ABSTRACT
Kounis syndrome is an acute coronary syndrome triggered by allergic mediators that induce coronary vasoconstriction and thrombosis, leading to further myocardial damage and anaphylactic shock. Kounis syndrome is also a rare but severe adverse reaction to the COVID-19 vaccine, a phenomenon that underscores the importance of collecting and analyzing similar cases to improve treatment and prognosis. Through comprehensive searches of the Web of Science, Embase, and PubMed databases, this study aimed to gather detailed patient data on patients who developed Kounis syndrome after receiving the COVID-19 vaccine and to further investigate the possible underlying mechanisms using currently available studies. A total of 15 patients (8 females, 7 males) were found. We analyzed comprehensive patient data, including demographics, vaccination details, time of onset of illness after vaccination, clinical manifestations, treatment and outcomes, duration of illness, and relevant examination results. Analysis of these data combined with known allergy-related mechanisms indicated that, regardless of the vaccine type, the first dose of the vaccine was more likely to cause Kounis syndrome than subsequent doses. Therefore, early diagnosis and clinical symptomatic treatment are particularly crucial for managing the severity of Kounis syndrome and preventing further cardiac complications. Additionally, when an unusual and severe allergic reaction occurs within a few hours after vaccination, it is important to closely monitor the development of cardiac-related symptoms and prepare clinically for the potential onset of Kounis syndrome.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which emerged at the end of 2019, is a highly transmissible and pathogenic virus.Citation1 The virus caused a pandemic of an acute respiratory disease known as coronavirus disease 2019 (COVID-19),Citation2 posing a global threat to human well-being and public security.Citation3
The development and global approval of more than two dozen COVID-19 vaccines more than a year after the virus’ emergence played a crucial role in curbing the transmission of COVID-19 and reducing the death toll due to COVID‐19.Citation4–6 These vaccines utilized a variety of platforms, such as adenovirus vectors, mRNA, subunits, and inactivated SARS-CoV-2 or DNA technology. To date, only eight of these vaccines have received emergency use authorization (EUA) from the World Health Organization (WHO). Notable examples include the BNT162b2 mRNA-based vaccine from BioNTech/Pfizer, which utilizes specifically modified sequences of the spike protein-encoding gene; the AZD1222/ChAdOx1 vaccine from Oxford/AstraZeneca and the ChAdOx1_nCoV19 vaccine, marketed as Covishield, which are nonreplicating adenovirus vector-based DNA vaccines; and CoronaVac from Sinovac Biotech, which is an inactivated virus vaccine. The ChAdOx1 nCoV-19 vaccine (AZD1222) is a vectored vaccine developed at Oxford University, and consists of a replication-deficient chimpanzee adenoviral vector ChAdOx1, containing the full-length structural surface glycoprotein (spike protein) of SARS-CoV-2. The receptor-binding domain (RBD) and the N-terminal domain (NTD) are the main targets of the antibody response induced by the vaccines and the mutations in the domains may cause the immune escape. While these vaccines offer significant benefits, it is crucial to remain vigilant about their potential adverse effects.Citation7,Citation8
Kounis syndrome is a coronary artery disease associated with allergies, hypersensitivity, anaphylaxis and allergic-like reactions.Citation9,Citation10 Kounis syndrome is classified into three types, vasospastic angina pectoris, anaphylactic myocardial infarction and stent thrombosis, in which eosinophils and/or mast cells infiltrate the occluded thrombus.Citation8,Citation10 Its known manifestations and etiological agents are constantly expanding.Citation11 In addition to being induced by drugs and environmental exposures,Citation12 Kounis syndrome can also be induced by vaccines.Citation13 Treatment of Kounis syndrome follows existing guideline recommendations based primarily on electrocardiographic criteria, as no specific recommendations are currently available. The different types of Kounis syndrome are treated based on the symptoms, and treatments can be classified as pharmacological (including antiallergic, anticoagulant and cardiac-related medications) or nonpharmacological (including aspiration thrombectomy and balloon angioplasty).Citation14,Citation15
Although Kounis syndrome is an extremely uncommon clinical occurrence,Citation7,Citation16 cases of Kounis syndrome after vaccination have gradually been reported since COVID-19 vaccines became widely available, which raises similar clinical concerns. This study analyzed pooled case reports of Kounis syndrome after COVID-19 vaccination to better evaluate its clinical manifestations and medical examination features and to improve the ability of clinicians to recognize this rare syndrome in the early stage.Citation17
Methods
Search strategies
We searched the PubMed, Web of Science and Embase public databases up to January 15, 2024, for case reports of patients with Kounis syndrome associated with COVID-19 vaccination. Additionally, we reviewed all the references of the above case reports to ensure that no cases were missed. Our search formula in Web of Science was as follows: TS=(covid OR cov OR SARS-CoV-2 OR coronavirus) AND (vaccine OR vaccination OR vaccinium) AND (Kounis syndrome OR Anaphylactic myocardial infarction OR Allergic myocardial infarction). For the purpose of broad screening, we did not limit the field and identified the studies mentioned above.Citation18
Data extraction
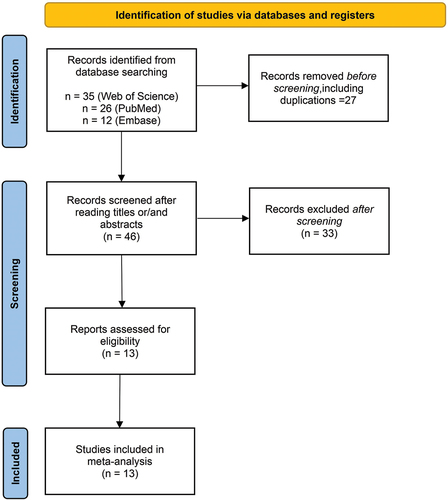
A total of 73 articles were retrieved (35 from Web of Science, 26 from PubMed, and 12 from Embase). After filtering out duplicate case reports (n = 50), reports unrelated to Kounis syndrome (n = 17), and studies that were not case reports (n = 31), 13 case reports comprising 15 patients were eventually included ().
We extracted relevant patient information, including age, sex, region, past medical history, vaccine type, number of vaccine doses, time of onset after vaccination, clinical presentation, examination results (including ECG, coronary angiography, cardiac enzymology, and echocardiography), and treatment.
Results
Basic information
A total of 15 patients who developed Kounis syndrome after COVID-19 vaccination were included in the study. The details are summarized in . Most of the 15 patients were from Asia and Europe (four from India; two from Turkey; two from China; and one each from Portugal, Italy, Sri Lanka, Kuwait, and Poland), with two patients from the United States, with an average age of 59 years. Seven patients received the Oxford-AstraZeneca AZD1222 COVID-19 vaccine, 4 patients received the Pfizer-BioNTech mRNA vaccine, 3 patients received the Sinovac Life Sciences Beijing vaccine, and 1 patient received the Moderna COVID-19 vaccine. Twelve patients experienced symptoms after the first dose of the vaccine, 2 patients experienced symptoms after the second dose, and only one patient experienced symptoms after the third dose. Eleven patients had past medical histories of cardiac (including angina and heart disease), allergic, or chronic conditions (including hypertension and diabetes).
Table 1. Basic information of the 15 included patients.
Clinical characteristics
The clinical presentations and major characteristics of the 15 patients are summarized in . The time between vaccination and the onset of symptoms ranged from 15 minutes to 12 days, with a median of 40 minutes. Patient onset can be divided into systemic and localized types. Four patients had systematic onset, including discomfort and itch. The localized onsets are as follows: 11 patients presented with chest pain, tightness, and discomfort; 6 patients presented with sweating; 5 patients presented with respiratory symptoms, including shortness of breath, dyspnea and cough, accompanied by heart rate changes with uncertain direction of heart rate variability (4 patients) and blood pressure decreases (3 patients); 3 patients presented with shock; 2 patients presented with skin changes, with discrete micropapular rash on the chest and flushing in 1 patient each; and 2 patients presented with nausea and vomiting.
Table 2. Patients from respective case reports included in the research.
Imaging results
The imaging results are shown in the table. Coronary angiography revealed vascular disease in seven of the 15 patients (3 patients with both thrombus and stenosis, one patient with stenosis, and one patient with thrombus). Of the other 8 patients, 3 had unknown angiographic results, 2 did not undergo angiography examination because of allergies, 2 had normal results, and 1 had a stent. Echocardiography revealed cardiac dysfunction or ventricular wall motion abnormalities in 13 patients.
Nonimaging results
Nonimaging examinations included electrocardiography and cardiac enzymology. None of the 15 patients presented with nonsinus arrhythmias, and sinus tachycardia was observed in only 1 patient. In addition, 14 patients showed ST-segment changes, which varied from lead to lead. T-wave inversions with QRS waveform changes were observed in 4 patients. In the myocardial enzymology tests, we collected data on cardiac troponin-I and troponin-T levels and creatine kinase-MB fractions. All recorded results from these tests were positive, indicating significant myocardial involvement.
Treatment and recovery
Treatments included pharmacological and nonpharmacological treatments. Of the patients who received pharmacological treatment, antithrombotic drugs were used in 12 patients (antiplatelet drugs in 7 patients and anticoagulants in 5 patients); antiallergic drugs were used in 5 patients; hormones were used in 5 patients; and cardiac-related medications were used in 5 patients, including medications for angina pectoris in 3 patients and myocardial infarction in 2 patients. The remaining drugs can be categorized as lipid-lowering drugs, antihypertensive drugs or antipyretic and analgesic drugs. The mode of administration of the 15 patients included intramuscular, intravenous, drug-coated balloon and implantable drug-eluting stent administration.
Nine of the fifteen patients received nonpharmacological treatments, and some patients received several treatments at the same time. Of the 9 patients, 4 underwent balloon dilatation, 3 underwent aspiration thrombectomy, 3 received oxygen therapy, and 3 underwent percutaneous coronary intervention (PCI), with the remaining six patients not receiving nonpharmacological related treatments.
Of the 16 patients, 15 were successfully cured, and one died; the median course of disease was approximately 5 days.
Discussion
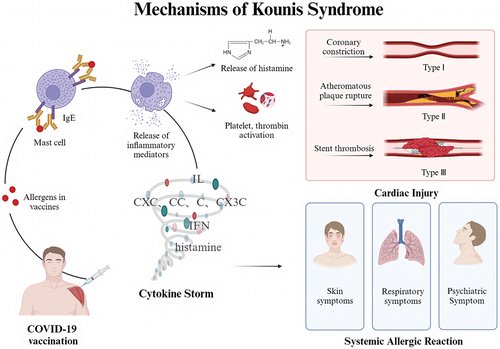
The release of allergic mediators induces coronary vasoconstriction and thrombosis, which can lead to further myocardial damage and acute coronary syndrome associated with anaphylaxis, known as Kounis syndrome,Citation19,Citation20 which is classified into 3 types: type I Kounis syndrome is characterized by coronary artery spasm with normal coronary arteries but no contributing cause of coronary artery disease; type II Kounis syndrome is characterized by atheromatous plaque rupture or coronary spasm with a preexisting atheromatous disease; and type III Kounis syndrome is characterized by stent thrombosisCitation21,Citation22 (). Kounis syndrome following COVID-19 vaccination is rare. As of October 2023, 68158,988 global COVID-19 vaccination doses have been administered, and we identified only 15 cases that met the criteria, although there is a possibility of incomplete case reporting. This finding is sufficient to support the rarity of Kounis syndrome among such a large number of vaccination doses.
To better discuss the Kounis syndrome-related cases, we summarized the basic case information and presenting symptoms. The majority of the patients were from Asia and Europe, and there was no significant correlation between the onset of Kounis syndrome and sex. According to the available cases, Kounis syndrome occurred after the first, second or booster doses of the COVID-19 vaccine but mainly developed after the first dose of the vaccine. Near half of the vaccines administered to the 15 patients were non‐replicating adenovirus vector‐based DNA vaccine. Most patients developed clinical manifestations associated with Kounis syndrome within 2 hours of vaccination and progressed rapidly.
As shown in , Kounis syndrome patients present with clinical manifestations of intense allergic reactions, including generalized itching, dyspnea, sweating and general malaise.Citation23 Inflammatory mediators released during anaphylactic injury, postinflammatory cellular activation, and multidirectional stimulus interactions are the main causative factors of Kounis syndrome.Citation24 Inflammatory mediators include histamine, neutral proteases, platelet-activating factor, arachidonic acid products, and a variety of cytokines and chemokines released during allergic activation, and inflammatory cells include interconnections and interactions among mast cells, macrophages, and T lymphocytes.Citation25,Citation26 Since 20% of the platelet subpopulation with high- and low-affinity IgE surface receptors are involved in this process, Kounis syndrome is not a single-organ disease but a multisystemic and multiorgan arterial clinical condition that may affect the skin, respiratory system and so on.Citation24 In addition to allergic symptoms, the patients presented with signs of acute coronary syndrome, including chest tightness, precordial pain, and restlessness. The heart, especially the coronary arteries, may be the main target of allergic inflammatory mediators;Citation25 the release of these mediators can lead to coronary artery spasm or induce further coronary artery damage, such as plaque rupture,Citation27 resulting in elevated cardiac troponin levels and causing the cardiac symptoms described above.Citation28 Notably, most patients had hypotension-related manifestations, which may have been caused by the combination of allergic reactions and myocardial injury factorsCitation29 ().
The examinations commonly used to diagnose Kounis syndrome are electrocardiogram (ECG), cardiac enzyme analysis, coronary angiography, and echocardiogram. The clinical ECG manifestations in patients with Kounis syndrome are ST-segment changes, and laboratory tests are positive for troponin. In addition, in most patients, echocardiographic findings suggest abnormal ventricular wall motion; the degree of reduced ejection function varies depending on disease progression. Most patients who underwent coronary angiography had thrombi or coronary stenosis. Coronary angiography results were missing in a total of five cases in this article: two patients did not undergo the test due to contrast allergy, one patient refused to undergo the test, and the reason for missing coronary angiography results in the remaining two cases was unknown. All of the above abnormalities may be associated with myocardial injury and myocardial infarction due to allergic reactions. In addition, ECG findings such as arrhythmias, elevated or depressed ST segments, any degree of cardiac block,Citation25 and a variety of electrocardiographic changes may be associated with the cardiac symptoms of Kounis syndrome.Citation30 In terms of laboratory findings, in addition to cardiac troponin, increases in serum tryptase, histamine, and cardiac biomarkers are particularly useful findings.Citation24,Citation27
Both cardiac and allergy treatments are recommended for the treatment of Kounis syndrome; however, the drugs for cardiac manifestations can exacerbate allergies, while the drugs for allergic symptoms can exacerbate cardiac insufficiency.Citation31 Treatment of acute coronary syndrome due to Kounis syndrome caused by COVID-19 vaccination should follow the existing guidelines based on ECG criteria, as no specific recommendations are currently available. For the different subtypes of Kounis syndrome, the appropriate treatment depends on the symptoms.Citation32 In type I subtype, coronary vasospasm can be relieved by vasodilators such as calcium chloride channel inhibitorsCitation33 (diltiazem was used in the present study) and nitrates. Other signs of hypersensitivity, such as asthma and skin rashes, can facilitate the recognition of an anaphylactic reaction at an early stage and lead to the judicious administration of corticosteroidsCitation33 such as hydrocortisone and antihistamines such as diphenhydramine; both drug classes were widely used in the cases reported here. In type II Kounis syndrome, treatment consists of an acute coronary event regimen as well as corticosteroids and antihistamines; in patients with type III Kounis syndrome, treatment includes the current acute myocardial infarction regimen; urgent aspiration of in-stent thrombus; and the use of antihistamines, corticosteroids, and mast-cell stabilizers if necessary.Citation21,Citation33
In summary, Kounis syndrome is a rare and serious adverse reaction that occurs after vaccination for COVID-19.Citation34 In this paper, a review of vaccination-related Kounis syndrome was conducted using the known cases. The specific etiology and pathway mechanisms of Kounis syndrome caused by the COVID-19 vaccine deserve further investigation. Considering the difficulty in confirming the diagnosis of Kounis syndrome in some patients,Citation35 as well as the small number of patients who were diagnosed but not reported, our study was not able to include all of the patients who developed Kounis syndrome after vaccination, so the statistical results have some limitations. In addition, the number of patients with Kounis syndrome is very small compared to the number of vaccinated patients, and a definitive association between vaccination and Kounis syndrome still needs to be confirmed by additional case‒control and direct experimental tests. However, given the symptoms of COVID-19, vaccination remains an encouraging option for susceptible populations despite the potential associated rare complications, such as Kounis syndrome.Citation36
Conclusion
In this article, we reviewed patients with Kounis syndrome that developed after COVID-19 vaccination and explored the possible underlying mechanisms involved. Early diagnosis and clinical symptomatic treatment are particularly crucial for managing the severity of Kounis syndrome and preventing further cardiac complications, and patients who develop abnormal and severe allergic reactions within hours of vaccination should be closely monitored for cardiac-related symptoms and clinically prepared for possible Kounis syndrome.
Author contribution
Chengjie Zhao and Ruoyan Lei contributed equally as the first authors. Mingyi Zhao contributed as a correspondence author. Siyang Liu contributed as an ordinary author. Mingyi Zhao, Chengjie Zhao, and Ruoyan Lei designed the study, Chengjie Zhao, Ruoyan Lei, and Siyang Liu carried out the manuscript searches and participated in data processing; Mingyi Zhao contributed to polishing articles, Chengjie Zhao, Ruoyan Lei, Siyang Liu and Mingyi Zhao revised the manuscript. All authors agree to be accountable for all aspects of the work.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data generated in this article are included in the paper and supplementary materials.
Additional information
Funding
References
- Talic S, Shah S, Wild H, Gasevic D, Maharaj A, Ademi Z, Li X, Xu W, Mesa-Eguiagaray I, Rostron J, et al. Effectiveness of public health measures in reducing the incidence of Covid-19, SARS-CoV-2 transmission, and COVID-19 mortality: systematic review and meta-analysis. BMJ (Clin Res Ed). 2021;375:e068302. doi:10.1136/bmj-2021-068302.
- Ma T, Liu D, Lyu K, Gao T, Shi D, Zhao L, Shen S, Tian Y, Xu S, Zhou H, et al. Establishment and application of national reference panels for SARS-CoV-2 antigen detection kit. Biosaf Health. 2023;5(6):326–10. doi:10.1016/j.bsheal.2023.10.002.
- Hu B, Guo H, Zhou P, Shi ZL. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19(3):141–54. doi:10.1038/s41579-020-00459-7.
- Feikin DR, Higdon MM, Abu-Raddad LJ, Andrews N, Araos R, Goldberg Y, Groome MJ, Huppert A, O’Brien KL, Smith PG, et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet (London, England). 2022;399(10328):924–44. doi:10.1016/S0140-6736(22)00152-0.
- Vitiello A, Ferrara F, Troiano V, La Porta R. COVID-19 vaccines and decreased transmission of SARS-CoV-2. Inflammopharmacology. 2021;29(5):1357–60. doi:10.1007/s10787-021-00847-2.
- Zhang Y, Wu G, Chen S, Ju X, Yimaer W, Zhang W, Lin S, Hao Y, Gu J, Li J, et al. A review on COVID-19 transmission, epidemiological features, prevention and vaccination. Med Rev (2021). 2022;2(1):23–49. doi:10.1515/mr-2021-0023.
- Mohamed K, Rzymski P, Islam MS, Makuku R, Mushtaq A, Khan A, Ivanovska M, Makka SA, Hashem F, Marquez L, et al. COVID-19 vaccinations: the unknowns, challenges, and hopes. J Med Virol. 2022;94(4):1336–49. doi:10.1002/jmv.27487.
- Menni C, Klaser K, May A, Polidori L, Capdevila J, Louca P, Sudre CH, Nguyen LH, Drew DA, Merino J, et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID symptom study app in the UK: a prospective observational study. Lancet Infect Dis. 2021;21(7):939–49. doi:10.1016/S1473-3099(21)00224-3.
- Wang C, Deng Z, Song L, Sun W, Fang W, Li Z. Analysis of clinical characteristics of Kounis syndrome induced by contrast media. Am J Emerg Med. 2022;52:203–7. doi:10.1016/j.ajem.2021.12.036.
- Tomasiak-Lozowska MM, Klimek M, Lis A, Moniuszko M, Bodzenta-Lukaszyk A. Markers of anaphylaxis – a systematic review. Adv Med Sci. 2018;63(2):265–77. doi:10.1016/j.advms.2017.12.003.
- Kounis N. Kounis syndrome: an update on epidemiology, pathogenesis, diagnosis and therapeutic management. Clin Chem Lab Med (CCLM). 2016;54(10):1545–59. doi:10.1515/cclm-2016-0010.
- Engheta M, Urbanczyk J, Fidone E, Escobedo Y, Mixon T. Kounis syndrome presenting as ST elevation acute myocardial infarction. Proc (Baylor Univ Med Center). 2021;34(4):500–2. doi:10.1080/08998280.2021.1907095.
- García AG, Romero EC, Martínez S, Antón ME, Gómez SD, Rodriguez F. Kounis syndrome in a patient after administration of influenza vaccine. Allergy. 2013;68:514–5.
- Hung MJ, Yeh CT, Kounis NG, Koniari I, Hu P, Hung MY. Coronary artery spasm-related heart failure syndrome: literature review. Int J Mol Sci. 2023;24(8):24. doi:10.3390/ijms24087530.
- Kounis NG, Koniari I, de Gregorio C, Velissaris D, Petalas K, Brinia A, Assimakopoulos SF, Gogos C, Kouni SN, Kounis GN, et al. Allergic reactions to current available COVID-19 vaccinations: pathophysiology, causality, and therapeutic considerations. NATO Adv Sci Inst Se. 2021;9(3):221. doi:10.3390/vaccines9030221.
- Ozdemir IH, Ozlek B, Ozen MB, Gunduz R, Bayturan O. Type 1 Kounis syndrome induced by inactivated SARS-COV-2 vaccine. J Emerg Med. 2021;61(4):71–6. doi:10.1016/j.jemermed.2021.04.018.
- Xu K, Gao B, Li J, Xiang Y, Cao L, Zhao M. Clinical features, diagnosis, and management of COVID-19 vaccine-associated Vogt-Koyanagi-Harada disease. Hum Vaccines Immunother. 2023;19(2):2220630. doi:10.1080/21645515.2023.2220630.
- Zhang HQ, Cao BZ, Cao QT, Hun M, Cao L, Zhao MY. An analysis of reported cases of hemophagocytic lymphohistiocytosis (HLH) after COVID-19 vaccination. Hum Vaccines Immunother. 2023;19(2):2263229. doi:10.1080/21645515.2023.2263229.
- Kounis NG, Cervellin G, Koniari I, Bonfanti L, Dousdampanis P, Charokopos N, Assimakopoulos SF, Kakkos SK, Ntouvas IG, Soufras GD, et al. Anaphylactic cardiovascular collapse and Kounis syndrome: systemic vasodilation or coronary vasoconstriction? Annals of translational medicine. Ann Transl Med. 2018;6(17):332. doi:10.21037/atm.2018.09.05.
- Allam C, Saouma M, Chlawit R. Kounis syndrome must be considered in the differential diagnosis of myocardial infarction following COVID-19 vaccination. QJM: Monthly J Assoc of Phys. 2022;115(11):783–4. doi:10.1093/qjmed/hcac001.
- Roumeliotis A, Davlouros P, Anastasopoulou M, Tsigkas G, Koniari I, Mplani V, Hahalis G, Kounis N. Allergy associated myocardial infarction: a comprehensive report of clinical presentation, diagnosis and management of Kounis syndrome. Vaccines. 2021;10(1):10. doi:10.3390/vaccines10010038.
- Giovannini M, Koniari I, Mori F, Barni S, Novembre E, Kounis NG. Kounis syndrome: Towards a new classification. Int J Cardiol. 2021;341:13–14. doi:10.1016/j.ijcard.2021.04.018.
- Ralapanawa DM, Kularatne SA. A case of Kounis syndrome after a hornet sting and literature review. BMC Res Notes. 2014;7(1):867. doi:10.1186/1756-0500-7-867.
- Kounis NG, Koniari I, Velissaris D, Tzanis G, Hahalis G. Kounis syndrome—not a single-organ arterial disorder but a multisystem and multidisciplinary disease. Balkan Med J. 2019;36(4):212–21. doi:10.4274/balkanmedj.galenos.2019.2019.5.62.
- Kounis NG. Kounis syndrome: an update on epidemiology, pathogenesis, diagnosis and therapeutic management. Clin Chem Lab Med (CCLM). 2016;54(10):1545–59. doi:10.1515/cclm-2016-0010.
- Alblaihed L, Huis in ‘t Veld MA. Allergic acute coronary syndrome—Kounis syndrome. Emerg Med Clin North Am. 2022;40(1):69–78. doi:10.1016/j.emc.2021.08.010.
- Kounis NG, Koniari I, de Gregorio C, Assimakopoulos SF, Velissaris D, Hung MY, Mplani V, Saba L, Brinia A, Kouni SN, et al. COVID-19 disease, women’s predominant non-heparin vaccine-induced thrombotic thrombocytopenia and Kounis syndrome: a passepartout Cytokine storm interplay. Biomedicines. 2021;9(8):959. doi:10.3390/biomedicines9080959.
- Ye Q, Wang B, Mao J. The pathogenesis and treatment of the `Cytokine storm’ in COVID-19. J Infect. 2020;80(6):607–13. doi:10.1016/j.jinf.2020.03.037.
- Fajgenbaum DC, June CH, Longo DL. Cytokine Storm. N Engl J Med. 2020;383(23):2255–73. doi:10.1056/NEJMra2026131.
- Alblaihed L, Huis in ‘t Veld MA. Allergic acute coronary syndrome—Kounis syndrome. Immunol Allergy Clin North Am. 2023;43(3):503–12. doi:10.1016/j.iac.2022.10.010.
- Doğan V, Mert G, Biteker FS, Mert KU, Biteker M. Treatment of Kounis syndrome. Int J Cardiol. 2015;181:133–4. doi:10.1016/j.ijcard.2014.11.171.
- Cevik C, Nugent K, Shome GP, Kounis NG. Treatment of Kounis syndrome. Int J Cardiol. 2010;143(3):223–6. doi:10.1016/j.ijcard.2010.02.040.
- Abdelghany M, Subedi R, Shah S, Kozman H. Kounis syndrome: a review article on epidemiology, diagnostic findings, management and complications of allergic acute coronary syndrome. Int J Cardiol. 2017;232:1–4. doi:10.1016/j.ijcard.2017.01.124.
- Renda F, Landoni G, Trotta F, Piras D, Finco G, Felicetti P, Pimpinella G, Pani L. Kounis syndrome: an analysis of spontaneous reports from international pharmacovigilance database. Int J Cardiol. 2016;203:217–20. doi:10.1016/j.ijcard.2015.10.003.
- Maloberti A, Pansera F, Sala O, Fusco R, Pierri A, Bossi I, Giannattasio C. Kounis syndrome: report of two cases with peculiar presentation and diagnostic issues. High Blood Press Cardiovasc Prev. 2019;26(2):145–9. doi:10.1007/s40292-019-00312-w.
- Meo SA, Bukhari IA, Akram J, Meo AS, Klonoff DC. COVID-19 vaccines: comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and moderna vaccines. Eur Rev Med Pharmacol Sci. 2021;25(3):1663–9. doi:10.26355/eurrev_202102_24877.
- Allam C, Kounis NG, Chlawit R, Saouma M, Badaoui G. Kounis syndrome following COVID-19 vaccination. Proc (Baylor Univ Med Center). 2022;35(3):369–70. doi:10.1080/08998280.2022.2034226.
- Fialho I, Mateus C, Martins-Dos-Santos G, Pita J, Cabanelas N, Baptista SB, Roque D. Recurrent Kounis syndrome – a life-threatening event after COVID-19 vaccine administration. J Cardiol Cases. 2022;25(6):400–3. doi:10.1016/j.jccase.2022.01.014.
- Minciullo PL, Amato G, Vita F, Pioggia G, Gangemi S. ATAK complex (Adrenaline, Takotsubo, Anaphylaxis, and Kounis Hypersensitivity-Associated Coronary Syndrome) after COVID-19 vaccination and review of the literature. NATO Adv Sci Inst Se. 2023;11(2):11. doi:10.3390/vaccines11020322.
- Farook AM, Priyankara D. Kounis syndrome in a patient following AstraZeneca coronavirus disease 2019 vaccination: a case report. J Med Case Rep. 2023;17(1):289. doi:10.1186/s13256-023-04022-9.
- Deng Y, Peng Z, Peng X. Type I Kounis syndrome induced by COVID-19 vaccination in China: a case report. BMC cardiovascular disorders. BMC Cardiovasc Disord. 2023;23(1):267. doi:10.1186/s12872-023-03289-6.
- Sanci E, Orcen C, Celik OM, Ozen MT, Bozyel S. Kounis syndrome associated with BNT162b2 mRNA COVID-19 vaccine presenting as ST-elevation acute myocardial infarction. Anatol J Cardiol. 2022;26(1):69–74. doi:10.5152/AnatolJCardiol.2021.1212.
- Maadarani O, Bitar Z, Elzoueiry M, Nader M, Abdelfatah M, Zaalouk T, Mohsen M, Elhabibi M. Myocardial infarction post COVID-19 vaccine – coincidence, Kounis syndrome or other explanation – time will tell. JRSM Open. 2021;12(8):20542704211025259. doi:10.1177/20542704211025259.
- Chatterjee S, Ojha UK, Vardhan B, Tiwari A. Myocardial infarction after COVID-19 vaccination-casual or causal? Diabetes Metab Syndr. 2021;15(3):1055–6. doi:10.1016/j.dsx.2021.04.006.
- Boivin Z, Martin J. Untimely myocardial infarction or COVID-19 vaccine side effect. Cureus. 2021;13:e13651. doi:10.7759/cureus.13651.
- Tajstra M, Jaroszewicz J, Gąsior M. Acute coronary tree thrombosis after vaccination for COVID-19. JACC Cardiovasc Interv. 2021;14(9):103–4. doi:10.1016/j.jcin.2021.03.003.
- Ou W, Wang B, Zhang G, Ding L, Liu Z, Wu K, Sun G, Huang C, Li Z, Feng S, et al. Acute myocardial infarction after inactivated COVID-19 vaccination: a case report and literature review. Front Cardiovasc Med. 2023;10:1123385. doi:10.3389/fcvm.2023.1123385.
- Srinivasan KN, Sathyamurthy I, Neelagandan M. Relation between COVID-19 vaccination and myocardial infarction – Casual or coincidental? IHJ Cardiovasc Case Rep (CVCR). 2021;5(2):71–4. doi:10.1016/j.ihjccr.2021.05.003.