ABSTRACT
There is limited literature on current human papillomavirus (HPV) vaccination in the Asia-Pacific region. This integrative literature review was conducted to describe HPV vaccination programs in Hong Kong, Indonesia, Japan, South Korea, Malaysia, the Philippines, Singapore, Taiwan, Thailand, and Vietnam. Program descriptions, recommendations, f unding, and coverage data were extracted. Twenty-five citations were included. As of 2022, eight of the 10 areas of interest include HPV in their national immunization program (NIP) for school-aged girls; full implementation in Indonesia is expected in 2023 whereas Vietnam’s NIP does not include HPV. Singapore also includes HPV vaccination for women (18–26 years). None of the HPV vaccination programs include males. In most areas (n = 7), programs include only one vaccine option. While female HPV NIPs are present in the Asia-Pacific region, opportunities remain to strengthen NIPs in broader populations (e.g., males, catch-up cohorts) to expand public health impact and provide gender equity in HPV vaccination.
Introduction
Human papillomavirus (HPV) is one of the most common sexually transmitted infections.Citation1–3 There are more than 100 types of HPV, including low-risk types (HPV 6 and 11) that are associated with benign genital warts and recurrent respiratory papillomatosis and high-risk types (HPV 16, 18, 31, 33, 45, 52, and 58) that account for most cases of cervical, head and neck, penile, and anal cancers.Citation4
Three types of HPV vaccines are available worldwide.Citation5 The bivalent (2vHPV) vaccine protects against HPV types 16 and 18, which are responsible for approximately 70% of cervical cancer cases globally. The quadrivalent (4vHPV) vaccine protects against HPV types 16 and 18 as well as HPV 6 and 11, which are responsible for 90% of genital warts cases worldwide. The nonavalent (9vHPV) vaccine protects against the types in the 4vHPV vaccine and five additional oncogenic HPV types (HPV 31/33/45/52/58), allowing for direct protection against nine HPV types responsible for 90% of genital warts and an additional 20% of HPV-related cervical cancers.Citation5
While a declining trend in the incidence/prevalence of HPV infection and HPV-related diseases has been observed since the introduction of HPV vaccinations for girls in many countries,Citation6,Citation7 the burden of HPV-related diseases among men remains.Citation8 Direct protection against HPV infection and associated diseases in males may further reduce HPV-related diseases in females.Citation9 In recent years, several countries have introduced gender-neutral vaccination programs into their national immunization schedule to provide greater and more equitable prevention of HPV-related diseases in their populations.Citation10–14
Although the Asia-Pacific (AP) region contributes more than half of the global burden of HPV-related disease,Citation15 literature on current HPV vaccination programs in the region is limited. The objective of this integrative literature review was to provide data on the current status of HPV vaccination programs (recommendations, funding, coverage) in select areas in the AP region.
Methods
Search strategy and data sources
An integrative literature review was conducted to identify publications regarding HPV vaccination programs in select AP areas (Hong Kong, Indonesia, Japan, South Korea, Malaysia, Philippines, Singapore, Taiwan, Thailand, and Vietnam). Journal articles and conference abstracts published from January 1, 2000, to February 25, 2022, in MEDLINE and Embase were searched via ProQuest (Supplemental Table S1) using a search strategy created by the study authors. Additional searches were conducted within national government/ministry of health websites and gray literature sources for each of the countries of interest.
Study selection and data extraction
Literature reporting vaccination programs from Hong Kong, Indonesia, Japan, South Korea, Malaysia, the Philippines, Singapore, Taiwan, Thailand, and Vietnam was included. Mainland China was not included in this review as literature from this area will be summarized in a separate publication by the study authors. Studies were eligible for inclusion if they included data on the current HPV vaccination programs in each area (including national immunization program [NIP] start year, f und ing body, vaccinations, recommendations, dosing schedule, and coverage). Publications in non-English languages were translated using native speakers or Google. Two reviewers independently screened the titles and abstracts of identified citations and selected full-text articles for potential inclusion. Reviewers extracted relevant data from each included publication; data were reconciled to assure accuracy. Relevant data were extracted into a Microsoft Excel database.
Results
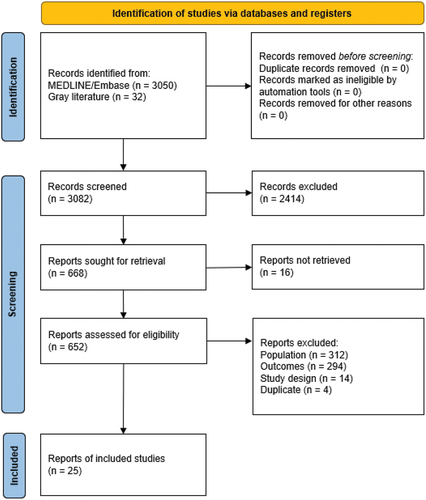
No data were identified from 3050 publications from MEDLINE and Embase. Of the 32 publications identified in the gray literature, 25 citations regarding NIPs and HPV vaccine recommendations available in the selected AP areas were included ().
outlines the NIP programs among the select AP areas. Vaccinations for HPV are included in the NIPs for school-aged girls (primarily 9–14 years of age) without catch-up in 80% (8/10) of the included AP areas: Hong Kong, Japan, Malaysia, the Philippines, Singapore, South Korea, Taiwan, and Thailand. Vaccinations for HPV are currently not part of the NIP in Vietnam. Indonesia is in an introductory phase of adding the HPV vaccine to the NIP, with implementation planned in all provinces and districts/cities in 2023.Citation41 Additionally, Singapore has a National Adult Immunization Schedule that includes women between 18 and 26 years old. Japan and South Korea have catch-up cohorts for some adult women as well (). Males were not included in HPV vaccination programs in any of the 10 included areas.
Table 1. National immunization programs for HPV.
As of 2022, of the nine AP areas with HPV vaccines as part of their NIPs (Hong Kong, Indonesia, Japan, Malaysia, the Philippines, Singapore, South Korea, Taiwan, and Thailand), bivalent and quadrivalent vaccines were utilized in 4/9 AP areas and 5/9 AP areas, respectively, while nonavalent vaccine was included in 2/9 areas. Moreover, one vaccine option is included in the NIP for seven areas: 9vHPV in Hong Kong and Taiwan (for Taiwan, this replaced 2vHPV in September 2022); 4vHPV in Japan, the Philippines, and Thailand; and 2vHPV in Singapore and Malaysia. The NIP in South Korea includes 2vHPV and 4vHPV. In 5/9 areas, the NIP dosing schedule mentioned girls receiving the second dose 6 months after the initial dose.
Discussion
Our review adds to the currently available literature by providing more recent data on NIPs for HPV in the AP region. The global burden of cervical cancer, as measured by the number of cases and deaths, is highest in Asia.Citation42 HPV vaccination is recognized as one of the most effective strategies for control of HPV-related cancers.Citation42 As of 2022, the available HPV vaccination programs in the AP region are often for a single sex (females) and single cohort (without multiple year catch-up programs), and few have existed for more than 10 years.
The impact of HPV vaccination on cervical cancer rates is already being seen in high-income European countries with early adoption of HPV vaccination programs, such as Sweden (2007), Denmark (2008), and the United Kingdom (2008).Citation43–45 In Malaysia, which was one of the earliest AP countries to introduce an NIP for HPV (2010), there are early indications that the HPV NIP may be reducing the burden of cervical cancer as well. In a cross-sectional study comparing HPV prevalence rates in women, there was a 91% decline in bivalent vaccine–targeted HPV prevalence (HPV16/18) and 87% reduction in quadrivalent vaccine–targeted HPV prevalence (HPV6/11/16/18) among young women aged 18 to 24 years from 2019 to 2020 compared to those aged 18 to 24 years from 2013 to 2015.Citation46
In contrast, suspension of proactive recommendations for HPV vaccination in Japan from June 2013 through November 2021 caused vaccination rates to plummet to less than 1%, which has led to a significant increase in HPV infection rates in young Japanese women.Citation47,Citation48 The HPV vaccine crisis has been estimated to increase the future incidence and death from cervical cancer in Japan, including an estimated 5000 to 5700 additional deaths, and recent studies have shown catch-up vaccination is unlikely to be able to redress the full impact of the vaccination gap.Citation49–51
These data highlight the beneficial impact HPV vaccination could have on the burden of HPV-related disease in the region. Future research should continue to compare results before and after the introduction of HPV vaccinations in NIPs in the AP region. However, such comparisons would require registry and surveillance systems to see the impact of the HPV NIPs. Moreover, this review provides a snapshot of HPV NIP programs in the AP region, especially among middle-income countries (MICs). However, many HPV programs are renewed annually, and despite achieving targets of HPV vaccine availability to adolescent girls, lower MICs in particular face many challenges in implementing and sustaining these programs, including vaccine supply constraints, confusion on eligibility, access issues, the political climate, and rumors/misinformation.Citation51
Limitations of this review must also be noted. First, this review was conducted in February 2022; government vaccination recommendations may have changed since the review was conducted. Second, this review looked at specific AP areas, which may not align across the entire AP region. Future reviews could focus on other countries not included in this review, including Mainland China. The literature search in MEDLINE and Embase did not yield any eligible articles, and relevant data were identified through the gray literature search of government websites. As the objective of this review was to summarize the most current recommendations, f und ing, coverage, and dosing schedules in NIPs, the data in the identified MEDLINE/Embase studies were largely outdated compared with those found on national/global health websites. Future research on this topic could potentially incorporate additional databases (e.g., Global Health) that may provide additional literature not captured in MEDLINE or Embase.
The epidemiologic and economic burdens of HPV are well described in women, but the full burden may be underestimated due to the limited availability of data in men. Most of the AP areas included in our analysis have female NIPs, but none include males in HPV vaccination programs. Reduction of HPV-related disease in men with HPV vaccination has been seen in real-world studies and clinical trials.Citation52–54 Countries in the AP region should therefore consider strengthening their NIPs by introducing male vaccination to further reduce female HPV-related disease burden and provide direct protection against HPV to males who are vulnerable to HPV infection and associated diseases.Citation8,Citation9 Across all genders, there is also a need to increase awareness of HPV vaccination among young adults. In a 2021 systematic literature review on vaccine attitudes among adolescents in Asia, HPV and vaccine awareness was generally low across 20 publications, and included publications demonstrated high variability in the percentage of respondents who intended to be vaccinated against HPV (range: 38% to 95%).Citation55
The ongoing COVID-19 pandemic has caused a sustained drop in childhood immunizations worldwide, including HPV.Citation56 It has highlighted the fragility of the current immunization programs: According to the World Health Organization, over one-quarter of the HPV vaccine coverage that was achieved globally in 2019 had been lost by 2022, and 3.5 million more children missed the first dose of the HPV vaccine compared to 2019.Citation56 More effort is needed to increase the resilience of HPV vaccination programs. In addition to addressing pandemic-related disruptions, such as the effect of school closures on school-based NIPs, countries must intensify catch-up vaccination efforts to reach missed children. Gender-neutral vaccination programs could also increase the resilience of HPV vaccination programs and accelerate HPV prevalence reduction.Citation57 All these approaches will require political commitment from national governments and increased domestic resource allocation to strengthen and sustain NIPs.Citation56
In 2020, the World Health Organization started a global initiative to eliminate cervical cancer. The goal is to reduce cervical cancer incidence to below four cases per 100,000 women-years in every country.Citation58 Results of a recent modeling study suggest that high HPV vaccination coverage is the most powerful intervention to reduce the burden of HPV and could lead to cervical cancer elimination in most low-income and lower MICs by the end of the century.Citation59 Strong political commitment to primary HPV cancer prevention could make elimination an achievable goal in the AP region.
Conclusions
While HPV NIPs are present in females in the AP region, opportunities remain to strengthen NIPs in broader populations (e.g., males, catch-up cohorts) to expand public health impact and provide gender equity in HPV vaccination. Highly populous countries with high disease burden within the region, such as Indonesia and Vietnam, have not yet introduced full NIPs for HPV. A multi-stakeholder effort and political will are required to reduce the disease burden in these countries. As HPV vaccination programs in the AP region continue to grow, government commitment is also required to start planning how to track the real-world impact of vaccination.
Author contributions
Conception and design of the study: PJL, WP, YHW, IS
Acquisition of data: OZ, AS, FD, MB
Analysis and interpretation of data: OZ, AS, FD, MB, YHW, IS
Drafting the manuscript: OZ, MB
Revising critically for important intellectual content: PJL, WP, YHW, IS
Final approval of version to be submitted: All authors
Supplement HPV NIP in AP.docx
Download MS Word (22.1 KB)Acknowledgments
Medical writing and editorial support were provided by Catherine Mirvis from OPEN Health, Bethesda, MD, and funded by the study sponsor.
Disclosure statement
PJL: Received study f und ing from Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. WP: Received study f und ing from Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and from GSK. IS: Employee of MSD Thailand. YHW: Employee of MSD Taiwan. OZ, FD, AS, MB: Employees of OPEN Health.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2024.2362449
Additional information
Funding
References
- Hebnes JB, Olesen TB, Duun-Henriksen AK, Munk C, Norrild B, Kjaer SK. Prevalence of genital human papillomavirus among men in Europe: systematic review and meta-analysis. J Sex Med. 2014 Nov. 11(11):2630–6. doi:10.1111/jsm.12652.
- Newman P, Lacombe-Duncan A. Human papillomavirus vaccination for men: advancing policy and practice. Future Virol. 2014;9(12):1033–47. doi:10.2217/fvl.14.91.
- Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine. 2006 Aug 31;24(Suppl 3):S3/11–25. doi:10.1016/j.vaccine.2006.05.111.
- de Sanjosé S, Serrano B, Tous S, de Sanjosé S, Alejo M, Lloveras B, Quirós B, Clavero O, Vidal A, Ferrándiz-Pulido C, et al. Burden of human papillomavirus (HPV)-related cancers attributable to HPVs 6/11/16/18/31/33/45/52 and 58. JNCI Cancer Spectr. 2018;2(4). doi:10.1093/jncics/pky045.
- Mo Y, Ma J, Zhang H, Shen J, Chen J, Hong J, Xu Y, Qian C. Prophylactic and therapeutic HPV vaccines: current scenario and perspectives. Front Cell Infect Microbiol. 2022;12:12. 2022-July-04. doi:10.3389/fcimb.2022.909223.
- Brisson M, Benard É, Drolet M, Bogaards JA, Baussano I, Vänskä S, Jit M, Boily M-C, Smith MA, Berkhof J, et al. Population-level impact, herd immunity, and elimination after human papillomavirus vaccination: a systematic review and meta-analysis of predictions from transmission-dynamic models. Lancet Public Health. 2016 Nov;1(1):8–17. doi:10.1016/S2468-2667(16)30001-9.
- Drolet M, Benard É, Perez N, Brisson M , Boily M-C, Baldo V, Brassard P, Brotherton JML, Callander D, et al. HPV Vaccination Impact Study Group. Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: updated systematic review and meta-analysis. Lancet. 2019 Aug 10;394(10197):497–509. doi:10.1016/S0140-6736(19)30298-3.
- de Martel C, Georges D, Bray F, Ferlay J, Clifford GM. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob Health. 2020 Feb;8(2):180–90. doi:10.1016/S2214-109X(19)30488-7.
- Lieblong BJ, Montgomery BEE, Su LJ, Nakagawa M. Natural history of human papillomavirus and vaccinations in men: a literature review. Health Sci Rep. 2019;2(5):e118. doi:10.1002/hsr2.118.
- Brotherton JML, Giuliano AR, Markowitz LE, Dunne EF, Ogilvie GS. Monitoring the impact of HPV vaccine in males—considerations and challenges. Papilloma Res. 2016 Dec;2:106–11. doi:10.1016/j.pvr.2016.05.001.
- Kmietowicz Z. Boys in England to get HPV vaccine from next year. BMJ. 2018 Jul 24;362:k3237. doi:10.1136/bmj.k3237.
- Parellada C, Perez Carrega M, Carvalho A, Massoc MA, Prieto E, Monsanto H, Cashat-Cruz M. Evolution of gender-neutral HPV vaccination in national immunization calendars in Latin America and the Caribbean, abstract Fri-Station 01.3. Int J Infect Dis. 2018;73(Suppl):82. doi:10.1016/j.ijid.2018.04.3612.
- Takla A, Wiese-Posselt M, Harder T, Meerpohl JJ, Röbl-Mathieu M, Terhardt M, van der Sande M, Wichmann O, Zepp F, Klug SJ. Background paper for the recommendation of HPV vaccination for boys in Germany. Bundesgesundheitsblatt - Gesundheitsforschung - Gesundheitsschutz. 2018;61(9):1170–86. 2018/09/01. doi:10.1007/s00103-018-2791-2.
- World Health Organization. Weekly Epidemiological Record [Online]. [Accessed 2019 Apr 4]. https://www.who.int/wer/2017/wer9219/en/.
- Parkin DM, Louie KS, Clifford G. Burden and trends of type-specific human papillomavirus infections and related diseases in the Asia Pacific region. Vaccine. 2008 Aug 19;26(Suppl 12):1–16. doi:10.1016/j.vaccine.2008.05.010.
- Family Health Service DoH. Schedule of Hong Kong childhood immunisation programme. [Accessed 2022 Jul]. https://www.fhs.gov.hk/english/main_ser/child_health/child_health_recommend.html.
- The Government of the Hong Kong Special Administrative Region. SFH chairs seventeenth meeting of cancer coordinating committee. [Accessed 2022 Jul]. https://www.info.gov.hk/gia/general/202206/07/P2022060700797.htm?fontSize=1.
- Ministry of Health Republic of Indonesia. Ministry of health adds 3 types of routine immunization vaccines, one of them is HPV. 2020. https://www.kemkes.go.id/article/view/22042400001/kemenkes-tambah-3-jenis-vaksin-imunisasi-rutin-salah-satunya-hpv.html.
- Ministry of Health Indonesia. Post-introduction evaluation of hpv vaccine programme in indonesia. [Accessed 2018 Nov 19]. https://www.who.int/docs/default-source/searo/indonesia/hpv-evaluation-vaccine-programme-post-introduction-final-report-nov2018.pdf?sfvrsn=9b63f1e9_2.
- Ministry of Health Labour and Welfare. Human papillomavirus infection-cervical cancer (cervical cancer) and HPV vaccine. [Accessed 2022 Jul]. https://www.mhlw.go.jp/bunya/kenkou/kekkaku-kansenshou28/index.html.
- Center for Strategic & International Studies. HPV vaccination in Japan: the continuing debate and global impacts. [Accessed 2022 Aug 8]. https://www.csis.org/analysis/hpv-vaccination-japan-0.
- Buang SN, Ja’afar S, Pathmanathan I, Saint V. Human papillomavirus immunisation of adolescent girls: improving coverage through multisectoral collaboration in Malaysia. BMJ. 2018;363:k4602. doi:10.1136/bmj.k4602.
- Immunise 4 Life. The Malaysian National Immunisation Programme (NIP). [Accessed 2022 Jul]. https://immunise4life.my/the-malaysian-national-immunisation-programme-nip/.
- Bruni LAG, Albero G, Serrano B, Mena M, Collado JJ, Gómez D, Muñoz J, Bosch FX, de Sanjosé S. Human papillomavirus and related diseases in philippines. Barcelona, Spain: ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre). 2021.
- Department of Health (DOH). 2013. Department memorandum no. 2013-0291: Guidelines in the implementation of human papillomavirus (HPV) vaccination in selected schools; 2013 August [accessed 2021 October 22]. https://doh.gov.ph/sites/default/files/publications/DOHGuidelinesSchoolbasedHPVvaccination.pdf.
- Phua LC, Choi HCW, Wu J, Jit M, Low J, Ng K, Pearce F, Hall C, Abdul Aziz MI. Cost-effectiveness analysis of the nonavalent human papillomavirus vaccine for the prevention of cervical cancer in Singapore. Vaccine. 2021;39(16):2255–63. doi:10.1016/j.vaccine.2021.03.040.
- Ministry of Health Singapore. National childhood immunisation schedule. [Accessed 2022 Jul]. https://www.moh.gov.sg/resources-statistics/nationally-recommended-vaccines.
- Ministry of Health Singapore. MOH establishes national adult immunisation schedule; extends use of Medisave for vaccines under the schedule. [Accessed 2022 Jul]. https://www.moh.gov.sg/news-highlights/details/moh-establishes-national-adult-immunisation-schedule-extends-use-of-medisave-for-vaccines-under-the-schedule.
- Vijaya K, Goei AHY. Improved population coverage of the human papillomavirus vaccine after implementation of a school-based vaccination programme: the Singapore experience. Singapore Med J. 2023;64(5):294–301. doi:10.11622/smedj.2022053.
- Ministry of Health Singapore. Changes to the national childhood immunisation schedule. [Accessed 2022 Aug 17]. https://www.cfps.org.sg/assets/1-Circular-for-GPs/4-MOH-Cir-No-180-2020-13Jul20-Updates-to-NCIS-doctors-1.pdf.
- Ministry of Health Singapore. Updates to the national adult immunisation schedule. [Accessed 2022 Aug 17]. https://www.primarycarepages.sg/GPRepository/Circular%20Documents/MOH%20Cir%20No%20182_2020_13Jul20_Updates%20to%20NAIS_doctors.pdf.
- Garland S. A significant measure of HPV vaccine effectiveness in a high-risk population in Korea prior to a national immunization program. J Gynecol Oncol. 2020;31(1):32. doi:10.3802/jgo.2020.31.e32.
- Korean Disease Control and Prevention Agency. National immunization program for children. [Accessed 2022 Jul]. https://www.kdca.go.kr/contents.es?mid=a30333000000.
- World Health Organization. HPV vaccine included in national immunization programme. [Accessed 2022 Jul]. https://app.powerbi.com/view?r=eyJrIjoiNDIxZTFkZGUtMDQ1Ny00MDZkLThiZDktYWFlYTdkOGU2NDcwIiwidCI6ImY2MTBjMGI3LWJkMjQtNGIzOS04MTBiLTNkYzI4MGFmYjU5MCIsImMiOjh9.
- Korean Disease Control and Prevention Agency. HPV national vaccination support project. [Accessed 2022 Aug 17]. https://nip.kdca.go.kr/irgd/introduce.do?MnLv1=3&MnLv2=6.
- Health Promotion Administration Taiwan. QA: know HPV. [Accessed 2022 Jul]. https://www.hpa.gov.tw/Pages/List.aspx?nodeid=1799.
- Lee CC, Chen TS, Wu TZ, Huang LM. A human papillomavirus public vaccination program in Taiwan: the Kinmen County experience. J Formos Med Assoc. 2012 Dec;111(12):682–5. doi:10.1016/j.jfma.2012.10.004.
- Bruni L, Albero, G, Serrano B, Mena M, Collado JJ, Gómez D, Muñoz J, Bosch FX, de Sanjosé S. Human Papillomavirus and Related Diseases in Thailand. Summary Rep. ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre). 2023 Mar 10.
- World Health Organization. Vaccination schedule for Thailand. [Accessed 2022 Jul]. https://immunizationdata.who.int/pages/schedule-by-country/tha.html?DISEASECODE=&TARGETPOP_GENERAL=.
- Termrungruanglert W, Khemapech N, Vasuratna A, Havanond P, Deebukkham P, Kulkarni AS, Pavelyev A. The epidemiologic and economic impact of a quadrivalent human papillomavirus vaccine in Thailand. PLOS ONE. 2021;16(2):e0245894. doi:10.1371/journal.pone.0245894.
- Singh D, Vignat J, Lorenzoni V, Eslahi M, Ginsburg O, Lauby-Secretan B, Arbyn M, Basu P, Bray F, Vaccarella S, et al. Global estimates of incidence and mortality of cervical cancer in 2020: a baseline analysis of the WHO global cervical cancer elimination initiative. Lancet Glob Health. 2023;11(2):197–206. doi:10.1016/S2214-109X(22)00501-0.
- Falcaro M, Castañon A, Ndlela B, Checchi M, Soldan K, Lopez-Bernal J, Elliss-Brookes L, Sasieni P. The effects of the national HPV vaccination programme in England, UK, on cervical cancer and grade 3 cervical intraepithelial neoplasia incidence: a register-based observational study. Lancet. 2021;398(10316):2084–92. doi:10.1016/S0140-6736(21)02178-4.
- Kjaer SK, Dehlendorff C, Belmonte F, Baandrup L. Real-world effectiveness of human papillomavirus vaccination against cervical cancer. JNCI J Nat Cancer Inst. 2021;113(10):1329–35. doi:10.1093/jnci/djab080.
- Lei J, Ploner A, Elfström KM, Wang J, Roth A, Fang F, Sundström K, Dillner J, Sparén P. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383(14):1340–8. 2020/10/01. doi:10.1056/NEJMoa1917338.
- Khoo SP, Muhammad Ridzuan Tan NA, Rajasuriar R, Nasir NH, Gravitt P, Ng CW, Woo YL. Changes in genital human papillomavirus (HPV) prevalence among urban females a decade after the Malaysian HPV vaccination program. PLOS One. 2022;17(12):e0278477. doi:10.1371/journal.pone.0278477.
- Haruyama R, Obara H, Fujita N. Japan resumes active recommendations of HPV vaccine after 8.5 years of suspension. Lancet Oncol. 2022;23(2):197–8. doi:10.1016/S1470-2045(22)00002-X.
- Sekine M, Yamaguchi M, Kudo R, Hanley SJB, Ueda Y, Adachi S, Kurosawa M, Miyagi E, Hara M, Enomoto T, et al. Suspension of proactive recommendations for HPV vaccination has led to a significant increase in HPV infection rates in young Japanese women: real-world data. Lancet Reg Health West Pac. 2021 Nov. 16:100300. doi:10.1016/j.lanwpc.2021.100300.
- Simms KT, Hanley SJB, Smith MA, Keane A, Canfell K. Impact of HPV vaccine hesitancy on cervical cancer in Japan: a modelling study. Lancet Publ Health. 2020 Apr. 5(4):223–34. doi:10.1016/s2468-2667(20)30010-4.
- Yagi A, Ueda Y, Nakagawa S, Ikeda S, Tanaka Y, Sekine M, Miyagi E, Enomoto T, Kimura T. Potential for cervical cancer incidence and death resulting from Japan’s current policy of prolonged suspension of its governmental recommendation of the HPV vaccine. Sci Rep. 2020 Sep 29. 10(1):15945. doi:10.1038/s41598-020-73106-z.
- Yagi A, Ueda Y, Nakagawa S, Ikeda S, Kakuda M, Hiramatsu K, Miyoshi A, Kobayashi E, Kimura T, Mizushima T, et al. Can catch-up vaccinations fill the void left by suspension of the governmental recommendation of HPV vaccine in Japan? Vaccines. 2022 Sep 2;10(9):1455. doi:10.3390/vaccines10091455.
- Tsu VD, LaMontagne DS, Atuhebwe P, Bloem PN, Ndiaye C. National implementation of HPV vaccination programs in low-resource countries: lessons, challenges, and future prospects. Prevent Med. 2021 Mar;144:106335. doi:10.1016/j.ypmed.2020.106335.
- Goldstone SE, Giuliano AR, Palefsky JM, Lazcano-Ponce E, Penny ME, Cabello RE, Moreira ED, Baraldi E, Jessen H, Ferenczy A, et al. Efficacy, immunogenicity, and safety of a quadrivalent HPV vaccine in men: results of an open-label, long-term extension of a randomised, placebo-controlled, phase 3 trial. Lancet Infect Dis. 2022;22(3):413–25. doi:10.1016/S1473-3099(21)00327-3.
- Palefsky JM, Giuliano AR, Goldstone S, Moreira ED, Aranda C, Jessen H, Hillman R, Ferris D, Coutlee F, Stoler MH, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365(17):1576–85. doi:10.1056/NEJMoa1010971.
- Tsentemeidou A, Fyrmpas G, Stavrakas M, Vlachtsis K, Sotiriou E, Poutoglidis A, Tsetsos N. Human papillomavirus vaccine to end oropharyngeal cancer. A systematic review and meta-analysis. Sex Transm Dis. 2021;48(9):700–7. doi:10.1097/OLQ.0000000000001405.
- Wang L, Liang Y, Zhang X, Yang J. Vaccine attitudes among young adults in Asia: a systematic review. Hum Vaccines Immunother. 2021;17(4):1142–55. 2021/04/03. doi:10.1080/21645515.2020.1810486.
- World Health Organization. COVID-19 pandemic fuels largest continued backslide in vaccinations in three decades. 2022 Dec 15. https://www.who.int/news/item/15-07-2022-covid-19pandemic-fuels-largest-continued-backslide-in-vaccinations-in-three-decades.
- Elfström KM, Lazzarato F, Franceschi S, Dillner J, Baussano I. Human papillomavirus vaccination of boys and extended catch-up vaccination: effects on the resilience of programs. J Infect Dis. 2016;213(2):199–205. doi:10.1093/infdis/jiv368.
- World Health Organization. Global strategy to accelerate the elimination of cervical cancer as a public health problem. 2020. https://www.who.int/publications/i/item/9789240014107.
- Brisson M, Kim JJ, Canfell K, Drolet M, Gingras G, Burger EA, Martin D, Simms KT, Bénard É, Boily M-C, et al. Impact of HPV vaccination and cervical screening on cervical cancer elimination: a comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet. 2020;395(10224):575–90. doi:10.1016/S0140-6736(20)30068-4.