficiencies in the United States. Clin Infect Dis. 2022;75(1):155–8. doi:10.1093/cid/ciab917.
- Ghaswalla PK, Bengtson LGS, Marshall GS, Buikema AR, Bancroft T, Schladweiler KM, Koep E, Novy P, Hogea CS. Meningococcal vaccination in patients with newly diagnosed asplenia in the United States. Vaccine. 2021;39(2):272–81. doi:10.1016/j.vaccine.2020.11.068.
- Ghaswalla PK, Marshall GS, Bengtson LGS, Buikema AR, Bancroft T, Koep E, Novy P, Hogea CS. Meningococcal vaccination rates among people with a new diagnosis of HIV infection in the US. JAMA Netw Open. 2022;5(4):e228573. doi:10.1001/jamanetworkopen.2022.8573.
- de Oliveira Costa J, Gianacas C, Beard F, Gonzalez-Chica D, Chidwick K, Osman R, MacIntyre CR, Havard A. Cumulative annual coverage of meningococcal B vaccination in Australian general practice for three at-risk groups, 2014 to 2019. Hum Vaccin Immunother. 2021;17(10):3692–701. doi:10.1080/21645515.2021.1923349.
- Orangzeb S, Watle SV, Caugant DA. Adherence to vaccination guidelines of patients with complete splenectomy in Norway, 2008-2020. Vaccine. 2023;41(31):4579–85. doi:10.1016/j.vaccine.2023.06.034.
- Martinón-Torres F, Bernatowska E, Shcherbina A, Esposito S, Szenborn L, Marti MC, Hughes S, Faust SN, Gonzalez-Granado LI, Yu LM. et al. Meningococcal B vaccine immunogenicity in children with defects in complement and splenic function. Pediatrics. 2018;142(3):e20174250. doi:10.1542/peds.2017-4250.
- van den Broek B, van Els C, Kuipers B, van Aerde K, Henriet SS, de Groot R, de Jonge MI, Langereis JD, van der Flier M. Multi-component meningococcal serogroup B (MenB)-4C vaccine induces effective opsonophagocytic killing in children with a complement deficiency. Clin Exp Immunol. 2019;198(3):381–9. doi:10.1111/cei.13368.
- Robin C, Redjoul R, Terrade A, Deghmane AE, Cabanne L, Cordonnier C, Taha MK. Immunogenicity and safety of the meningococcal B recombinant (4CMenB) vaccine in allogeneic hematopoietic cell transplantation recipients. Clin Microbiol Infect. 2022;28(12):1609–14. doi:10.1016/j.cmi.2022.06.024.
- Isitt C, Bartolf A, Andrews N, Athaide S, Pryce-Williams R, Townsend-Payne K, Borrow R, Ladhani S, Heath PT, Cosgrove CA. The propositive study: Immunogenicity and safety of a four-component recombinant protein-based vaccine against MenB and a quadrivalent conjugate MenACWY vaccine in people living with HIV. HIV Med. 2023;24(9):979–89. doi:10.1111/hiv.13495.
- San Francisco Ramos A, Isitt C, Athaide S, Ladhani SN, Andrews NJ, Townsend-Payne K, Holland A, Louth J, Borrow R, Heath PT. et al. Propositive follow-up: Long-term immune responses to the 4CMenB and MenACWY vaccines in people living with HIV. HIV Med. 2024;25(3):370–80. doi:10.1111/hiv.13586.
- Hernando Real S, Vega Castano S, Pajares Garcia R, Meije Y. Meningococcemia in vaccinated patient under treatment with eculizumab. Enferm Infecc Microbiol Clin. 2017;35(3):200–1. doi:10.1016/j.eimc.2016.02.015.
- World Health Organization. Defeating meningitis by 2030: a global road map. 2021 [accessed 2024 Mar 15]. https://www.who.int/publications/i/item/9789240026407.
- Taha MK, Martinon-Torres F, Köllges R, Bonanni P, Safadi MAP, Booy R, Smith V, Garcia S, Bekkat-Berkani R, Abitbol V. Equity in vaccination policies to overcome social deprivation as a risk factor for invasive meningococcal disease. Expert Rev Vaccines. 2022;21(5):659–74. doi:10.1080/14760584.2022.2052048.
- Taha MK, Weil-Olivier C, Bouée S, Emery C, Nachbaur G, Pribil C, Loncle-Provot V. Risk factors for invasive meningococcal disease: a retrospective analysis of the French national public health insurance database. Hum Vaccin Immunother. 2021;17(6):1858–66. doi:10.1080/21645515.2020.1849518.
- Gras-Le Guen C, Vignier N, Kochert F, Javouhey E, Launay E, Dufour V, Gaudelus J, Launay O, Stahl JP, Tattevin P. et al. Why should the meningococcal B vaccine be recommended, and therefore reimbursed, for infants in France? Infect Dis Now. 2021;51(5):407–9. doi:10.1016/j.idnow.2021.05.001.
- Borrow R, Taha MK, Giuliani MM, Pizza M, Banzhoff A, Bekkat-Berkani R. Methods to evaluate serogroup B meningococcal vaccines: from predictions to real-world evidence. J Infect. 2020;81(6):862–72. doi:10.1016/j.jinf.2020.07.034.
- Obergfell KP, Seifert HS. Mobile DNA in the pathogenic Neisseria. Microbiol Spectr. 2015;3(1):MDNA3–0015–2014. doi:10.1128/microbiolspec.MDNA3-0015-2014.
- Rotman E, Seifert HS. The genetics of Neisseria species. Annu Rev Genet. 2014;48(1):405–31. doi:10.1146/annurev-genet-120213-092007.
- Plikaytis BD, Stella M, Boccadifuoco G, DeTora LM, Agnusdei M, Santini L, Brunelli B, Orlandi L, Simmini I, Giuliani M. et al. Interlaboratory standardization of the sandwich enzyme-linked immunosorbent assay designed for MATS, a rapid, reproducible method for estimating the strain coverage of investigational vaccines. Clin Vaccine Immunol. 2012;19(10):1609–17. doi:10.1128/cvi.00202-12.
- Donnelly J, Medini D, Boccadifuoco G, Biolchi A, Ward J, Frasch C, Moxon ER, Stella M, Comanducci M, Bambini S. et al. Qualitative and quantitative assessment of meningococcal antigens to evaluate the potential strain coverage of protein-based vaccines. Proc Natl Acad Sci USA. 2010;107(45):19490–5. doi:10.1073/pnas.1013758107.
- Muzzi A, Brozzi A, Serino L, Bodini M, Abad R, Caugant D, Comanducci M, Lemos AP, Gorla MC, Křížová P. et al. Genetic Meningococcal Antigen Typing System (gMATS): a genotyping tool that predicts 4CMenB strain coverage worldwide. Vaccine. 2019;37(7):991–1000. doi:10.1016/j.vaccine.2018.12.061.
- Medini D, Stella M, Wassil J. MATS: Global coverage estimates for 4CMenB, a novel multicomponent meningococcal B vaccine. Vaccine. 2015;33(23):2629–36. doi:10.1016/j.vaccine.2015.04.015.
- Tzanakaki G, Hong E, Kesanopoulos K, Xirogianni A, Bambini S, Orlandi L, Comanducci M, Muzzi A, Taha MK. Diversity of Greek meningococcal serogroup B isolates and estimated coverage of the 4CMenB meningococcal vaccine. BMC Microbiol. 2014;14(1):111. doi:10.1186/1471-2180-14-111.
- Vogel U, Taha MK, Vazquez JA, Findlow J, Claus H, Stefanelli P, Caugant DA, Kriz P, Abad R, Bambini S. et al. Predicted strain coverage of a meningococcal multicomponent vaccine (4CMenB) in Europe: a qualitative and quantitative assessment. Lancet Infect Dis. 2013;13(5):416–25. doi:10.1016/s1473-3099(13)70006-9.
- Waśko I, Hong E, De Paola R, Stella M, Moschioni M, Taha MK, Skoczyńska A. High predicted strain coverage by the multicomponent meningococcal serogroup B vaccine (4CMenB) in Poland. Vaccine. 2016;34(4):510–5. doi:10.1016/j.vaccine.2015.11.070.
- Bodini M, Brozzi A, Giuliani M, Nohynek H, Vainio A, Kuusi M, De Paola R, Pizza M, Medini D, Toropainen M. et al. Genomic characterization of invasive meningococcal serogroup B isolates and estimation of 4CMenB vaccine coverage in Finland. mSphere. 2020;5(5):e00376–20. doi:10.1128/mSphere.00376-20.
- Křížová P, Musílek M, Vacková Z, Kozáková J, Claus H, Vogel U, Medini D. Predicted strain coverage of a new protein-based meningococcal vaccine in the Czech Republic. Epidemiol Mikrobiol Imunol. 2014;63(2):103–6.
- Tsang RSW, Law DKS, De Paola R, Giuliani M, Stella M, Zhou J, Deng S, Boccadifuoco G, Giuliani MM, Serino L. Culture-confirmed invasive meningococcal disease in Canada, 2010 to 2014: Characterization of serogroup B Neisseria meningitidis strains and their predicted coverage by the 4CMenB vaccine. mSphere. 2020;5(2):e00883–19. doi:10.1128/mSphere.00883-19.
- Parikh SR, Newbold L, Slater S, Stella M, Moschioni M, Lucidarme J, De Paola R, Giuliani M, Serino L, Gray SJ. et al. Meningococcal serogroup B strain coverage of the multicomponent 4CMenB vaccine with corresponding regional distribution and clinical characteristics in England, Wales, and Northern Ireland, 2007-08 and 2014-15: a qualitative and quantitative assessment. Lancet Infect Dis. 2017;17(7):754–62. doi:10.1016/s1473-3099(17)30170-6.
- Mulhall RM, Bennett D, Cunney R, Borrow R, Lucidarme J, Findlow J, Jolley KA, Bray J, Maiden MCJ, Moschioni M. et al. Potential coverage of the 4CMenB vaccine against invasive serogroup B Neisseria meningitidis isolated from 2009 to 2013 in the Republic of Ireland. mSphere. 2018;3(4):e00196–18. doi:10.1128/mSphere.00196-18.
- Abad R, Medina V, Stella M, Boccadifuoco G, Comanducci M, Bambini S, Muzzi A, Vazquez JA. Predicted strain coverage of a new meningococcal multicomponent vaccine (4CMenB) in Spain: Analysis of the differences with other European countries. PLOS ONE. 2016;11(3):e0150721. doi:10.1371/journal.pone.0150721.
- Simões MJ, Bettencourt C, De Paola R, Giuliani M, Pizza M, Moschioni M, Machado J. Predicted strain coverage of a meningococcal multicomponent vaccine (4CMenB) in Portugal. PLOS ONE. 2017;12(5):e0176177. doi:10.1371/journal.pone.0176177.
- Hong E, Terrade A, Muzzi A, De Paola R, Boccadifuoco G, La Gaetana R, Deghmane AE, Pizza M, Serino L, Taha MK. Evolution of strain coverage by the multicomponent meningococcal serogroup B vaccine (4CMenB) in France. Hum Vaccin Immunother. 2021;17(12):5614–22. doi:10.1080/21645515.2021.2004055.
- Tozer SJ, Smith HV, Whiley DM, Borrow R, Boccadifuoco G, Medini D, Serruto D, Giuliani MM, Stella M, De Paola R. et al. High coverage of diverse invasive meningococcal serogroup B strains by the 4-component vaccine 4CMenB in Australia, 2007-2011: Concordant predictions between MATS and genetic MATS. Hum Vaccin Immunother. 2021;17(9):3230–8. doi:10.1080/21645515.2021.1904758.
- Freudenburg-de Graaf W, Knol MJ, van der Ende A. Predicted coverage by 4CMenB vaccine against invasive meningococcal disease cases in the Netherlands. Vaccine. 2020;38(49):7850–7. doi:10.1016/j.vaccine.2020.10.008.
- Lodi L, Moriondo M, Nieddu F, Ricci S, Guiducci S, Lippi F, Canessa C, Calistri E, Citera F, Giovannini M. et al. Molecular typing of group B Neisseria meningitidis’ subcapsular antigens directly on biological samples demonstrates epidemiological congruence between culture-positive and -negative cases: A surveillance study of invasive disease over a 13-year period. J Infect. 2021;82(4):28–36. doi:10.1016/j.jinf.2020.12.034.
- Frosi G, Biolchi A, Lo Sapio M, Rigat F, Gilchrist S, Lucidarme J, Findlow J, Borrow R, Pizza M, Giuliani MM. et al. Bactericidal antibody against a representative epidemiological meningococcal serogroup B panel confirms that MATS underestimates 4CMenB vaccine strain coverage. Vaccine. 2013;31(43):4968–74. doi:10.1016/j.vaccine.2013.08.006.
- Stella M, Giuliani M, Biolchi A, Tomei S, De Paola R, Bai X, Borrow R, Lucidarme J, La Gaetana R, Toneatto D. et al. Does vaccination with 4CMenB convey protection against meningococcal serogroup B strains not predicted to be covered by MATS? A study of the UK clonal complex cc269. Hum Vaccin Immunother. 2020;16(4):945–8. doi:10.1080/21645515.2019.1688039.
- Abad R, Biolchi A, Moschioni M, Giuliani MM, Pizza M, Vázquez JA. A large portion of meningococcal antigen typing system-negative meningococcal strains from Spain is killed by sera from adolescents and infants immunized with 4CMenB. Clin Vaccine Immunol. 2015;22(4):357–60. doi:10.1128/cvi.00669-14.
- Viviani V, Biolchi A, Pizza M. Synergistic activity of antibodies in the multicomponent 4CMenB vaccine. Expert Rev Vaccines. 2022;21(5):645–58. doi:10.1080/14760584.2022.2050697.
- Kleinschmidt A, Vadivelu K, Serino L, Neidig N, de Wergifosse B. Endogenous complement human serum bactericidal assay (enc-hSBA) for vaccine effectiveness assessments against meningococcal serogroup B. NPJ Vaccines. 2021;6(1):29. doi:10.1038/s41541-021-00286-8.
- Welsch JA, Senders S, Essink B, Klein T, Smolenov I, Pedotti P, Barbi S, Verma B, Toneatto D. Breadth of coverage against a panel of 110 invasive disease isolates, immunogenicity and safety for 2 and 3 doses of an investigational MenABCWY vaccine in US adolescents - Results from a randomized, controlled, observer-blind phase II study. Vaccine. 2018;36(35):5309–17. doi:10.1016/j.vaccine.2018.07.016.
- Muzzi A, Bodini M, Topaz N, Masignani V, Vadivelu K, Marjuki H, Wang X, Serino L, Medini D. Genetic features of a representative panel of 110 meningococcal B isolates to assess the efficacy of meningococcal B vaccines. mSphere. 2022;7(5):e0038522. doi:10.1128/msphere.00385-22.
- EU Clinical Trials Register. Clinical trial results: A phase III, randomized, controlled, observer-blind study to demonstrate effectiveness, immunogenicity and safety of GSK’s meningococcal Group B and combined ABCWY vaccines when administered to healthy adolescents and young adults. 2024 [accessed 2024 Mar 15]. https://www.clinicaltrialsregister.eu/ctr-search/trial/2019-001666-15/results.
- ClinicalTrials.gov. Effectiveness of GlaxoSmithKline Biologicals S.A’s meningococcal group B and combined ABCWY vaccines in healthy adolescents and young adults. 2024 [accessed 2024 Mar 15]. https://www.clinicaltrials.gov/ct2/show/NCT04502693.
- New York State Department Of Health AIDS Institute. Immunizations for adults with HIV. Meningococcal Serogroup B. 2023 [accessed 2024 Mar 15]. https://www.hivguidelines.org/guideline/hiv-immunizations/?mytab=tab_10&mycollection=hiv-care.
- Mahase E. Routinely offer MenB jabs to prevent gonorrhoea, UK vaccine committee recommends. BMJ. 2023;383:2696. doi:10.1136/bmj.p2696.
- Pinto Cardoso G, Lagrée-Chastan M, Caseris M, Gaudelus J, Haas H, Leroy JP, Bakhache P, Pujol JF, Werner A, Dommergues MA. et al. Overview of meningococcal epidemiology and national immunization programs in children and adolescents in 8 Western European countries. Front Pediatr. 2022;10:1000657. doi:10.3389/fped.2022.1000657.
- Herrera-Restrepo O, Clements DE, Conley WJ, Marshall GS. Expert perspectives on the vaccination of individuals who are at increased risk of meningococcal disease due to medical conditions: a podcast. Infect Dis Ther. 2023;12(4):1019–27. doi:10.1007/s40121-023-00778-1.
- Ballalai I, Dawson R, Horn M, Smith V, Bekkat-Berkani R, Soumahoro L, Vicic N. Understanding barriers to vaccination against invasive meningococcal disease: a survey of the knowledge gap and potential solutions. Expert Rev Vaccines. 2023;22(1):457–67. doi:10.1080/14760584.2023.2211163.
- McMillan M, Chandrakumar A, Wang HLR, Clarke M, Sullivan TR, Andrews RM, Ramsay M, Marshall HS. Effectiveness of meningococcal vaccines at reducing invasive meningococcal disease and pharyngeal Neisseria meningitidis carriage: a systematic review and meta-analysis. Clin Infect Dis. 2021;73(3):609–19. doi:10.1093/cid/ciaa1733.
- McMillan M, Koehler AP, Lawrence A, Sullivan TR, Bednarz J, MacLennan JM, Maiden MCJ, Ladhani SN, Ramsay ME, Trotter C. et al. B Part of it school leaver study: A repeat cross-sectional study to assess the impact of increasing coverage with meningococcal B (4CMenB) vaccine on carriage of Neisseria meningitidis. J Infect Dis. 2022;225(4):637–49. doi:10.1093/infdis/jiab444.
- Marshall HS, Andraweera PH, Ward J, Kaldor J, Andrews R, Macartney K, Richmond P, Krause V, Koehler A, Whiley D. et al. An observational study to assess the effectiveness of 4CMenB against meningococcal disease and carriage and gonorrhea in adolescents in the Northern Territory, Australia - study protocol. Vaccines (Basel). 2022;10(2):309. doi:10.3390/vaccines10020309.
- Carr J, Plested E, Aley P, Camara S, Davis K, MacLennan JM, Gray S, Faust SN, Borrow R, Christensen H. et al. ‘Be on the TEAM’ study (teenagers against meningitis): protocol for a controlled clinical trial evaluating the impact of 4CMenB or MenB-fHbp vaccination on the pharyngeal carriage of meningococci in adolescents. BMJ Open. 2020;10(10):e037358. doi:10.1136/bmjopen-2020-037358.