ABSTRACT
The objective of the study is to analyze the implementation effect of the Live Attenuated Varicella Vaccine (VarV) Vaccination Program for eligible children in Bao’an District, Shenzhen, and evaluate the vaccine effectiveness. Children’s vaccination data was obtained from the Shenzhen Immunization Planning Information Management System, while varicella case data came from the China Disease Prevention and Control Information System. The Joinpoint regression method examined vaccination rate trends, and a retrospective cohort study assessed vaccine effectiveness. After program implementation, VarV vaccination rates significantly increased, surpassing provincial and national averages. Overall incidence declined 54.6% across age groups, with the largest reductions among 7- and 6-year-olds. One year post-vaccination, single-dose vaccine effectiveness was 91.1% (95% CI: 79.2% to 96.2%). However, two doses remained 91.4% effective(95% CI: 89.1% to 93.2%) after 7 years. Overall, Shenzhen’s VarV program achieved positive results. For children under six, routine immunization with two doses of VarV should be strengthened. Furthermore, we recommend that physicians conduct thorough inquiries to ascertain patients’ vaccination history and previous varicella infections. This will enable doctors to provide tailored vaccination recommendations based on comprehensive, practical evaluations.
Varicella is a common acute infectious disease caused by the Varicella-Zoster Virus (VZV), mainly transmitted via respiratory tract or direct contact, Clinical manifestations include fever and a widespread rash/papules, Sever cases can lead to pneumonia, encephalitis, etc. Outbreaks often occur in schools/childcare facilities, compromising children’s health and education and posing a significant public health issue.Citation1,Citation2 The live attenuated VarV developed by Japanese researcher Takahashi in 1974, is currently the most effective varicella prevention method.Citation3,Citation4 VarV is included in 51 countries’ national immunization programs worldwide.Citation5 However, vaccination strategies vary significantly in different countries and regions. Most European nations, such as Finland, Germany, Greece, Hungary, Iceland, and Spain, have adopted a two-dose VarV vaccine in their national immunization programs, while many developing countries only recommend voluntary, self-paid vaccination without including VarV in national programs.Citation6 Only a few developing countries, such as Argentina and Peru, have incorporated single-dose VarV into their national immunization programs.Citation7
China introduced imported live attenuated varicella vaccines in 1997, with several domestically produced versions approved for market since 2000. VarV is currently not part of China’s national immunization program; two doses are recommended for voluntary, self-paid vaccination.Citation8,Citation9 Imported vaccines cost around $45, while domestic versions are around $20. Data indicates that among children aged 1–14 in China, the vaccination rates for one dose and two doses of the VarV vaccine are 52.7% and 11.4%, respectively. There are significant regional disparities in vaccination rates, Developed areas such as Beijing and Shanghai exceed 80% coverage, while remote regions such as Xinjiang and Gansu remain below 20%.Citation8 Varicella is currently China’s most commonly reported vaccine-preventable childhood illness, with an increasing incidence from 35.50/100,000 in 2016 to 70.14/100,000 in 2019.Citation10
To varicella incidence and mitigate its disease burden and social impact, regions like Beijing, Shanghai, Tianjin, Jiangsu, and Huzhou in Zhejiang have successively incorporated the varicella vaccine into their local immunization programs, providing citizens with two free doses.Citation8 Since January 2019, Shenzhen has implemented a free, two-dose varicella vaccination program for eligible children born on or after December 1, 2014. The vaccination program consists of two doses, with the first dose administered free of charge at 12 months and the second dose at 4 years old. The organizational and departmental responsibilities for the VarV immunization program are comprehensively structured as follows: The Municipal Health Commission oversees the organization, coordination, supervision, and evaluation of the program. The Finance Departments at both municipal and district levels are tasked with providing the necessary financial support. Disease Control Centers (Municipal and District Levels) are responsible for the unified bidding, procurement, storage, transportation, and deployment of vaccines. These centers also handle technical training and guidance, and they actively monitor and manage any suspected adverse reactions to the vaccines. Vaccination Units play a crucial role by administering vaccines to eligible target populations.Since the launch of the VarV program, the Shenzhen Health and Wellness Department has vigorously publicized the VarV program, using a multifaceted approach to increase community engagement and support. Their methods include community outreach, educational-programs in schools, media campaigns, collaborations with partner organizations, and the dissemination of Public Service Announcements. This strategic publicity aims to maximize public awareness and increase the community’s willingness to be vaccinated. To date, over 2.3 million doses have been administered across 5 years (2019–2023), a 30.3% annual increase versus pre-implementation. Vaccines from three local Chinese vaccine companies are purchased and used. The detailed vaccine information can be accessed in the Supplementary Material, S1.
This study focuses on the Bao’an District in Shenzhen, examining the vaccination status of children born between 2012 and 2020. It aims to analyze and evaluate the implementation effects of the free varicella vaccine immunization program for eligible children in Shenzhen, assessing the vaccine’s protective efficacy against regional varicella outbreaks. This study is the first evaluation research in China focusing on the implementation of free varicella vaccine immunization programs for eligible children, providing critical insights and benchmarks for the promotion and execution of similar preventive vaccination projects.
Object and methods
Data sources
Population Data and Children’s Immunization History: These are obtained from children’s vaccination certificates/cards or the “Shenzhen Immunization Planning Information Management System.” Information on Infectious Disease Outbreaks: This is sourced from the “China Disease Prevention and Control Information System Infectious Disease Reporting Information Management System.”
Methods
Data spanning from 2012 to 2020 pertaining to the birthdates, genders, and residences of children from Bao’an District, Shenzhen, along with their varicella vaccination records, were systematically gathered using the “Shenzhen Immunization Planning Information Management System.” This compilation included personal demographics, varicella diagnosis, and dates of onset from 2012 through 2022. The data set was enriched by integrating case details from the “China Disease Prevention and Control Information System, Infectious Disease Reporting Information Management System“ through data linkage via ID card numbers, names, genders, and birthdates.
Relevant definitions
Chickenpox case definition
As per the CDC, a clinical case of chickenpox is characterized by a rash of spots, papules, vesicles, and scabs that appear sequentially on the skin and mucous membranes, accompanied by fever, itching, and an epidemiological linkage.
Vaccination rate
Varicella 1-dose (VarV) vaccination rate for children born in a given year (%) = Number of children who received only 1 dose of VarV/Number of children born in that given year × 100%. Varicella 2-dose (VarV) vaccination rate for children born in a given year (%) = Number of children who received 2 doses of VarV/Number of children born in that given year × 100%.VarV Vaccinated (%) = Number of children who received at least 1 dose of VarV/Number of children born in that given year × 100%.
Vaccine Effectiveness (VE)
Using a retrospective cohort study method, children born in 2012 are categorized based on VarV vaccination status into the 1-dose VarV group, 2-dose VarV group, and unvaccinated VarV group. Incidence density = Number of cases/Person-years of follow-up. VE(%) = [(Incidence density in the unvaccinated group − Incidence density in the vaccinated group)/Incidence density in the unvaccinated group] × 100%. Person-years are calculated using the life table method, assuming no loss to follow-up, with varicella onset as the endpoint.
Statistical analysis
Data entry was managed with Excel 2003, while statistical analysis was performed using SPSS software (version 22.0, IBM Inc.). This analysis included computing VarV vaccination rates, Vaccine Effectiveness (VE), 95% Confidence Interval (CI), and incidence rate comparisons using the chi-square test (significance level α = 0.05). Join point regression analysisCitation11 was employed to explore Annual Percentage Changes (APC) and turning points in vaccination rates over the span from 2012 to 2020. Due to the recommendation of receiving the first and second doses of VarV at ages 1–2 and 4–6, respectively, in Shenzhen, this study analyzes the 1-dose and 2-dose vaccination rates for children born in 2012–2020 and 2012–2018, respectively.
Ethics approval
Our study was approved by the Ethics Committee of Baoan District Center for Disease Control and Prevention, Shenzhen, China. The study complied with the guidelines of the Declaration of Helsinki.
Results
Basic information
From 2012 to 2020, a total of 473,233 children were recorded in Bao’an District, Shenzhen, comprising 247,879 males (52.4%), and 225,354 females (47.6%).
Varicella epidemiological characteristics
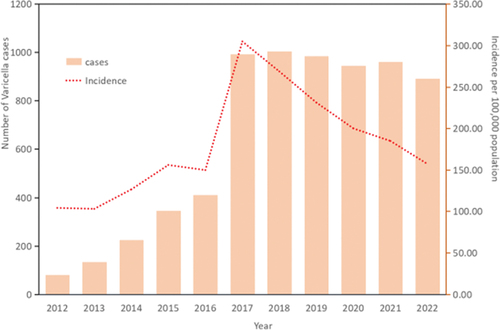
Between 2012 and 2022, a total of 6,973 varicella cases were reported in children born from 2012 to 2020 in Bao’an District, Shenzhen. The average reported incidence rate of varicella in children was 180.46 per 100,000, ranging from 102.97 per 100,000 to 304.73 per 100,000, as shown in .
Temporal distribution
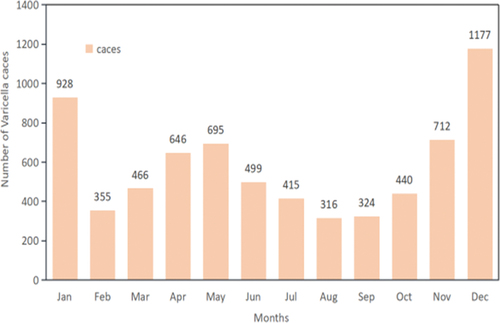
The incidence of varicella exhibited a “bimodal” pattern, peaking from October to January and again from April to May. These periods accounted for 3,257 cases (46.7%) and 1,341 cases (19.2%), respectively, as depicted in .
Demographic distribution
The majority of the reported varicella cases, 6,962 (99.8%), were clinically diagnosed, with the remaining 11 cases (0.2%) confirmed via laboratory tests. All cases were mild and non-fatal. The distribution between genders showed a male-to-female ratio of 1.34 (3,990 males to 2,983 females). The average incidence rates were significantly higher in males (1,609.71 per 100,000) than in females (1,323.70 per 100,000). Preschool children accounted for the highest proportion of cases at 39.0% (2717cases), followed by other children and students at 36.3% (2,528 cases) and 24.8% (1,728 cases), respectively.
VarV vaccination
Vaccination rate and timeliness
In Bao’an District of Shenzhen, the vaccination rate for varicella vaccine among children is 88.9%, and the rate for receiving one dose within 2 years is 70.5%, while the rate for receiving two doses within 6 years is 32.0%, as shown in and . Join point regression analysis indicates that the varicella vaccination rate shows an overall increasing trend (AAPC = 2.1%, CI: 1.3–2.9, p < .05). A turning point occurred in 2016, where the rate increased from 2012 to 2016 (APC = 5.1%,p < .05), but there was no statistically significant difference from 2016 to 2020. The two-dose varicella vaccination rate shows an overall increasing trend from 2012 to 2016 (AAPC = 29.8%, CI:1.4–66.2,P < .05).
Figure 3. Varicella vaccination rate of children born during 2012–2020 in Bao ‘an district, Shenzhen City.
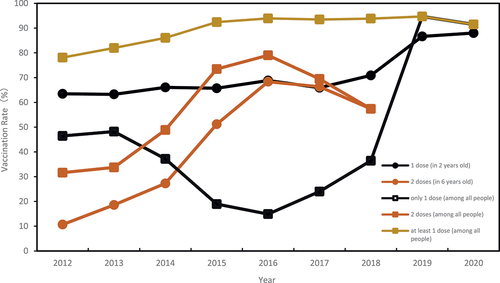
Table 1. The coverage rate and VE of VarV in children born during 2012–2020 in Shenzhen.
Vaccination rate and incidence rate
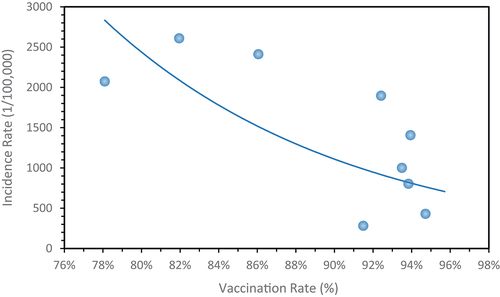
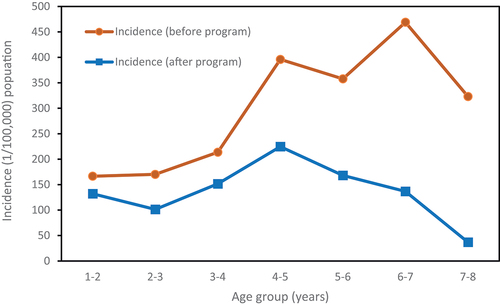
The analysis demonstrates a significant negative correlation (r = − 0.67, p < .05) between the rising annual vaccination rate of VarV and the overall incidence rate of varicella, which shows a declining trend as depicted in . After the implementation of the VarV vaccination program in Shenzhen (after January 1, 2019), the overall incidence rate in the age group of 1–7 age group decreased by 54.6% compared to before the program, with a statistically significant difference (p < .01). The age groups of 7 and 6 years old had the largest reductions in varicella incidence rates, decreasing by 88.6% and 70.9%. There were statistically significant differences in the varicella incidence rates before and after the VarV program in all age groups (p < .01), as illustrated in .
Varicella cases and VarV vaccination
In Baoan District, 52.0% of children diagnosed with varicella had previously received the VarV vaccine, as indicated in . Additionally, after the initiation of the VarV vaccination program in Shenzhen, some children without prior VarV immunization who contracted varicella were subsequently vaccinated with either one or two doses of VarV.Among all cases assessed, 417 children (6.0%, 417/6,973) who had contracted varicella were vaccinated with one dose of VarV post-recovery, and 178 these children (2.6%, 178/6,973) received a second dose.
VE (Vaccine effectiveness)
A retrospective cohort study was conducted on children born in Bao’an District, Shenzhen, in 2012 to calculate the vaccine effectiveness of VarV. The results show that after receiving one or two doses of VarV, the vaccine effectiveness decreases each year. The VE of the one-dose group has dropped to below 64.5% by the fourth year (58.4% − 69.9%), but the VE of the two-dose VarV group remains at a high level, reaching 91.4% by the seventh year (89.1% − 93.2%). These findings underscore the superior long-term protection offered by the two-dose regimen over the single-dose approach ().
Table 2. Vaccine effectiveness of VarV in children born in Bao ‘an District, Shenzhen in 2012.
Discussion
From 2012 to 2022, the Bao’an District of Shenzhen documented a total of 6,973 chickenpox cases among children born between 2012 and 2020, resulting in an average incidence rate of 180.46 per 100,000 people. This rate is relatively high compared to other regions in China and aligns with the trends observed in Guangdong Province.Citation12 In Shenzhen, the seasonality of varicella showcases a bimodal pattern: the first peak spans from October to January and the second from April to May, coinciding with school terms and heightened student concentration, facilitating respiratory disease transmission. The implementation of the VarV vaccination program for eligible children in Shenzhen has yielded positive results.The introduction of the VarV Program for eligible children has significantly improved vaccination rates. For children born after December 2014, over 92.4% received the VarV, and 73.5% of these received the two doses, exceeding averages in Guangdong Province and China. However, first-dose coverage trails behind Shanghai.Citation8
We found that since 2012, the rates for one-dose within two years and two-dose within six years have increased, although they still fall short of Shenzhen’s routine immunization schedule program requirements. Between 2020 and 2022, both the incidence and vaccination rates of varicella in Bao’an District, Shenzhen, showed a decline when compared to previous years. This decrease likely resulted from the disruptions caused by the COVID-19 pandemic, including school closures and other public health interventions. Analysis indicates a clear inverse relationship between varicella incidence and VarV vaccination rates; as vaccination coverage increased, the incidence of varicella significantly declined.
After the implementation of the VarV vaccination program, there was a 54.6% reduction in the incidence of varicella among children, a finding in line with other epidemiological studies. For instance, research conducted in Turkey,Citation13 revealed a decrease in varicella incidence by 91.7% among children ≤5 years old, and 39.5% among those aged 6–17 years following VarV integration into their national immunization program. Likewise, a study from the United StatesCitation14,Citation15 noted an 84.6% reduction in varicella cases among children aged 12 months to 12 years from 2005 to 2013, attributed to a robust two-dose VarV regimen. This trend may also be accentuated by the inclusion of older children who received both wild-type varicella and attenuated varicella vaccine viruses.
The study results demonstrated that while the effectiveness of one and two-dose VarV vaccination waned over time, the two-dose regimen maintained good vaccine effectiveness up to 7 years post-vaccination. Corroborating evidence comes from a U.S. studyCitation16 which found that two VarV doses could reduce severe varicella cases and outbreaks by 10%. Adhering to the recommended two-doses schedule is advised to achieve long-lasting immunity. Notably, 52.0% of varicella cases occurred in vaccinated children, slightly higher than other regions. However, as highlighted by the U.S. CDC and NCIRD,Citation17 this phenomenon is expected in highly vaccinated populations where overall incidence declines but breakthrough cases in vaccinees persist.Citation18 Post-implementation of Shenzhen’s program, 6.0% of children with prior varicella received unnecessary VarV vaccination after recovery. Primary VZV infection typically confers lifelong immunity, effectively protecting against reinfection.Citation19–21 Hence, revaccination is generally unnecessary for individuals with a clear varicella history. It is recommended that clinicians at vaccination centers thoroughly assess and confirm a patient’s history of varicella before providing vaccination recommendations tailored to individual circumstances.
Conclusion
The implementation of the free VarV program for school-aged children in Bao’an District, Shenzhen, has delivered impressive outcomes. Since the start of this project, vaccination rates have shown a consistent upward trend, while morbidity rates have significantly declined, indicating the effectiveness of the two-dose regimen. This program not only underscores the Shenzhen Municipal Government’s strong commitment to child health but also highlights the pivotal role of evidence-based preventive measures in managing infectious diseases. Continuing the VarV program is likely to further decrease the incidence of varicella among children, thereby mitigating outbreaks and lessening the disease’s familial and social impacts. We advocate for the expansion of this program across the nation, allowing a greater number of school-aged children to benefit from this policy. Such an expansion would not only extend health protection for children, but also enhance the overall strategy for varicella control in China, contributing positively to the nation’s health objectives.
Nevertheless, several challenges persist in the program’s execution. Specifically, by age six, the coverage rates for the recommended two doses and timely vaccination schedules require improvement. We recommend that healthcare providers intensify the screening for previous varicella exposure among vaccine recipients and provide tailored vaccination advice based on comprehensive assessments.
Limitations
This evaluation work still has some limitations. Firstly, our implementation effect analysis of the Live Attenuated Varicella Vaccination Project are unique nationwide, this could result in cognitive biases due to regional characteristics. Secondly, the research data collected through passive monitoring systems may not include cases that did not seek medical attention. Most reported varicella cases are clinically diagnosed, lacking laboratory confirmation. Third, the VarV varicella program in Shenzhen has been implemented for four years, and the observation years for birth cohorts are limited. Further observation and research are needed to explore differences in disease incidence across different age groups and long-term trends. Fifth,no vaccination titers were determined to assess seroprotection to demonstrate the effectiveness of vaccination. Finally, the accuracy of vaccination rate estimates can lead to the underestimation or overestimation of VarV Vaccine Effectiveness (VE). It may also introduce selection bias, affecting the reliability of the results.
Supplementary Material
Download MS Word (13.6 KB)Acknowledgments
Ziqi Wang and Linxiang Chen contributed to study design, data collection, analyze statistics, data interpretation, and manuscript writing. Fangfang Lu helped in revising and improving writing.Jing Peng and Fang Huang helped develop the study. The original draft was written by Ziqi Wang. XuXie, Dongfeng Kong the preeminent biostatisticians in China, and they participated in and guided the entire project design, data collection, statistical analysis, and graphical presentation. The article’s final version has been approved by all writers. We thanked XuXie and Dongfeng Kong for his assistance with this research.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2024.2364485
Additional information
Funding
References
- Wang M, Li X, You M, Wang Y, Liu X, Li Z, Zhao W, Jiang Z, Hu Y, Yin D. Epidemiological characteristics of varicella outbreaks — China, 2006–2022. China CDC Wkly. 2023;5(52):1161–7. doi:10.46234/ccdcw2023.218.
- Zhu YF, Li YF, Du Y, Zeng M. Epidemiological characteristics of breakthrough varicella infection during varicella outbreaks in Shanghai, 2008–2014. Epidemiol Infect. 2017;145(10):2129–36. doi:10.1017/S0950268817000772.
- Takahashi M, Otsuka T, Okuno Y, Asano Y, Yazaki T, Isomura S. Live vaccine used to prevent the spread of varicella in children in hospital[J]. The Lancet. 1974;2(7892):1288–90. doi:10.1016/s0140-6736(74)90144-5.
- Takahashi M. Development of a live varicella vaccine–past and future[J]. Jpn J Infect Dis. 2000;53(2):47–55.
- WHO. WHO vaccine-preventable diseases: monitoring system. 2020 global summary[EB/OL] (2020-07-15); 2020 Jan 16. https://apps.who.int/immunization_monitoring/globalsummary/schedules.
- European Centre for Disease Control and Prevention. Varicella: Recommended vaccinations; 2023. [EB/OL]. https://vaccine-schedule.ecdc.europa.eu/Scheduler/ByDisease?SelectedDiseaseId=11&SelectedCountryIdByDisease=-1.
- Wolfson LJ, Castillo ME, Giglio N, Meszner Z, Molnar Z, Vazquez M, Wysocki J, Altland A, Kuter BJ, Rickard J, et al. Varicella healthcare resource utilization in middle income countries: a pooled analysis of the multi-country MARVEL study in Latin America & Europe[J]. Hum Vaccines Immunother. 2019;15(4):932–41. doi:10.1080/21645515.2018.1559687.
- Hu Q, Zhang Q, Li Y, Zheng H, Liu Q, Tang L, Wang X, Yang H, Wen N, Yin Z, et al. Varicella vaccine coverage levels among 1-14-year-old children in China in 2020: a cross sectional survey[J]. Chin J Vaccines Immun. 2022;28(2):169–73. in Chinese. doi:10.19914/j.CJVI.2022033.
- Lu L, Wang C, Suo L, Li J, Liu W, Pang X, Seward JF. Varicella disease in Beijing in the era of voluntary vaccination, 2007 to 2010[J]. Pediatric Infect Dis J. 2013;32(8):314–8. doi:10.1097/INF.0b013e31828d948b.
- Dong PM, Wang M, Liu YM. Varicella epidemiology in China, 2016-2019[J]. Chin J Vaccines Immun. 2020;26(4):403–6. in Chinese doi:10.19914/j.cjvi.2020.04.011.
- Kim HJ, Fay MP, Feuer EJ, Midthune, DN. Permutation tests for joinpoint regression with applications to cancer rates[J]. Stat Med. 2000;19(3):335–51. doi:10.1002/(sici)1097-0258(20000215)19:3<335:aid-sim336>3.0.co;2-z.
- Li YH. The impact of different varicella vaccination strategies on varicella in Guangdong Province[D]. Guangdong Province, China: Southern Medical University; 2023. in Chinese. doi:10.27003/d.cnki.gojyu.2023.001001.
- Soysal A, Gonullu E, Yildiz İ, Karaböcüoğlu M. Incidence of varicella and herpes zoster after inclusion of varicella vaccine in national immunization schedule in Turkey: time trend study[J]. Hum Vaccines Immunother. 2021;17(3):731–7. doi:10.1080/21645515.2020.1788861.
- Lopez AS, Zhang J, Marin M. Epidemiology of Varicella During the 2-Dose Varicella Vaccination Program - United States, 2005-2014[J]. MMWR Morb Mortal Wkly Rep. 2016;65(34):902–5. doi:10.15585/mmwr.mm6534a4.
- Marin M, Guris D, Chaves SS, Schmid S, Seward JF. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP)[J]. MMWR Recomm Rep. 2007;56(RR–4):1–40.
- Shapiro ED, Marin M. The Effectiveness of Varicella Vaccine: 25 Years of Postlicensure Experience in the United States[J]. J Infect Dis. 2022;226(Suppl 4):425–30. doi:10.1093/infdis/jiac299.
- Guris D, Jumaan AO, Mascola L, Watson B, Zhang J, Chaves S, Gargiullo P, Perella D, Civen R, Seward J. Changing Varicella Epidemiology in Active Surveillance Sites—United States, 1995–2005. J Infect Dis. 2008;197(Suppl 2):71–5. doi:10.1086/522156.
- Duncan JR, Witkop CT, Webber BJ, Costello AA. Varicella seroepidemiology in United States air force recruits: A retrospective cohort study comparing immunogenicity of varicella vaccination and natural infection[J]. Vaccine. 2017;35(18):2351–7. doi:10.1016/j.vaccine.2017.03.054.
- Musharrafieh UM, Nuwayhid IA, Hamadeh GN, Steitieh SW, Bizri ARN. Immunity to chickenpox among school adolescents in Lebanon and options for vaccination[J]. Epidemiol Infect. 2002;129(3):607–15. doi:10.1017/s0950268802007938.
- Bakker KM, Eisenberg MC, Woods RJ, Martinez ME. Identifying optimal vaccination scenarios to reduce varicella zoster virus transmission and reactivation[J]. BMC Med. 2022;20(1):387. doi:10.1186/s12916-022-02534-7.
- Gershon AA, Breuer J, Cohen JI, Cohrs RJ, Gershon MD, Gilden D, Grose C, Hambleton S, Kennedy PGE, Oxman MN, et al. Varicella zoster virus infection[J]. Nat Rev Dis Primers. 2015;1(1):15016. doi:10.1038/nrdp.2015.16.