ABSTRACT
To assess the impact of vaccines on clinical outcomes among hospitalized COVID-19-infected patients requiring oxygen supplementation during the Beijing Omicron outbreak. We conducted a retrospective cohort study at Beijing Chaoyang Hospital, Capital Medical University, from November 15, 2022, to March 31, 2023. Vaccination statuses were categorized into 3 doses, 2 doses, and unvaccinated (0 dose). The primary outcome was 28-day all-cause mortality. Secondary outcomes included poor outcomes, intensive care unit admission, cardiovascular thromboembolism events, and hospital readmission. Among the included patients, 117 were 2 doses, 285 received booster doses, and 503 were unvaccinated. After propensity score inverse probability weighting, the 3 doses group showed a significantly lower 28-day all-cause mortality compared to the unvaccinated group (inverse probability of treatment weighting-adjusted HR: 0.64, 95% CI: 0.50–0.81). No significant difference was observed in all-cause mortality between the 2 doses and unvaccinated groups. No significant differences were observed in secondary outcome analyses when comparing the 3 doses or 2 doses group to the unvaccinated group. Subgroup analysis revealed significant benefits of booster vaccination in patients with shorter symptom duration, lower Charlson Comorbidity Index, and without immunosuppression status. Our study highlights the significant reduction in all-cause mortality among hospitalized Omicron-infected patients who received a third dose vaccine. These findings underscore the importance of prioritizing booster vaccinations, especially among the elderly. Further research is warranted to confirm and extend these observations.
Introduction
Since the emergence of the coronavirus disease 2019 (COVID-19), the escalating number of global infections and associated fatalities has raised grave concerns worldwide, affecting more than 700 million individuals all around the world.Citation1 To confer protection against the spread of the virus and reduce symptomatic infections, vaccines were widely distributed to prevent COVID-19 infection and progression among non-infected individuals. The positive effect of vaccines against infection, incidence of symptomatic infection, hospitalization, and all-cause mortality has been proven by lots of studies.Citation2–4
Global rates of vaccination have been greatly improved, by administrating mRNA vaccines, viral vector vaccines, protein subunit vaccines, and inactivated virus vaccines.Citation4 China’s COVID-19 vaccination strategy primarily relies on the use of inactivated vaccines, leading to a high rate of 2 doses per individual, with coverage rates of 89.8% and a boosted vaccination rate of 32.6% as of February 2022.Citation5 While the ongoing emergence of spontaneous mutations in SARS-CoV-2 variants, represented by Omicron, caused unprecedented numbers of vaccine-breakthrough infections and re-infections.Citation6,Citation7 Because of the increased infectivity and resistance to neutralizing antibodies induced by vaccination, the high vaccine coverage was insufficient to prevent the spread of Omicron. Gradually, the concern regarding the protective efficacy of vaccines has shifted from preventing infection to slowing the disease progression and reducing the severity of COVID-19.Citation8
Evidence for the effectiveness of vaccines among hospitalized COVID-19 patients, who often exhibit distinct characteristics compared to individuals with mild or moderate disease, remains insufficient. Previous studies mostly focused on specific populations, including those in the intensive care unit (ICU),Citation3,Citation9 pregnant and postpartum women,Citation10 and immunocompromised patients.Citation11 Only one study conducted in SpainCitation12 investigated the effectiveness of vaccines in patients who need oxygen support. However, the small sample size limited the strength of the evidence. Thirdly, most of the data based on non-inactivated virus vaccines, such as mRNA vaccines, are not directly applicable to countries where inactivated virus vaccines constitute the primary vaccination approach.Citation13,Citation14 In this context, it is crucial to evaluate the effectiveness of vaccines, especially inactivated vaccines, for hospitalized severe COVID-19-infected patients who need oxygen supplementation.
To build the knowledge gap for the efficacy of vaccines for infected patients with severe to critical COVID-19, we performed a retrospective analysis of all hospitalized COVID-19 patients with oxygen supplementation.
Methods
Study design
The retrospective cohort study was conducted at Beijing Chaoyang Hospital, Capital Medical University from October 15, 2022, to March 31, 2023. This study was approved by the institutional review board and ethics committee of Beijing Chaoyang Hospital, Capital Medical University (2023–2-27-10). All patients were informed of the study’s purpose by telephone. The study was registered at www.clinicaltrials.gov (NCT05792865).
Eligibility criteria and data source
We included all individuals ≥18 years old who were hospitalized with a documented COVID-19 infection. COVID-19 infection was defined as a diagnosis of COVID-19 upon hospital admission or a positive PT-PCR or rapid antigen test for SARS-CoV-2 infection on the date of admission. Exclusion criteria included: 1) did not require supplemental oxygen therapy on admission; 2) without data on vaccination; 3) received the most recent vaccination within 14 days.
Baseline characteristics, diagnoses, medications, procedures, laboratory tests, dates of ICU admission, and date of registered death, were obtained from the routine electronic health record database of the hospital information system. Results of laboratory tests that were the first test upon admission were collected. The vaccination status and missing data such as Body Mass Index (BMI) were collected via telephone. The exposure variable was vaccination status, including 3 doses, 2 doses, and unvaccinated (vaccinated with less than two doses).
Outcomes
The primary outcome was 28-day all-cause mortality. The secondary outcomes included a composite of poor outcomes including invasive mechanical ventilation and death, as well as ICU admission, cardiovascular thromboembolism events, and hospital re-admission.
Statistical analysis
Baseline demographic and clinical data are reported as the mean (standardized difference) or frequency (percentage) and compared using the chi-squared test or Student’s t-test, as appropriate. Hazard ratios (HRs) with 95% confidence intervals (CIs) for each outcome were estimated using Cox regression models. The mortality and disease progression probabilities were plotted using Kaplan – Meier curves and evaluated using the log-rank test. An inverse probability of treatment weighting (IPTW)-adjusted Cox regression model was created to adjust for potential confounding factors. Propensity scores were estimated by logistic regression according to baseline covariates. The weight of the 2 doses and 3 doses group was the inverse of (1 - propensity score), and the weight of the unvaccinated group was the inverse of the propensity score. Covariates used in the IPTW model were selected using all baseline characteristics. Standardized differences were used to assess the differences between the two groups before and after IPTW adjustment. A standardized difference value of < .10 indicated a good balance between the two groups.
Subgroup analyses were performed for the major outcome regarding age, sex, BMI, duration of symptoms to hospitalization, Charlson Comorbidity Index (CCI), and immunosuppression (defined as any diagnosis of oncology, post-organ transplantation, or rheumatic system disease).
Statistical analysis was performed using Stata version 16.0. A two-sided p-value of 0.05 was used to identify statistically significant differences.
Results
Between October 15, 2022, and March 31, 2023, a total of 1120 patients met the inclusion criteria and were enrolled in the cohort. After excluding patients, 117 and 285 patients received 2 doses and 3 doses, compared with 503 unvaccinated patients (). Baseline characteristics by vaccination status are shown in Table S1. Before IPTW was carried out, the 3 doses group showed a lower mean age (69.8 ± 13.7 years) compared with the unvaccinated group (76.5 ± 12.2 years) and the 2 doses group (76.1 ± 10.9 years) (). Both the 2 doses group and 3 doses group had a lower proportion of females at baseline (27.5% and 24.8% versus 40.2%). The prevalence of certain comorbidities varied among the groups.
Figure 1. Study population flowchart displaying the inclusion of patients and selection of cohorts according to vaccination status.
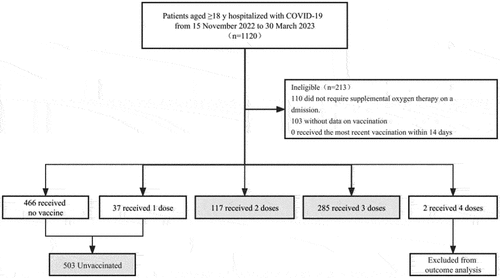
Table 1. Baseline characteristics before and after the inverse probability weighting of propensity scores.
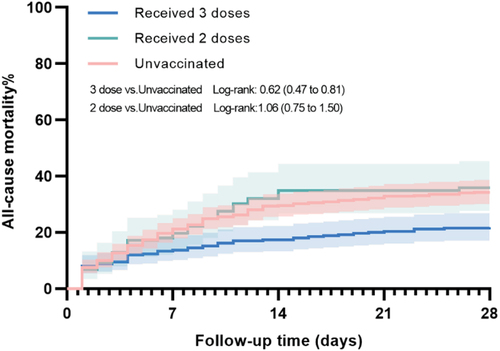
Following IPTW, reduced all-cause mortality within 28 days was found in the 3 doses group compared with the unvaccinated group with an adjusted HR of 0.64, 95%CI 0.50–0.81, unadjusted log-rank p < .001 (, ). The all-cause mortality within 14 days was also significantly also reduced in the 3 doses group compared with the unvaccinated group (IPTW-adjusted HR 0.35, 95%CI 0.25–0.49, unadjusted log-rank p = .734). There was no statistical difference between the 2 doses group and the unvaccinated group for all-cause mortality within 28 days (IPTW-adjusted HR 0.91, 95%CI 0.72–1.16) and 14 days (IPTW-adjusted HR 1.08, 95%CI 0.84–1.40).
Table 2. Clinical outcomes of hospitalized 2 doses and 3 doses COVID-19 patient compared to unvaccinated group.
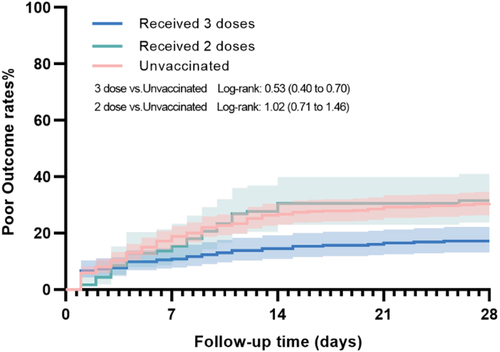
No significant differences were found in poor outcomes between booster vaccinated and unvaccinated group (IPTW-adjusted HR 0.82, 95%CI 0.67–1.01, unadjusted log-rank p < .001) or 2 doses and unvaccinated group (IPTW-adjusted HR 0.85, 95%CI 0.68–1.06, unadjusted log-rank p = .924) (, ). Similar result insignificant results were observed in the analysis regarding ventilator support and ICU admission in the two groups when compared to the unvaccinated group.
Figure 3. 28-day cumulative incidence of the poor outcome consisted of ventilator support and all-cause mortality.
Compared with the unvaccinated group, we found no association in cardiovascular thromboembolic events (IPTW-adjusted HR 0.60, 95%CI 0.23–1.56) and readmission rate (IPTW-adjusted HR 0.68, 95%CI 0.42–1.09) in the 3 doses group. No statistically significant reduction in the incidence of cardiovascular thromboembolic events (IPTW-adjusted HR 0.48, 95%CI 0.14–1.63) and readmission rate (IPTW-adjusted HR 0.55, 95%CI 0.30–1.00) was found between the 2 doses group and unvaccinated group.
As for subgroup analysis for all-cause mortality within 28 days, we found significant differences between subgroups regarding the duration of symptoms to hospitalization (p < .001), CGI score (p < .001), and immunosuppression status (p = .008) (). Booster vaccination could significantly reduce all-cause mortality within 28 days for patients with disease onset to hospitalization ≤5 days (IPTW-adjusted HR 0.61, 95%CI 0.50–0.74), CCI < 3 (IPTW-adjusted HR 0.64, 95%CI 0.53–0.78), and without immunosuppression status (IPTW-adjusted HR 0.84, 95%CI 0.77–0.91). There was a significant subgroup difference regarding immunosuppression status in the subgroup analysis comparing the 2 doses group and the unvaccinated group (p = .044).
Table 3. Subgroup analysis of all-death mortality within 28 days in 3 doses group compared with unvaccinated group.
Third dose vaccine, age, BMI, CCI, chronic lung disease, neurological disease, and nirmatrelvir-ritonavir use were risk factors associated with all-death mortality in all vaccinated patients (Table S2). Sensitivity analysis using multivarite Cox regression showed similar result with the main analysis (Table S3).
Discussion
The results of this retrospective cohort study showed the positive impact of early administration of 3 doses vaccines during the Beijing Omicron outbreak from December 2022 to March 2023. Among patients hospitalized due to Omicron infection and requiring oxygen support, those who received 3 doses of inactivated vaccine exhibited a significantly lower mortality rate compared to those who were unvaccinated. However, no statistically significant difference was observed in the 28-day all-cause mortality rate between patients who had completed 2 doses of the vaccine and the unvaccinated hospitalized population. Our findings provide compelling evidence supporting the role of booster-inactivated vaccines in improving outcomes for hospitalized patients with Omicron infection.
Our study was different from the prior study conducted in IsraelCitation13 and the United States,Citation15 which reported that among critically ill hospitalized COVID-19 patients who had not completed their vaccine regimen, older individuals tended to receive the third vaccine dose. In our cohort, those who received 3 doses were notably younger and in better health than those who received 2 doses or remained unvaccinated. The findings indicated that despite public initiatives promoting the administration of booster vaccinations, especially among older adults and high-risk individuals since late 2021, vaccine hesitancy regarding the third dose persists. This aligned with the previous research from Hong Kong,Citation16 which identified a bias in vaccine administration, with healthier individuals more likely to receive the vaccine, particularly among those aged 60 years and above.Citation17 Such inherent biases may lead to an overestimation of the third dose’s efficacy. To address this concern, we applied rigorous propensity score inverse probability weighting to balance baseline differences between the vaccinated and unvaccinated groups, reducing the impact of confounding factors. Our subgroup analysis, stratified by age, consistently reveals the protective effects of the third vaccine dose in both age groups above and below 65 years, further reinforcing the robustness and generalizability of our findings. The comprehensive evidence presented in our study enriches the public health value of the third vaccine dose, not only for its efficacy in reducing mortality among hospitalized infections but also for its potential to alleviate healthcare system burden and public apprehensions.
In our study, most of the patients received inactivated vaccines, specifically CoronaVac or Sinopharm’s vaccine, which are known for their efficacy in preventing COVID-19 infection and lowering the risk of hospitalization.Citation4,Citation16,Citation18,Citation19 However, the impact of inactivated vaccine on adverse outcomes in Omicron-infected hospitalized patients remains insufficiently investigated. A Hong Kong study compared CoronaVac and BNT162b2 efficacy concerning severe outcomes among hospitalized patients with SARS-CoV-2 Omicron infection.Citation20 The findings demonstrated vaccine effectiveness against death of 28.3%, 51.0%, and 75.9% for one, two, and three doses, respectively, suggesting potential enhancement with the third dose. Nonetheless, the case-control study design limited its level of evidence. Large-scale studies from CanadaCitation21 and IranCitation9 revealed an inverse dose-response relationship between vaccine dosage and in-hospital mortality but did not specifically examine the finding for inactivated vaccines. In contrast, a multi-center cohort investigation conducted in Spain and ArgentinaCitation12 has corroborated the efficacy of vaccines in mitigating in-hospital mortality among individuals afflicted with COVID-19 necessitating oxygen supplementation. This study also scrutinized the potency of the Sinopharm vaccine. However, due to the limited sample size, the evidence remained insufficient. Evaluating the efficacy of inactivated vaccines, widely used in China and developing countries, for hospitalized patients with Omicron infection is crucial. Our study provides evidence supporting the implementation of booster doses of inactivated vaccines to reduce all-cause mortality and poor outcomes in hospitalized patients. And further support the efficacy of inactivated vaccine for reducing mortality in hospitalized COVID-19 patients.
We did not find statistically significant improvement in the incidence of clinical outcomes when comparing the 2 doses group to the unvaccinated group. This could potentially be linked to declining vaccine-induced antibody levels over time, which aligned with prior research that suggested a waning of protection against COVID-19 in elderly patients after six months following the second vaccine dose.Citation22–24 The mean time since the last vaccine dose was 6.9 months and 5.0 months for those 2 doses and 3 doses. A parallel pattern emerged in an Israeli study exploring the efficacy of the third and fourth vaccine doses in preventing adverse outcomes among hospitalized Omicron-infected patients.Citation13 The results indicated a comparable occurrence rate of adverse events between the group receiving the third dose and the unvaccinated cohort (49% and 51%, respectively; p = .72). In contrast, patients who received four doses exhibited improved outcomes compared to the unvaccinated group (34% vs. 51%; p < .01). Our findings underscored the critical importance of continued vaccine booster administration to sustain adequate antibody levels against the novel virus variants, particularly in vulnerable populations.
The results of our data analysis showed significant interactions between the time from symptom onset to hospitalization, CCI, immunosuppressive status, and the effectiveness of the booster vaccine. Among hospitalized patients with shorter symptom-to-hospitalization intervals, lower CCI scores, and without immunosuppressive conditions, the beneficial impact of the booster dose was notably more pronounced. These findings aligned with expectations, as prior evidenceCitation25 has established a positive correlation between longer symptom-to-hospitalization intervals and heightened COVID-19 hospital mortality rates. Similarly, higher CCI scores and immunosuppressive states were associated with reduced survival rates among hospitalized patients,Citation13,Citation22 implying that the protective effect of the booster dose may be limited in these populations. Consequently, we recommend further research to robustly validate the vaccine’s efficacy in these specific populations. Moreover, our findings demonstrated the protective effects of the booster dose in both patients aged >65 years and younger ones. This finding supports the use of booster vaccine dose for older individuals, highlighting its crucial role in protecting this vulnerable population.
Most global studies focus on mRNA vaccines, and inactivated vaccines are widely used in many parts of the world, such as China, Turkey, Indonesia, Morocco, and Brazil.Citation26,Citation27 A case-control study conducted in Morocco found that the inactivated vaccine was highly protective against COVID-19 infection with vaccine effectiveness of 64% beyond the sixth month.Citation26 So far, no study has reported on the efficacy of booster-dose inactivated vaccines for hospitalized COVID-19 patients requiring oxygen supplementation outside China. Our findings could enrich the understanding of the booster vaccine’s role in other countries. Furthermore, studies investigating its efficacy in different races are needed.
Benefiting from the government’s highly effective epidemic control measures, Beijing had not experienced widespread Omicron variant outbreaks before December 2022. This study has a significant advantage as most of the included patients experienced their first Omicron infection. By contrast, previous investigationCitation9,Citation12,Citation21,Citation28 involved populations exposed to multiple waves of outbreaks, which could introduce biases in assessing vaccine efficacy due to previous viral infections and passive enhanced immune status.
Our study also has several limitations: 1) This is a single-center, retrospective study so the findings may not fully represent the vaccine’s protective efficacy during the Omicron outbreak. 2) Due to limited medical resources, some cases in our study might have been underdiagnosed. Additionally, certain patients may have been unable to be admitted to the intensive care unit due to bed shortages or may have refused invasive mechanical ventilation. These factors could result in incomplete disease progression outcomes in our study population. 3) We also excluded patients with no data on vaccine status, which could introduce some degree of bias to the study. To address this, we compared the baseline characteristics of the excluded population with those included in the final analysis and found no significant differences (Table S4).
Conclusion
Our findings revealed a significant reduction in all-cause mortality among patients who received 3 vaccine doses, compared to those who were unvaccinated. This protective effect was not found in patients who received two vaccine doses. These results highlight the importance of booster vaccination, particularly for the elderly, as a new wave of COVID-19 approaches.
Supplemental material.docx
Download MS Word (41.1 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2024.2361500
Additional information
Funding
References
- WHO. Coronavirus dashboard. [Accessed: 2023 Jaly 10]. https://covid19whoint/.
- Tsang NNY, So HC, Cowling BJ, Leung GM, Ip DKM. Effectiveness of BNT162b2 and CoronaVac COVID-19 vaccination against asymptomatic and symptomatic infection of SARS-CoV-2 omicron BA.2 in Hong Kong: a prospective cohort study. Lancet Infect Dis. 2023;23(4):421–7. doi:10.1016/S1473-3099(22)00732-0.
- Grapsa E, Adamos G, Andrianopoulos I, Tsolaki V, Giannakoulis VG, Karavidas N, Giannopoulou V, Sarri K, Mizi E, Gavrielatou E. Association between vaccination status and mortality among intubated patients with COVID-19–related acute respiratory distress syndrome. JAMA Netw Open. 2022;5(10):e2235219. doi:10.1001/jamanetworkopen.2022.35219.
- Graña C, Ghosn L, Evrenoglou T, Jarde A, Minozzi S, Bergman H, Buckley BS, Probyn K, Villanueva G, Henschke N. Efficacy and safety of COVID-19 vaccines. Cochrane Database Syst Rev. 2022;12(3):Cd015477. doi:10.1002/14651858.CD015477.
- Council JPaCMoS. Joint prevention and control mechanism of. State Council Authority publishing; https://www.gov.cn/xinwen/gwylflkjz183/index.htm.
- Murphy TJ, Swail H, Jain J, Anderson M, Awadalla P, Behl L, Brown PE, Charlton CL, Colwill K, Drews SJ. The evolution of SARS-CoV-2 seroprevalence in Canada: a time-series study, 2020–2023. Cmaj. 2023;195(31):1030–7. doi:10.1503/cmaj.230249.
- Islam MA, Shahi S, Marzan AA, Amin MR, Hasan MN, Hoque MN, Ghosh A, Barua A, Khan A, Dhama K. Variant-specific deleterious mutations in the SARS-CoV-2 genome reveal immune responses and potentials for prophylactic vaccine development. Front Pharmacol. 2023;14:1090717. doi:10.3389/fphar.2023.1090717.
- Miteva D, Kitanova M, Batselova H, Lazova S, Chervenkov L, Peshevska-Sekulovska M, Sekulovski M, Gulinac M, Vasilev GV, Tomov L. The end or a new era of development of SARS-CoV-2 virus: genetic variants responsible for severe COVID-19 and clinical efficacy of the most commonly used vaccines in clinical practice. Vaccines. 2023;11(7):1181. doi:10.3390/vaccines11071181.
- Jamaati H, Karimi S, Ghorbani F, Panahi Y, Hosseini-Baharanchi FS, Hajimoradi M, Malek R, Noorali S, Mokhtari M, Khoundabi B. Effectiveness of different vaccine platforms in reducing mortality and length of ICU stay in severe and critical cases of COVID-19 in the Omicron variant era: a national cohort study in Iran. J Med Virol. 2023;95(3):e28607. doi:10.1002/jmv.28607.
- de Freitas Paganoti C, Alkmin da Costa R, da Costa RA, da Silva Costa F, Quintana SM, Graziela de Godoi L, de Freitas Paganoti C, de Godoi LG, Adriana Jiménez Monroy N, Sacramento Rodrigues A. COVID-19 vaccines confer protection in hospitalized pregnant and postpartum women with severe COVID-19: a retrospective cohort study. Vaccines. 2022;10(5):749. doi:10.3390/vaccines10050749.
- Turtle L, Thorpe M, Drake TM, Swets M, Palmieri C, Russell CD, Ho A, Aston S, Wootton DG, Richter A. Outcome of COVID-19 in hospitalised immunocompromised patients: an analysis of the WHO ISARIC CCP-UK prospective cohort study. PLoS Med. 2023;20(1):e1004086. doi:10.1371/journal.pmed.1004086.
- Huespe IA, Ferraris A, Lalueza A, Valdez PR, Peroni ML, Cayetti LA, Mirofsky MA, Boietti B, Gómez‐Huelgas R, Casas‐Rojo JM. COVID-19 vaccines reduce mortality in hospitalized patients with oxygen requirements: differences between vaccine subtypes. a multicontinental cohort study. J Med Virol. 2023;95(5):e28786. doi:10.1002/jmv.28786.
- Brosh-Nissimov T, Hussein K, Wiener-Well Y, Orenbuch-Harroch E, Elbaz M, Lipman-Arens S, Maor Y, Yagel Y, Chazan B, Hershman-Sarafov M. Hospitalized patients with severe coronavirus disease 2019 during the Omicron wave in Israel: benefits of a fourth vaccine dose. Clin Infect Dis. 2023;76(3):234–9. doi:10.1093/cid/ciac501.
- Busic N, Lucijanic T, Barsic B, Luksic I, Busic I, Kurdija G, Barbic L, Kunstek S, Jelic T, Lucijanic M. Vaccination provides protection from respiratory deterioration and death among hospitalized COVID-19 patients: differences between vector and mRNA vaccines. J Med Virol. 2022;94(6):2849–54. doi:10.1002/jmv.27666.
- Bohnert AS, Kumbier K, Rowneki M, Gupta A, Bajema K, Hynes DM, Viglianti E, O’Hare AM, Osborne T, Boyko EJ. Adverse outcomes of SARS-CoV-2 infection with delta and omicron variants in vaccinated versus unvaccinated US veterans: retrospective cohort study. BMJ. 2023;381:e074521. doi:10.1136/bmj-2022-074521.
- McMenamin ME, Nealon J, Lin Y, Wong JY, Cheung JK, Lau EHY, Wu, P, Leung, G.M, Cowling, BJ. Vaccine effectiveness of one, two, and three doses of BNT162b2 and CoronaVac against COVID-19 in Hong Kong: a population-based observational study. Lancet Infect Dis. 2022;22(10):1435–43. doi:10.1016/S1473-3099(22)00345-0.
- Xiao J, Cheung JK, Wu P, Ni MY, Cowling BJ, Liao Q. Temporal changes in factors associated with COVID-19 vaccine hesitancy and uptake among adults in Hong Kong: serial cross-sectional surveys. Lancet Reg Health West Pac. 2022;23:100441. doi:10.1016/j.lanwpc.2022.100441.
- Ng RWY, Sze RKH, Chong KC, Zhao S, Ling L, Lui G, Leung ASY, Yeung ACM, Ho WCS, Wong MCS. Effectiveness of mRNA and inactivated COVID-19 vaccines: a test-negative study in an infection-naïve Hong Kong population. J Infect. 2023;87(2):136–43. doi:10.1016/j.jinf.2023.05.020.
- Heidarzadeh A, Amini Moridani M, Khoshmanesh S, Kazemi S, Hajiaghabozorgi M, Karami M. Effectiveness of COVID-19 vaccines on hospitalization and death in Guilan, Iran: a test-negative case-control study. Int J Infect Dis. 2023;128:212–22. doi:10.1016/j.ijid.2022.12.024.
- Wei Y, Jia KM, Zhao S, Hung CT, Mok CKP, Poon PKM, Man Leung EY, Wang MH, Yam CHK, Chow TY. Estimation of vaccine effectiveness of coronaVac and BNT162b2 against severe outcomes over time among patients with SARS-CoV-2 Omicron. JAMA Netw Open. 2023;6(2):e2254777. doi:10.1001/jamanetworkopen.2022.54777.
- Grima AA, Murison KR, Simmons AE, Tuite AR, Fisman DN. Relative virulence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) among vaccinated and unvaccinated individuals hospitalized with SARS-CoV-2. Clin Infect Dis. 2023;76(3):409–15. doi:10.1093/cid/ciac412.
- Yan VKC, Wan EYF, Ye X, Mok AHY, Lai FTT, Chui CSL, Li X, Wong CKH, Li PH, Ma T. Waning effectiveness against COVID-19-related hospitalization, severe complications, and mortality with two to three doses of CoronaVac and BNT162b2: a case–control study. Emerg Microbes Infect. 2023;12(1):2209201. doi:10.1080/22221751.2023.2209201.
- Zeng G, Wu Q, Pan H, Li M, Yang J, Wang L, Wu Z, Jiang D, Deng X, Chu K. Immunogenicity and safety of a third dose of CoronaVac, and immune persistence of a two-dose schedule, in healthy adults: interim results from two single-centre, double-blind, randomised, placebo-controlled phase 2 clinical trials. Lancet Infect Dis. 2022;22(4):483–95. doi:10.1016/S1473-3099(21)00681-2.
- Levin EG, Lustig Y, Cohen C, Fluss R, Indenbaum V, Amit S, Doolman R, Asraf K, Mendelson E, Ziv A. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med. 2021;385(24):e84. doi:10.1056/NEJMoa2114583.
- Shimizu H, Kawase J, Hayashi M, Imaizumi K, Ito Y, Okazawa M. COVID-19 symptom-onset to diagnosis and diagnosis to treatment intervals are significant predictors of disease progression and hospitalization in high-risk patients: a real world analysis. Respir Investig. 2023;61(2):220–9. doi:10.1016/j.resinv.2023.01.002.
- Belayachi J, Obtel M, Mhayi A, Razine R, Abouqal R, Lau EHY. Long term effectiveness of inactivated vaccine BBIBP-CorV (Vero cells) against COVID-19 associated severe and critical hospitalization in Morocco. PLOS ONE. 2022;17(12):e0278546. doi:10.1371/journal.pone.0278546.
- Jin L, Li Z, Zhang X, Li J, Zhu F. CoronaVac: A review of efficacy, safety, and immunogenicity of the inactivated vaccine against SARS-CoV-2. Hum Vaccin Immunother. 2022;18(6):2096970. doi:10.1080/21645515.2022.2096970.
- Arbel R, Sergienko R, Friger M, Peretz A, Beckenstein T, Yaron S, Netzer D, Hammerman A. Effectiveness of a second BNT162b2 booster vaccine against hospitalization and death from COVID-19 in adults aged over 60 years. Nat Med. 2022;28(7):1486–90. doi:10.1038/s41591-022-01832-0.