ABSTRACT
A maternal vaccine and long-acting monoclonal antibody (mAb) were recently approved to protect infants against respiratory syncytial virus (RSV). We identified subgroups of pregnant people with different preferences for RSV preventives and respondent characteristics associated with subgroup membership. An online survey, including a discrete choice experiment (DCE), was conducted among US pregnant people. RSV preventive attributes included effectiveness, duration of protection during RSV season, injection recipient/timing, preventive type (vaccine or mAb), and type of visit required to receive injection. In DCE choice tasks, pregnant people selected between two hypothetical preventive profiles with varying attribute-levels and a no-preventive option. Logistic regression, including latent class analysis (LCA), was used to analyze the data. Of 992 pregnant people (mean age: 30.0 years), 60.3% were expecting their second/later birth. LCA identified three preference subgroups: ‘Effectiveness’ (preventive choice mostly driven by increases in effectiveness; 51.4% class membership probability), ‘Season’ (preventive choice mostly driven by improvement in duration of protection during the RSV season; 39.2% class membership probability), and ‘No Preventive’ (frequently chose no-preventive option; 9.4% class membership probability). ‘Effectiveness’ and ‘Season’ preferred maternal vaccine over mAb; mAb was preferred by ‘No Preventive.’ Perceiving RSV as serious for infants, higher health literacy, and lower household income were associated with ‘Effectiveness.’ Perceiving RSV as serious for pregnant people was associated with ‘Season.’ Perceiving RSV to not be serious for pregnant people and not being employed were associated with ‘No Preventive.’ Subgroups of pregnant people vary in preferences for RSV preventives. Most pregnant people preferred a maternal vaccine, although some may be more willing to accept alternative preventive options.
Introduction
Respiratory syncytial virus (RSV) causes lower respiratory tract infections (LRTI). For most individuals, RSV infection causes only mild symptoms, similar to the common cold. Nevertheless, RSV can result in severe illness among infants, particularly those who are born pre-term or who have other risk factors (e.g., low birth weight, <6 months of age), as well as in older adults, people with underlying cardiovascular or respiratory disease, and immunocompromised individuals.Citation1 Annually, RSV leads to approximately 58,000 hospitalizations and 100–500 deaths among children <5 years old in the United States (US).Citation2 Recently, a surge of RSV infections in the US has challenged pediatric hospital capacity and resources,Citation3 underscoring the need for preventive options to reduce the societal burden of RSV.
RSV prevention strategies for infants include maternal vaccination or administration of monoclonal antibodies (mAbs) to infants early in life.Citation4,Citation5 Maternal vaccination, which confers immunity to infants via the transmission of maternal antibodies through the placenta, is indicated for use in pregnant people during the third trimester of pregnancy;Citation6 with maternal vaccination, an infant is protected against RSV from the time of birth.Citation5
In contrast, mAbs confer immunity to the infant from the time of injection. Among currently available mAbs, the use of palivizumab is limited due to cost and the need for monthly injections during the RSV season. Long-acting mAbs (e.g., nirsevimab), which have a longer half-life than palivizumab, do not require repeated administration.Citation4 According to Advisory Committee on Immunization Practices (ACIP), nirsevimab is recommended for all infants aged <8 months who are born either during or just prior to their first RSV season, as well as for children aged 8–19 months who are entering their second RSV season if they are at higher risk of severe illness due to RSV.Citation7
Despite the urgent need to protect infants from severe RSV illness, immunization coverage for vaccine-preventable infections is suboptimal, with < 70% of pregnant people reporting that they intend to receive recommended vaccines (influenza, Tdap, or COVID-19) during their pregnancy or to have their infant immunized.Citation8,Citation9 Moreover, perceptions about vaccine safety and efficacy, knowledge and information about vaccines, and personal characteristics, such as race, ethnicity, household income, and number of children, impact pregnant people’s intentions to immunize themselves or their infant.Citation8–10 Hence, a key challenge for public health policymakers will be to overcome the many potential barriers to infant RSV immunization.
Research on preferences for RSV preventives is limited, given the very recent approval of a maternal vaccine and long-acting mAbs. A recent study found that nearly 9-in-10 pregnant people were willing to choose a preventive (maternal vaccination or infant mAb) over no preventive.Citation11 Effectiveness and duration of protection during the RSV season were the primary drivers of RSV preventive choice among pregnant people, irrespective of whether they were expecting their first or second/later birth.
Nevertheless, given the suboptimal immunization rates among pregnant people, further research is warranted to identify the characteristics of pregnant people with greater or lesser willingness to use currently available RSV preventives. Identifying preferences and priorities for infant RSV immunization among subgroups of pregnant people and understanding the characteristics of each subgroup will facilitate patient communication and the development of educational information targeted to what is important to different subgroups of pregnant people to support decisions on infant RSV prevention.
Therefore, the objective of this study was to identify subgroups of pregnant people with similar preferences and priorities for infant immunization and to identify the characteristics of each subgroup and how they vary across subgroups. To address this objective, we conducted a secondary analysis of data from Beusterien and colleagues.Citation11
Materials and methods
Study design and sample
A cross-sectional online survey, including a discrete choice experiment (DCE), was conducted between October 11 and November 11, 2022. We planned to recruit up to 1,000 pregnant people to participate in the study. Pregnant people, who were recruited via the LifePoints general panel, were included if they were aged ≥18 years, resided in the US, were currently pregnant at the time of the survey, were able to answer questions in English, and provided informed consent. To ensure diversity in key respondent characteristics, recruiting quotas were set to target approximately 400 pregnant people who were expecting their first birth and 400 expecting their second/later birth. Within each of these 2 quotas, we sought to recruit at least 100 pregnant people who were White, Black or African American, and Hispanic or Latina.
A formula used to determine sample size for aggregate level full-profile DCE modeling is nta/c > 1,000, where n is the number of respondents, t is the number of choice tasks, a is the number of alternatives (RSV preventive profiles) presented in each choice task, and c is equal to the largest number of levels (e.g., RSV preventive effectiveness could have four levels, 50%, 63%, 77%, and 90%) for any given attribute.Citation12 With a target sample size of 1,000 pregnant people, a maximum of four levels per attribute, two alternatives in each choice task, and 12 choice tasks, the formula result is 6,000, which is > 1,000, indicating that our target sample size was sufficient to estimate relatively precise preference weights from the DCE data.
Increasingly, DCEs have been used to quantify the preferences of patients, healthcare providers, and other decision-makers for health-related products, services, and outcomes, with the aim to facilitate stakeholder involvement in healthcare decision-making.Citation13 Regarding childhood immunization specifically, DCE studies often focus on parents’ preferences for having their children vaccinated and most frequently assess vaccine uptake and willingness to pay for vaccines.Citation14 The DCE approach stipulates that preventive options can be decomposed into features of interest, referred to as attributes.Citation15 The attractiveness of a preventive product to an individual depends on their relative preferences for the attributes associated with that particular product, which is expressed by the frequency with which they choose RSV preventive profiles with the preferred features in a series of choice tasks. This method allows us to quantify how important each attribute is in influencing pregnant people’s choice of RSV preventive and the trade-offs that they are willing to make among the attributes of RSV preventive options.
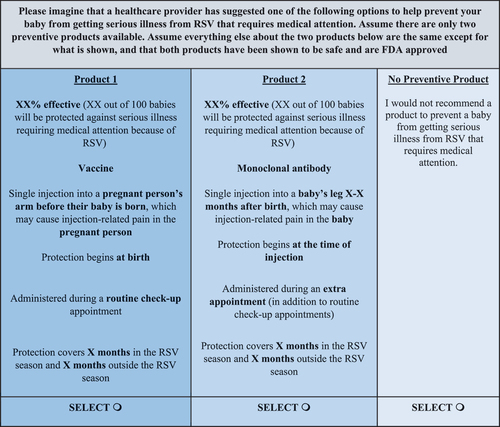
RSV preventive attributes of potential importance to pregnant people that are likely to differ among preventive options were identified via literature search, confirmed during qualitative interviews, and included in the DCE. presents the attributes and levels included in the DCE choice tasks. Prior to completing the DCE, pregnant people were presented with information about RSV and RSV preventive attributes.Citation16 Pregnant people completed a series of 12 DCE choice tasks, each including two hypothetical RSV preventive profiles with varying levels of each attribute and a no-preventive opt-out option. An example DCE choice task is shown in .
Table 1. Discrete choice experiment attributes and levels.
The survey also included questions regarding respondent characteristics including: sociodemographic characteristics (e.g., age, income, education, race/ethnicity); perceptions about the seriousness of RSV infection for pregnant people and for infants (rated from 1 = not at all serious to 5 = extremely serious, or ‘don’t know’); social support (“How easy is it for you to get help from family, friends, or neighbors if you need it?,” rated from 1 = very difficult to 5 = very easy); health literacy (“How confident are you filling out medical forms by yourself?,” rated from: 1 = extremely to 5 = not at all);Citation23 and number of prior births (first birth vs. second/later birth).
Study participants provided informed consent electronically. The final study protocol and informed consent documents were submitted to Sterling IRB (Atlanta, Georgia, USA), and an exemption determination was granted on 30 June 2022 (IRB ID #: 10193-MMaculaitis).
Statistical analysis
The analysis involved three steps: 1) a latent class analysis (LCA) to identify different subgroups or classes within the population who may have different distributions of preferences; 2) estimation of the probability of choosing between one of two preventive profiles versus the no-preventive option by latent class; and 3) examination of the relationship between individual respondent characteristics and the probability of membership in each of the LCA classes by regressing class membership probability on respondent characteristics.Citation24
The LCA involved using the DCE data to assess potential RSV preventive product preference heterogeneity among pregnant people.Citation25–27 The procedure for LCA involves running the Expectation-Maximization (EM) algorithm, which uses conditional logistic regression.Citation28 The EM algorithm first uses conditional logistical regression to fit a latent class solution conditioned on a specified number of classes and yields preference weights for each attribute-level in the DCE for each class. Second, the EM algorithm assigns a probability to each individual of belonging to each latent class. In this study, the LCA evaluated solutions for 1 to 5 latent classes. Identification of the optimal number of latent classes was based on the Bayesian Information Criteria (BIC), where lower values, as well as larger decreases in value with each subsequent class, indicate a more optimal solution and the size and differences of the preference weights across classes in each solution.Citation29
Based on the attribute-level preference weights generated for each latent class from the LCA, attribute relative importance (RI) estimates were computed for each latent class by dividing the range of preference weights for each attribute (preference weight of the most preferred level minus preference weight of the least preferred level) by the sum of the ranges of all attributes and standardizing to a 0–100% scale, where higher values indicate greater importance to RSV preventive choice.
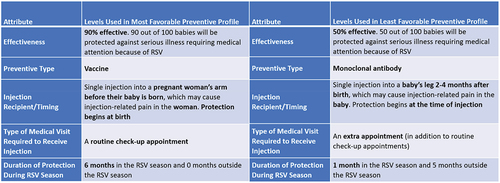
For each latent class, we estimated the probability of choosing two specific treatment profiles versus the no-preventive option using a preference share logit model. The profiles reflected a preventive profile that comprised all of the most favorable attribute-levels, based on the mean preference weights for the full sample (Most Favorable Preventive Profile), and a profile comprising all of the least favorable levels based on the mean preference weights for the full sample (Least Favorable Preventive Profile) ().Citation11 Within each class, attribute-level preference weights from the LCA were used to compute the probability of selecting the most favorable or least favorable preventative profiles versus the ‘no-preventative’ option.
Respondent characteristics, both for the total sample of pregnant people and weighted by the probability of membership in each class, were reported via descriptive statistics. A one-way analysis of variance (ANOVA) test was used to compare across latent classes on age, with chi-square tests used to compare across latent classes on all other respondent characteristics.Citation30 P-values <0.05, 2-tailed, were considered statistically significant.
A separate multinomial logistic regression model was used to predict the probability of latent class membership among potential predictors.Citation31 The potential set of independent predictor variables were prespecified and included sociodemographic characteristics, perceptions about the seriousness of RSV infection for pregnant people and for infants, social support, health literacy, and number of prior births. To select the independent variables, we first examined intercorrelations among the set of respondent characteristics (Spearman’s rho and phi coefficients). We then input the set of respondent characteristics into the multinomial regression model. All variables with p < .25 were retained in the final model, given that stricter thresholds for covariate selection may not identify all meaningful predictors.Citation32,Citation33 For this analysis, adjusted odds ratios (ORs) and 95% confidence intervals (CIs) are reported. This analysis allowed us to examine the independent contribution of each patient characteristic to latent class membership, while controlling for the effects of all other variables in the model.
Results
Overall, 992 pregnant people completed the study survey and were included in the analyses. reports respondent characteristics for the total sample and probability-weighted respondent characteristics by latent class. Nearly 40% of pregnant people in the total sample were expecting their first birth, with the majority (60.3%) expecting their second/later birth. A majority identified as White (69.9%), and approximately 1-in-5 pregnant people in the total sample identified as Black/African American.
Table 2. Respondent characteristics by latent class.
The LCA identified three classes with distinct preferences: Class 1 (‘Effectiveness Group’), Class 2 (‘Season Group’), and Class 3 (‘No Preventive Group’). The racial distribution differed across latent classes (p = .025), with the No Preventive Group having the highest proportion of Black/African American pregnant people (27.6%), followed by the Season Group (23.7%), and the Effectiveness Group (16.9%).
Additionally, the distributions for not employed (42.4%, 25.7%, and 25.3% for No Preventive Group, Effectiveness, and Season Group, respectively), household income of $90,001–$150,000 (21.1%, 15.7%, and 8.4% for Season Group, Effectiveness Group, and No Preventive Group, respectively), residing in an urban area (31.0%, 21.0%, and 19.8% for Season Group, Effectiveness Group, and No Preventive Group, respectively), and individual/family plan insurance (54.8%, 46.0%, and 38.3% for Effectiveness Group, Season Group, and No Preventive Group, respectively) differed across latent classes (all, p < .05). For items assessing health literacy and the perceived seriousness of RSV for infants and pregnant people, the distribution of some, but not all, response options significantly differed across latent classes (all, p < .05). No other differences in respondent characteristics were observed.
Preferences by latent class
presents the estimated preference weights for each DCE attribute level by latent class, and presents the attribute RI estimates by latent class. Overall, the biggest differences between latent classes were observed on effectiveness and duration of protection during RSV season. Although smaller in absolute terms, there were also differences between latent classes on injection recipient/timing and preventive type, with the levels of these two attributes varying in rank order by latent class. Specifically, vaccine was preferred to mAb in the Effectiveness Group and the Season Group, while mAb was preferred to vaccine in the No Preventive Group. In addition, earlier infant injection (≤14 days after birth) was preferred to later infant injection (2–4 months after birth) in the Effectiveness Group, while later infant injection was preferred in the No Preventive Group. There were no differences between latent classes in type of medical visit required to receive injection.
Figure 1. Attribute-level preference weights by latent class.
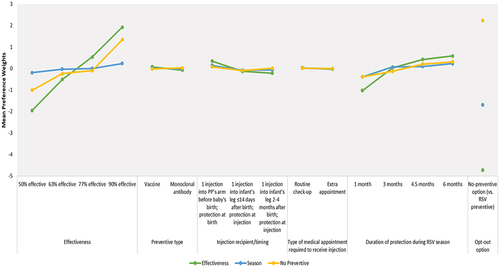
Figure 2. Attribute relative importance by latent class.
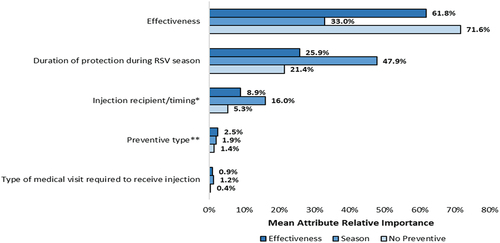
Class 1 (‘Effectiveness Group’), constituting 51.4% of overall class membership probability, prioritized greater effectiveness versus changes in all other DCE attributes (largest difference in preference weights [∆] was for a change from ‘50% effective’ to ‘63% effective:’ 1.95-[−0.51] = 1.44). An increase from ‘50% effectiveness’ to ‘90% effectiveness’ (RI = 61.8%) was most important to those in the Effectiveness Group; effectiveness was at least 2.4 times as important as each of the other attributes.
Class 2 (‘Season Group’), constituting 39.2% of overall class membership probability, prioritizes duration of protection during the RSV season over changes in the other DCE attributes (∆ for change from ‘1 month’ to ‘3 months:’ 0.07–[−0.39] = 0.46). An increase from ‘1 month’ to ‘6 months’ of protection during the RSV season (RI = 47.9%) was most influential to preventive choice among pregnant people in the Season Group and was at least 1.5 times as important as each of the other attributes. Additionally, changes in injection recipient/timing were more important to pregnant people in the Season Group, relative to those in the Effectiveness Group and No Preventive Group (RI = 16.0% vs. 8.9% and 5.3%, respectively). For all attributes, the preference weights for the Season Group were relatively flat; although the Season Group was most sensitive to improvements in duration of protection during the RSV season, they were still the least sensitive to this attribute across the three latent classes.
Class 3 (‘No Preventive Group’), constituting 9.4% of overall class membership probability, was most likely to choose the no-preventive option over a preventive option, regardless of the combination of attributes. The positive mean value of the preference weight for the no-preventive option (2.22) indicated that pregnant people in Class 3, on average, preferred the no-preventive option versus an RSV preventive. Nevertheless, improving effectiveness from ‘50% effective’ to ‘90% effective’ (RI = 71.6%) was of greatest importance to pregnant people in this group and was at least 3.3 times as important as each of the other attributes.
Opt-out versus preventive profile choice by latent class
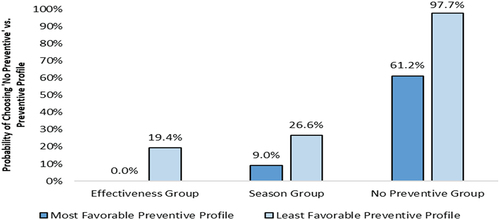
The probability of choosing the no-preventive option over an RSV preventive profile for each latent class is shown in . For the Effectiveness Group, 0.0% and 19.4% were estimated to choose the no-preventive option over the most and least favorable RSV preventive profiles, respectively. For the Season group, 9.0% and 26.6% were estimated to choose the no-preventive option over the most and least favorable RSV preventive profiles, respectively. It was estimated that 61.2% and 97.7% of those in the No Preventive Group would choose the no-preventive option over the most and least favorable preventive profiles, respectively.
Collectively, these results indicate that when an RSV preventive has highly preferred features, nearly all pregnant people in the Effectiveness Group and the Season Group would likely choose an RSV preventive. Furthermore, even with lower effectiveness, shorter duration of protection during RSV season, and other less preferred features, most pregnant people in the Effectiveness Group and the Season Group would still choose an RSV preventive. Most pregnant people in the No Preventive Group are anticipated to opt-out of immunization, regardless of the features of a given RSV preventive option. Yet, when an RSV preventive has highly preferred features, nearly 4-in-10 pregnant people in the No Preventive Group would potentially be willing to choose an RSV preventive.
Predictors of latent class membership
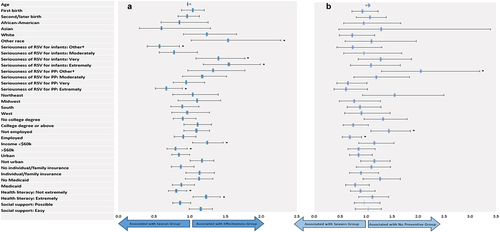
The respondent characteristics variables shown in were input into the LCA model to evaluate their associations with latent class membership in terms of adjusted ORs; the Season Group was treated as the reference group for the outcome variable; therefore, the likelihood of being in either the Effectiveness Group or No Preventive Group is expressed as compared to the Season Group. Pregnant people who perceived RSV as ‘very’ or ‘extremely’ serious for infants were significantly more likely to be members of the Effectiveness Group (ORs = 1.39, 95% CI: 1.09–1.77 and 1.54, 95% CI: 1.19–1.99, respectively), whereas those who perceived RSV to be ‘extremely’ serious for pregnant people were significantly less likely (OR = 0.68, 95% CI: 0.51–0.90) to be members of the Effectiveness Group (). Pregnant people who had high health literacy (‘extremely,’ OR = 1.22, 95% CI: 1.04–1.43) and household income <$60k (OR = 1.23, 95% CI: 1.04–1.47) were significantly more likely to be members of the Effectiveness Group. Those who perceived RSV as ‘not at all/slightly’ serious for pregnant people and pregnant people who were not employed were significantly more likely to be members of the No Preventive Group (ORs = 2.03, 95% CI: 1.30–3.19 and 1.42, 95% CI: 1.09–1.86, respectively).
Figure 3. Association of respondent characteristics and latent class membership for (a) effectiveness group versus the season group and (b) no preventive group versus the season group.
Discussion
The current study offers important insights on the factors underlying the willingness of pregnant people to immunize their infants against RSV. This is the first study to identify unique segments of pregnant people who differ in RSV preventive preferences. This analysis revealed three segments of pregnant people with different degrees of willingness to use RSV preventives. For one group (~51% of overall class membership probability), RSV preventive choice was mostly influenced by increases in effectiveness (‘Effectiveness Group’).
Conversely, there was a group of pregnant people (~39% of overall class membership probability) for whom RSV preventive choice was driven by improvement in duration of protection during the RSV season (‘Season Group’). Lastly, a smaller group of pregnant people (<10% of overall class membership probability), who frequently chose to opt-out of RSV immunization, was identified (‘No Preventive Group’). Collectively, these findings suggest that the choice to immunize infants against RSV is a function of both the attributes of the preventive options and the characteristics of the pregnant person. Notably, while the attributes of available RSV preventive options are fixed, the characteristics of pregnant people can potentially be used to inform targeted educational efforts to increase immunization coverage.
A recent study conducted by Gidengil and colleagues found that positive attitudes about vaccines, receiving/intending to receive certain immunizations (Tdap and influenza), and awareness that an infant will likely contract RSV in the first year of life were related to greater willingness among pregnant people to use an RSV preventive; living in the US South (vs. Northeast) was associated with less willingness to use an RSV preventive.Citation33 In contrast to Gidengil et al.,Citation34 our study relates pregnant person characteristics to both willingness to immunize and to the features of preventive options that drive willingness to immunize and preventive choice. Our results indicated that latent classes differed most on the importance of effectiveness and duration of protection during the RSV season; smaller differences were observed on injection recipient/timing and preventive type, with attribute-levels switching in rank order. Type of medical visit required to receive injection was least influential to preventive choice among all three latent classes.
Furthermore, we found that perceiving RSV as serious for infants was associated with membership in the Effectiveness Group, whereas perceiving RSV to be serious for pregnant people was associated with being a member of the Season Group. Having a high level of health literacy and lower household income were also correlated with membership in the Effectiveness Group. Perceiving RSV to not be serious for pregnant people and not being employed were associated with membership in the No Preventive Group. By determining these relationships, the present study not only identified additional characteristics than those reported by Gidengil et al.,Citation34 but also showed how those characteristics relate to the RSV preventive priorities of pregnant people.
Notably, a preventive with high effectiveness was strongly preferred by pregnant people in the No Preventive Group, explaining nearly three-quarters of the variation in their RSV preventive choice. These results suggest that, among the pregnant people who are less likely to choose a preventive, but who are willing to consider a preventive (as opposed to those who would never choose a preventive), efficacy is the primary driver of their decision. Likewise, efficacy was of greatest importance to preventive choice among the Effectiveness Group and was second most important among the Season Group, explaining 62% and 33% of the variation in preventive choice, respectively, for pregnant people in these two groups. These findings are consistent with previous DCE studies showing that vaccine effectiveness is highly influential to parents’ choice to vaccinate their children.Citation35,Citation36
The pattern of attribute-level preference weights indicated a preference for maternal vaccine over mAb among pregnant people in the Effectiveness Group and the Season Group, which together comprise over 90% of overall class membership probability. In contrast, mAb was preferred over maternal vaccine by those in the No Preventive Group, which accounts for about 10% of overall class membership probability. As such, it is possible that those who are reluctant to vaccinate may not have the same aversion to a preventive that is not a vaccine. Nonetheless, among all three groups of pregnant people, preventive type had relatively little influence on preventive choice compared to the other attributes included in the study.
It has been projected that the adoption of RSV preventives will reduce RSV hospitalizations in infants by up to 50%.Citation37 Still, sufficient uptake will be paramount to realizing these potential public health benefits. The current study’s findings suggest that healthcare providers and public health officials should emphasize RSV preventive effectiveness when advising pregnant people about immunization, especially among those who might otherwise be hesitant to seek immunization, including individuals who are not employed and those who do not recognize the seriousness of RSV for pregnant people. For a subset of pregnant people, it will also be important to highlight the duration of protection for different preventive options. Maternal vaccine was preferred by most pregnant people in this study; yet, among some pregnant people who are reluctant to receive vaccination, other preventive options, including long-acting mAbs, may potentially be viewed as more acceptable.
Limitations
The DCE did not include all factors that could potentially influence the preferences of pregnant people regarding RSV preventives, such as preventive product safety. Nevertheless, prior DCE studies on the preferences of parents for vaccinating their children have found that safety-related attributes are among the least influential drivers of vaccine choice.Citation35,Citation36 Further, vaccines must be demonstrably safe before they will be granted FDA approval in the US. Nonetheless, we cannot exclude the possibility that perceptions about safety could also be reflected in the preference of the No Preventive Group for mAbs over maternal vaccine to the extent that concerns about vaccine safety overshadow any concerns about the safety of mAbs.
Moreover, pregnant people may prefer to choose to opt-out of immunization because they are concerned about the safety of both types of preventives, either for themselves and/or their infant. Should this be the case, we would expect that a preventive with an unfavorable safety profile could increase the probability of opting out, particularly among those in the No Preventive Group, although this will need to be verified in future research specifically focusing on RSV preventives in the context of maternal and infant immunization.
Factors associated with preterm birth may influence the RSV preventive preferences of pregnant people, including having a previous preterm birth, a newborn with health issues, or problems with the present pregnancy that increase the risk of preterm birth. Given preterm infants are at greater risk of severe illness from RSV, discussing RSV prevention options with all pregnant people, but particularly those at increased risk for preterm birth, may be especially important. Future research will be needed to understand how these factors influence the RSV preventive preferences of pregnant people who are at higher risk for preterm birth to further inform public health education and communication efforts.
Hypothetical preventive profiles may differ from the clinical profiles of currently available preventives, although the profiles presented in the DCE aligned with the data published on those products at the time the study was conducted. Of note, hypothetical preventive profiles did not include an attribute to represent availability or accessibility of preventives, which could influence real-world preventive choice, given the limited supplies of nirsevimab for the 2023–2024 RSV season.Citation38 It is also possible that the implementation of an alternative approach to evaluate preference heterogeneity (e.g., random-parameters logit models) may have yielded a somewhat different pattern of correlations between preferences and respondent characteristics,Citation25 although the approach applied in the current study (LCA) was appropriate for addressing the research questions of interest with the observed data. At the same time, there is no reason to believe that the overall results and conclusions from an alternative approach would materially differ from those found in the current study. While the use of a convenience sample may potentially limit generalizability of results to the broader population of pregnant people in the US, the sample of pregnant people in the current study was generally similar to US national data in terms of age, number of prior births, education, and race.Citation39,Citation40
Conclusions
The current study provided evidence of naturally occurring subgroups of pregnant people who have similar RSV preventive preferences. Specifically, three segments of pregnant people who varied in their receptivity to RSV preventives were identified. RSV preventive effectiveness was important to preventive choice for all groups, although for some pregnant people, duration of protection during RSV season was the foremost consideration. While nearly all pregnant people preferred a maternal vaccine, a subset who did not prefer vaccination may potentially be more willing to accept alternative preventive options. This study not only identified the target groups who may be less willing to immunize but also facilitated a better understanding of the barriers to immunization that could be influenced by targeted information or education. Our findings suggest that patient educational efforts should focus on improving knowledge and awareness about the seriousness of RSV among pregnant people who are not currently employed to potentially increase the willingness to accept an RSV preventive option among the subset of pregnant people who are most likely to opt-out of immunization. Ultimately, these findings can help to inform the development of targeted public health initiatives, which appeal to subpopulations of pregnant people by differentially emphasizing effectiveness and duration of protection during RSV season, to increase uptake of RSV preventives and to ensure that immunization decision-making is in accord with pregnant people’s values and preferences.
Author contribution statement
All authors contributed to the conception and design of the study, interpretation of the data, and revising the manuscript critically for intellectual content. OW was responsible for data analysis; MCM was responsible for drafting the initial version of the manuscript. All authors approve of the version to be published and agree to be accountable for all aspects of the work.
Acknowledgments
The authors acknowledge Kari Yacisin and Jessica Atwell (employees of Pfizer Inc.) for their contributions to the various phases of the project.
Disclosure statement
MCM, KMB, OW, and LK are employees of Oracle Life Sciences, which received funds from Pfizer for study conduct and manuscript development. BH, AWL, JTV, JCC, JRC, and SP are employees of Pfizer and may own Pfizer stock; KMS was an employee of Pfizer at the time the study was conducted and may own Pfizer stock.
Additional information
Funding
References
- Centers for Disease Control and Prevention (CDC). RSV in infants and young children; 2022 Oct 28 [accessed 2023 May 26]. https://www.cdc.gov/rsv/high-risk/infants-young-children.html.
- Centers for Disease Control and Prevention (CDC). Increased interseasonal respiratory syncytial virus (RSV) activity in parts of the southern United States. 2021 June 10 [accessed 2023 May 26]. https://emergency.cdc.gov/han/2021/han00443.asp.
- Abbasi J. “This is our COVID”-what physicians need to know about the pediatric RSV surge. JAMA. 2022;328(21):2096–13. doi:10.1001/jama.2022.21638.
- Hammitt LL, Dagan R, Yuan Y, Baca Cots M, Bosheva M, Madhi SA, Muller WJ, Zar HJ, Brooks D, Grenham A, et al. Nirsevimab for prevention of RSV in healthy late-preterm and term infants. N Engl J Med. 2022 Mar 3. 386(9):837–46. doi:10.1056/NEJMoa2110275.
- Kampmann B, Madhi SA, Munjal I, Simões EAF, Pahud BA, Llapur C, Baker J, Pérez Marc G, Radley D, Shittu E, et al. Bivalent prefusion F vaccine in pregnancy to prevent RSV illness in infants. N Engl J Med. 2023 Apr 20. 388(16):1451–64. doi:10.1056/NEJMoa2216480.
- Food and Drug Administration (FDA). FDA approves first vaccine for pregnant individuals to prevent RSV in infants; 2023 Aug 21 [accessed 2023 Nov 13]. https://www.fda.gov/news-events/press-announcements/fda-approves-first-vaccine-pregnant-individuals-prevent-rsv-infants#:~:text=Abrysvo%20is%20approved%20for%20use,years%20of%20age%20and%20older.
- Jones JM, Fleming-Dutra KE, Prill MM, Roper LE, Brooks O, Sánchez PJ, Kotton CN, Mahon BE, Meyer S, Long SS, et al. Use of nirsevimab for the prevention of respiratory syncytial virus disease among infants and young children: recommendations of the advisory committee on immunization practices — United States. MMWR Morb Mortal Wkly Rep. 2023;72(34):920–5. doi:10.15585/mmwr.mm7234a4.
- Dudley MZ, Limaye RJ, Omer SB, O’Leary ST, Ellingson MK, Spina CI, Brewer SE, Chamberlain AT, Bednarczyk RA, Malik F, et al. Characterizing the vaccine knowledge, attitudes, beliefs, and intentions of pregnant women in Georgia and Colorado. Hum Vaccin Immunother. 2020 May 3;16(5):1109–17. doi:10.1080/21645515.2020.1717130.
- Skirrow H, Barnett S, Bell S, Riaposova L, Mounier-Jack S, Kampmann B, Holder B. Women’s views on accepting COVID-19 vaccination during and after pregnancy, and for their babies: a multi-methods study in the UK. BMC Pregnancy Childbirth. 2022 Jan 14;22(1):33. doi:10.1186/s12884-021-04321-3.
- Dudley MZ, Limaye RJ, Salmon DA, Omer SB, O’Leary ST, Ellingson MK, Spina CI, Brewer SE, Bednarczyk RA, Malik F, et al. Racial/ethnic disparities in maternal vaccine knowledge, attitudes, and intentions. Public Health Rep. 2021;136(6):699–709. doi:10.1177/0033354920974660.
- Beusterien KM, Law AW, Maculaitis MC, Will O, Kopenhafer L, Olsen P, Hauber B, Vietri JT, Cappelleri JC, Coulter JR, et al. Healthcare providers’ and pregnant people’s preferences for a preventive to protect infants from serious illness due to respiratory syncytial virus. Vaccines (Basel). 2024;12(5):560. doi:10.3390/vaccines12050560.
- Orme B. Getting started with conjoint analysis: strategies for product design and pricing research. 4th ed. Madison (WI): Research Publishers LLC; 2019.
- Soekhai V, de Bekker-Grob EW, Ellis AR, Vass CM. Discrete choice experiments in health economics: past, present and future. Pharmacoeconomic. 2019;37(2):201–26. doi:10.1007/s40273-018-0734-2.
- Michaels-Igbokwe C, MacDonald S, Currie GR. Individual preferences for child and adolescent vaccine attributes: a systematic review of the stated preference literature. Patient. 2017;10(6):687–700. doi:10.1007/s40271-017-0244-x.
- Wang Y, Wang Z, Wang Z, Li X, Pang X, Wang S. Application of discrete choice experiment in health care: a bibliometric analysis. Front Public Health. 2021 June 4;9:673698. doi:10.3389/fpubh.2021.673698.
- Centers for Disease Control and Prevention (CDC). Respiratory syncytial virus infection (RSV). 2022 Oct 28 [accessed 2023 May 26]. https://www.cdc.gov/rsv/index.html.
- Etti M, Calvert A, Galiza E, Lim S, Khalil A, Le Doare K, Heath PT. Maternal vaccination: a review of current evidence and recommendations. Am J Obstet Gynecol. 2022 Apr;226(4):459–74. doi:10.1016/j.ajog.2021.10.041.
- Griffin MP, Yuan Y, Takas T, Domachowske JB, Madhi SA, Manzoni P, Simões EAF, Esser MT, Khan AA, Dubovsky F, et al. Single-dose nirsevimab for prevention of RSV in preterm infants. N Engl J Med. 2020 Jul 30;383(5):415–25. doi:10.1056/NEJMoa1913556.
- Muňoz FM, Swamy GK, Hickman SP, Agrawal S, Piedra PA, Glenn GM, Patel N, August AM, Cho I, Fries L, et al. Safety and immunogenicity of a respiratory syncytial virus fusion (F) protein nanoparticle vaccine in healthy third-trimester pregnant women and their infants. J Infect Dis. 2019 Oct 22;220(11):1802–15. doi:10.1093/infdis/jiz390.
- World Health Organization (WHO). WHO preferred product characteristics for respiratory syncytial virus (RSV) vaccines. 2017 [accessed 2022 Mar 24]. https://www.who.int/publications/i/item/WHO-IVB-17.11.
- Schwarz TF, Johnson C, Grigat C, Apter D, Csonka P, Lindblad N, Nguyen TLA, Gao FF, Qian H, Tullio AN, et al. Three dose levels of a maternal respiratory syncytial virus vaccine candidate are well tolerated and immunogenic in a randomized trial in nonpregnant women. J Infect Dis. 2022 June 15;225(12):2067–76. doi:10.1093/infdis/jiab317.
- Baral R, Higgins D, Regan K, Pecenka C. Impact and cost-effectiveness of potential interventions against infant respiratory syncytial virus (RSV) in 131 low-income and middle-income countries using a static cohort model. BMJ Open. 2021 Apr 24;11(4):e046563. doi:10.1136/bmjopen-2020-046563.
- Chew LD, Bradley KA, Boyko EJ. Brief questions to identify patients with inadequate health literacy. Fam Med. 2004 Sep;36(8):588–94.
- Hauber AB, González JM, Groothuis-Oudshoorn CG, Prior T, Marshall DA, Cunningham C, IJzerman MJ, Bridges JFP. Statistical methods for the analysis of discrete choice experiments: a report of the ISPOR conjoint analysis good research practices task force. Value Health. 2016 June;19(4):300–15. doi:10.1016/j.jval.2016.04.004.
- Boeri M, Saure D, Schacht A, Riedl E, Hauber B. Modeling heterogeneity in patients’ preferences for psoriasis treatments in a multicountry study: a comparison between random-parameters logit and latent class approaches. Pharmacoeconom. 2020;38(6):593–606. doi:10.1007/s40273-020-00894-7.
- Vass C, Boeri M, Karim S, Marshall D, Craig B, Ho K-A, Mott D, Ngorsuraches S, Badawy SM, Mühlbacher A, et al. Accounting for preference heterogeneity in discrete-choice experiments: an ISPOR special interest group report. Value in Health. 2022;25(5):685–94. doi:10.1016/j.jval.2022.01.012.
- Zhou M, Thayer WM, Bridges JFP. Using latent class analysis to model preference heterogeneity in health: a systematic review. Pharmacoeconom. 2018;36(2):175–87. doi:10.1007/s40273-017-0575-4.
- Dempster AP, Laird NM, Rubin DB. Maximum likelihood from incomplete data via the EM algorithm. J R Stat Soc Series B Stat Methodol. 1977;39(1):1–22. doi:10.1111/j.2517-6161.1977.tb01600.x.
- Raftery AE. Bayesian model selection in social research. Sociol Methodol. 1995;25:111–63. doi:10.2307/271063.
- Rosner B. Fundamentals of biostatistics. 8th ed. Boston (MA): Cengage Learning; 2015.
- Kwak C, Clayton-Matthews A. Multinomial logistic regression. Nurs Res. 2002;51(6):404–10. doi:10.1097/00006199-200211000-00009.
- Bendel RB, Afifi AA. Comparison of stopping rules in forward “stepwise” regression. J Am Stat Assoc. 1977;72(357):46–53. doi:10.1080/01621459.1977.10479905.
- Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989;129(1):125–37. doi:10.1093/oxfordjournals.aje.a115101.
- Gidengil C, Jones J, Pike J, Prill M, Wodi P, Lindley M, Gedlinske A, Parker A, Scherer A. Willingness to receive maternal RSV vaccine and infant monoclonal RSV antibody. Poster presented at the Infectious Diseases Society for Obstetrics & Gynecology (IDSOG) Annual Meeting; 2023 Jul 27–29; Denver, CO, USA.
- Hoogink J, Verelst F, Kessels R, Van Hoek AJ, Timen A, Willem L, Beutels P, Wallinga J, De Wit GA. Preferential differences in vaccination decision-making for oneself or one’s child in the Netherlands: a discrete choice experiment. BMC Public Health. 2020;20(1):828. doi:10.1186/s12889-020-08844-w.
- Lavelle TA, Messonnier M, Stokley S, Kim D, Ramakrishnan A, Gebremariam A, Simon NJE, Rose AM, Prosser LA. Use of a choice survey to identify adult, adolescent and parent preferences for vaccination in the United States. J Patient Rep Outcomes. 2019;3(1):51. doi:10.1186/s41687-019-0135-0.
- Treskova M, Pozo-Martin F, Scholz S, Schönfeld V, Wichmann O, Harder T. Assessment of the effects of active immunisation against respiratory syncytial virus (RSV) using decision-analytic models: a systematic review with a focus on vaccination strategies, modelling methods and input data. Pharmacoeconomics. 2021;39(3):287–315. doi:10.1007/s40273-020-00991-7.
- Centers for Disease Control and Prevention (CDC). Limited availability of nirsevimab in the United States—interim CDC recommendations to protect infants from respiratory syncytial virus (RSV) during the 2023–2024 respiratory virus season. 2023 Oct 23 [accessed 2023 Dec 14]. https://emergency.cdc.gov/han/2023/han00499.asp.
- Centers for Disease Control and Prevention (CDC). National vital statistics reports - births: final data for 2020. 2022 Jul 2 [accessed 2023 Mar 25]. https://stacks.cdc.gov/view/cdc/112078.
- Centers for Disease Control and Prevention (CDC). National vital statistics reports - natality on CDC WONDER online database 2016-2021. 2022 June 17 [accessed 2023 Mar 25]. http://wonder.cdc.gov/natality-expanded-current.html.
Appendix
A
Figure A1. Example discrete choice experiment choice task.