ABSTRACT
A growing body of research indicates the promising potential of vaccines in both preventing and treating atherosclerosis (AS). To gain a comprehensive understanding of the current research landscape and emerging trends in this field, this study conducted a bibliometric analysis of publications on AS vaccines using the Web of Science Core Collection (WoSCC) database, based on the “bibliometric” R package and VOSviewer software. From 1991 to 2024, a total of 462 publications were identified in the WoSCC. The United States appeared as the leading contributor in terms of both total publications and citations. The Vaccine journal exhibited the highest publications output. Nilsson J from the Lund University in Sweden was the author with the most published articles, total citations and Hirsch index (H-index). Keywords analysis and thematic maps analysis revealed the passive immunotherapy (AS protective antibodies vaccines) was a hot mature theme, the active immunotherapy (AS antigens vaccines) was an emerging and booming theme, while the efficacy and safety of AS vaccines was a niche and well-developed theme. These findings offered valuable insights into the AS vaccination and provided guidance for future research in this domain.
Introduction
Atherosclerosis (AS), a chronic vascular disease driven by the imbalance of cholesterol homeostasis, inflammation and immunityCitation1, stands as the underlying pathology of cardiovascular disease (CVD), contributing to premature mortality and escalating healthcare expenditures worldwide.Citation2 The pursuit of effective strategies for treating and preventing AS has emerged as a focal point of research, particularly with recent attention drawn to inflammatory and immune mechanisms.Citation3 Inflammatory mediators ranging from pathogens, immunoglobulins, cytokines, and chemokines, as well as immune cells such as macrophages, lymphocytes, and dendritic cells, play pivotal roles in the initiation and progression of AS.Citation4,Citation5
In recent years, the preclinical development of AS vaccines has made rapid progress, lipid-lowering and anti-inflammatory monoclonal antibodies have been tested and applied in clinic, and the research of AS-related antigens and adjuvants has also become popular.Citation6,Citation7 However, the translation of these advancements into large-scale therapeutic interventions necessitates further clinical trials and testing in animal models that faithfully mimic human lipid metabolism.Citation8 Despite the burgeoning literature and research activity surrounding AS vaccines, a dearth of objective and comprehensive information persists, including data on publication counts, countries, institutions, journals, authors, as well as keyword clustering analysis and thematic hotspots in AS vaccine research.
Bibliometrics, renowned for its ability to quantitatively characterize fields of study and pinpoint research hotspots, serves as a valuable tool for illuminating primary issues and forecasting future trajectories.Citation9 Therefore, this study aims to comprehensively analyze the publications on AS vaccines in the Web of Science Core Collection (WoSCC) database, present research hotspots and cutting-edge trends, and propose feasible suggestions for future research directions.
Materials and methods
Dataset establishment
WoSCC database stands as one of the foremost comprehensive, systematic, and authoritative repositories globally, often utilized for visualizing scientific literature.Citation10 To procure data that is both representative and accurate, the retrieval parameters were delineated as follows: TS = (“vaccine*” OR “vaccination” OR “vaccinate*” OR “vaccinating”) AND (“Atherosclerosis” OR “Atheroscleroses” OR “Atherogenesis” OR “Atheromatous Plaque*” OR “Arteriosclerosis” OR “Coronary Disease*” OR “coronary artery disease” OR “Atherosclerotic Plaque*” OR Carotid Artery Disease* OR “Fibroatheroma*” OR “Arterial Fatty Streak*” OR “Atheroma*”). To facilitate subsequent analysis, the citation edition was confined to the Science Citation Index Expanded (SCI-Expanded), and only articles and reviews written in English were included. All bibliographic records, encompassing titles, authors, keywords, institutions, abstracts, references, publication year, etc., were archived in plain text format. Data retrieval was conducted on February 8th, 2024. Two authors (BCJ and RW) autonomously executed data identification and screening of the retrieved literature. In instances of disagreement, a third author (YHH) was consulted for arbitration. Given that the research data was derived from an open database, ethical considerations were not applicable.
Database analysis
The screened literature data was imported into Biblioshiny and VOSviewer (version 1.6.19) for visual analysis. Biblioshiny, powered by the R software 4.3.2 Bibliometrix package (version 4.1.4), offered an intuitive and well-structured interface to quantitatively analyze annual publications and visualize national cooperation, core journals, author publication timelines, as well as trend and thematic maps of keywords within the realm of AS vaccines. VOSviewer specializes in co-occurrence network visualization of scientific knowledge.Citation11 In this study, VOSviewer was leveraged to construct keyword co-occurrence network and density maps. In the co-occurrence network graph, keywords were represented as nodes, with larger nodes denoting higher frequency of keywords occurrences. Keywords sharing similar features were clustered together and assigned the same color ().
Furthermore, the Hirsch index (H-index) served as a metric for evaluating the academic output and influence of researchers, indicating that a researcher published h articles, each cited at least h times.Citation12 The multiple country publications (MCP) ratio was employed to gauge national cooperation,Citation13 while the impact factor (IF) and journal citation report (JCR) zoning were utilized to assess the quality of scientific literature.Citation14 Bradford’s law was applied to delineate the core journals in the field of AS vaccines.Citation15
Results
Publication and citation summary
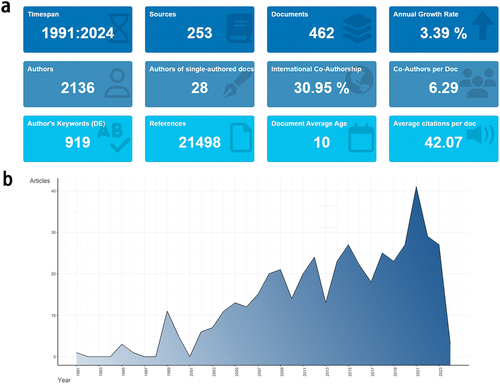
In 1991, the first research article related to AS and vaccines appeared. showcased 2,136 authors published a total of 462 papers related to AS vaccines between 1991 and 2024, comprising 294 articles and 168 reviews. Over this period, the average annual growth rate of published papers is 3.39%, a total of 919 keywords are involved 21,498 references are cited, and the average citation frequency of the literature is 42.07 times. The annual publication volume within distinct time frames serves as a quantitative indicator of the developmental trajectory within a research domain, and based on this, the evolution of the AS vaccine field can be segmented into three discernible stages (). The period spanning from 1991 to 2004 marked an initial stage, characterized by an annual publication volume of less than 12 articles. Notably, six years within this timeframe witnessed no publications, suggesting an absence of a cohesive research framework. The subsequent period, from 2005 to 2018, witnessed a gradual upward trajectory, with the annual publication volume hovering around 20 articles, albeit exhibiting a sluggish growth trend, indicative of a burgeoning interest in the field. The years 2019 to 2021 heralded a phase of rapid expansion, with the total number of publications in 2021 more than doubling in comparison to 2019. This surge may be attributed to the COVID-19 pandemic, which catalyzed a surge in vaccine development, subsequently fostering rapid advancements in the AS vaccine domain. The period from 2022 to 2023 was a phase of stabilized growth, characterized by a marginal decline in annual publication volume, yet still maintaining a threshold of over 25 articles per annum.
Analysis of countries or regions
The publications of AS vaccines emanated from 52 countries or regions. delineates the top 10 countries based on publication quantity and total citation (TC). The United States led in both metrics, garnering 113 articles and 7,660 TCs. Following suit were China (52 articles) and the Netherlands (34 articles) in terms of publication quantity, with Sweden (1,793 TCs) and the Netherlands (1,504 TCs) trailing behind in total citation. presents a global map of national cooperation, illustrating link lines between countries with more than five cooperative publications. The wider the link line, the higher the frequency of cooperation between the two countries. Notable collaborations include those between the United States and Sweden (15 articles), the Netherlands and Germany (13 articles), and the United States and Germany (10 articles). The proportion of MCP is an indicator to evaluate inter-country cooperation. Among the top 10 countries in terms of publication quantity, the Netherlands and Iran exhibit a higher proportion of MCP (≥50%), indicating strong cooperation between these two countries ().
Figure 3. Distribution map of countries. a) the Country collaboration map. b) the proportion of SCP and MCP in the top ten countries ranked by publication output. SCP: single country publications. MCP: multiple country publications.
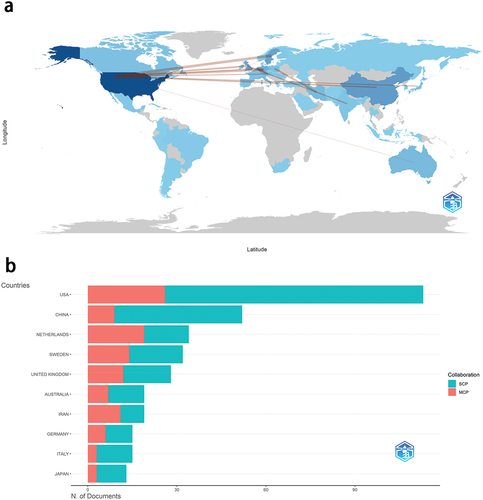
Table 1. Top 10 contributing countries/regions related to atherosclerosis vaccine.
Analysis of authors
In order to ascertain the most influential authors within this field, a ranking based on the total publications (TP), total citations (TC) and H-index was conducted (). provides the three-field plot of countries, institutions, and authors. Nilsson J, from Lund University in Sweden, is the foremost figure in terms of both publication quantity and impact, boasting the highest TP, TC, and H-index. His research primarily focuses on CVD and immunology. illustrates the publications trajectory of the main authors over time. Pioneers such as Liu JJ, Wu J, and Hansson GK initiated research in AS vaccines as early as 2004. Nilsson J had consistently published new articles from 2005 to 2021 establishing himself as a central figure in AS vaccine research. Banach M appeared as a prominent contributor in the past three years, with a surge in publications, signifying his ascent as a new force in this research domain.
Figure 4. Distribution map of authors. a) Three-Field Plot-country, institution, author. b) Author’s production over time.
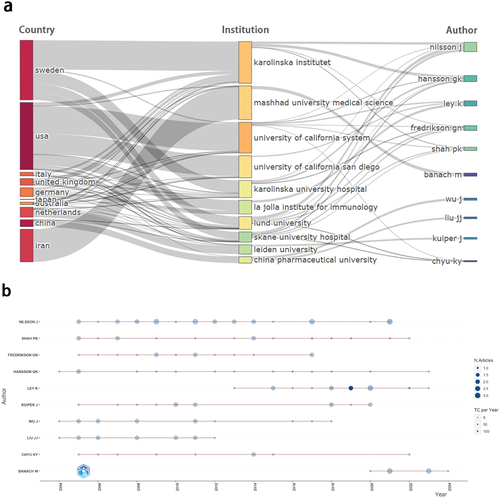
Table 2. The most influential authors in the field of atherosclerosis vaccine.
Analysis of journals and most cited publications
A total of 253 journals published studies relevant to AS vaccines. The top 10 journals collectively contributed 91 papers, representing approximately 20% of the total publications. Vaccine ranks first in the TP, with its primary research focus on immunology. Atherosclerosis follows closely behind, focusing primarily on heart and cardiovascular system. Atherosclerotic Thrombosis and Vascular Biology ranks third in TP, with a primary emphasis on blood diseases and vascular diseases. The top three journals with the highest IF are European Heart Journal (39.3), Circulation (37.8) and Circulation Research (20.1). (). shows the core journals in the field of AS vaccines calculated by the Bradford’s law.
Figure 5. Core sources by Bradford’s Law. The vertical axis: number of articles, the horizontal axis: journals.
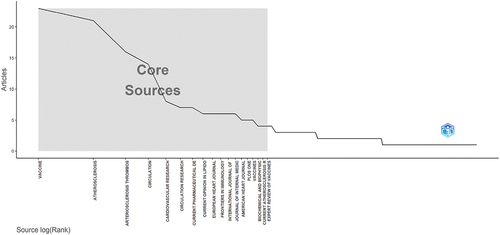
Table 3. The top 10 journals related to atherosclerosis vaccine.
Analyzing the most frequently cited publications provides valuable insights into critical advancements within the research field. showcases the 10 most cited papers. Topping the list is an article titled “Immunity and Inflammation in Atherosclerosis” authored by Wolf D et al. and published in Circulation Research in 2019. This paper delineated evidence of autoimmune reactions in AS, underscored the existing challenges in elucidating the mechanisms of AS vaccines and emphasized the necessity of addressing specific dosage, pathways, and adjuvant selection. The top 10 cited publications predominantly offer guidance for AS vaccine development, exploring themes such as inflammation, immunity, infection, and aging.
Table 4. Top 10 highest citation publications in the field of atherosclerosis vaccine.
Analysis of keywords
Keyword analysis was utilized to uncover research trends, identify hot topics, and monitor scientific progress. Synonyms were consolidated (such as “vaccines” to “vaccine”), resulting in the identification of 2,250 keywords from 462 articles, from which 164 keywords appeared more than 5 times. presents the keyword density visualization generated by VOSviewer, where higher frequencies are denoted by yellow colors. Prominent keywords include “atherosclerosis,” “inflammation,” “low density lipoprotein (LDL),” “immunization,” and “vaccine.” displays a co-occurrence network diagram of keywords, delineated into 6 clusters, which can be further distilled into 4 clusters with refined content. Cluster 1: AS vaccine and lipid regulation. Representative keywords include “vaccine,” “immunization,” “cholesterol,” and “proprotein convertase subtilisin/kexin type-9 serine protease (PCSK9),” etc. Cluster 2: AS vaccine and anti-inflammatory. Representative keywords include “inflammation,” “C-reactive protein,” “infection,” and “influenza vaccination,” etc. Cluster 3: Antigens related to AS vaccines. Representative keywords include “LDL,” “oxLDL,” “regulatory T-cell,” and “apo b-100,” etc. Cluster 4: The efficacy and risk of AS vaccines. Representative keywords include “prevention,” “risk,” “association,” and “disease,” etc.
Figure 6. Visualization of keywords. a) the density visualization of keywords. b) the cluster map of keywords.
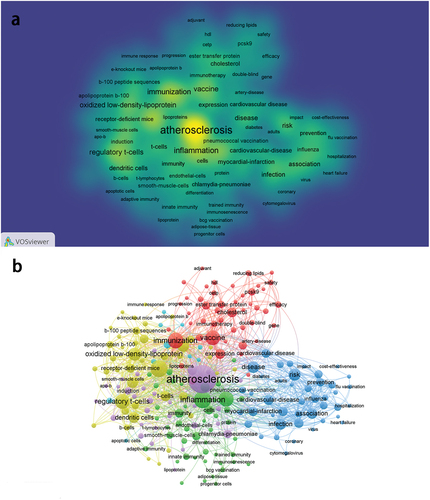
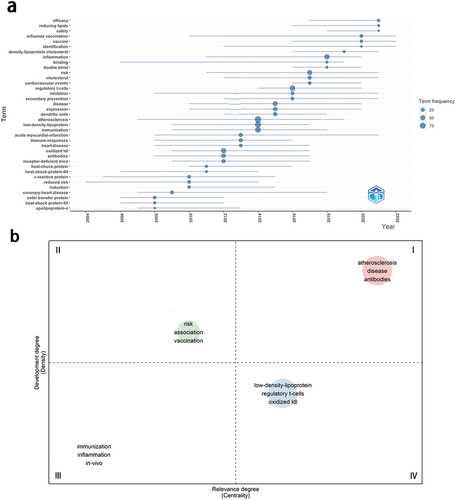
In the trend topic map (), keywords corresponding to circles represent high-frequency theme words of the year. Larger circles denote higher frequencies, while blue lines signify the time period of keyword appearance. Notable keywords in the past 5 years include “efficiency,” “reducing lipids,” “safety,” “influenza vaccination,” and “identification.”
The thematic map was applied to explore the evolutionary patterns of research topics and anticipate future research directions. The horizontal axis of the map denotes centrality, while the vertical axis represents density. Higher centrality values indicate themes occupying a core position and possessing close relationships with other themes, while higher density values suggest themes with greater maturity. The map is partitioned into four quadrants: the first quadrant is the core theme with high maturity, the second quadrant is the highly specialized niche theme that is becoming increasingly popular, the third quadrant refers to certain newly developed or soon-to-decline themes, and the fourth quadrant is the fundamental theme of significant importance that has not yet been developed. Bubbles marked with the most frequent keywords signify clustering, with their size directly proportional to the frequency of occurrence.Citation26 By synthesizing cluster map of keywords () and trend topic map () to analyze the thematic map (), the following insights were gleaned: The theme of AS protective antibody vaccines occupied the first quadrant, indicating its high centrality and status at the forefront of research. Themes situated in quadrant four (corresponding to clusters 1 and 3) hold substantial development potential and may evolve into future research hotspots. The theme of the efficacy and safety of AS vaccines (corresponding to cluster 4) resides in the second quadrant representing a niche topic characterized by strong professionalism and growing attention. The theme of AS vaccines and inflammation regulation is situated in the third quadrant (corresponding to cluster 2), which is an emerging topic in the process of development and maturity.
Discussion
Vaccines, as an advanced method of preventing and treating diseases, have shown tremendous potential in addressing AS, increasingly catching the eye of researchers.Citation27 In this study, R and VOSviewer software were utilized to analyze 462 articles on AS vaccines in the WoSCC database. Globally, the number of publications on AS vaccines had been steadily climbing. A total of 2,136 authors from 52 countries or regions contributed to this research field. The United States led significantly in publication output and citation frequency, underscoring its pivotal role in this domain. Regarding international cooperation, the United States, the Netherlands, Germany, Sweden, and Iran exhibited more frequent partnerships with other countries. The author’s analysis not only underscored the pioneering efforts of Liu JJ, Wu J, and Hansson GK in the AS vaccine research domain, highlighting their substantial contributions to antigen selection, administration routes, and the delineation of immune regulatory mechanisms, but also identified Nilsson J as the foremost contributor in terms of publication volume, citation frequency, and H-index (). Nilsson J’s research has been instrumental in identifying antigen-specific epitopes of AS vaccines,Citation28 unraveling the molecular mechanisms of peptide vaccines and their corresponding antibodies,Citation29 and investigating the preventive effects of influenza vaccines on cardiovascular events.Citation30 Among the top 10 journals with the highest number of publications in this field, 6 fell within the Q1 region. The IF of European heart journal was the highest, at 39.3, followed by Circulation with IF = 37.8, which mainly focused on the heart and cardiovascular system.
We compiled the most cited articles and conducted analyses by integrating keyword clustering maps (), trend topic maps (), and thematic maps (). Ultimately, we uncovered one hot mature topic, one niche and well-developed topic, and one rapidly evolving emerging topic. The ensuing discussion section seeks to delve into the current research challenges and future development directions across these three themes.
Hot mature topic: passive immunotherapy – vaccines for as protective antibodies
Monoclonal antibodies have demonstrated significant potential in preventing and managing AS plaques.Citation31 Current research predominantly targets monoclonal antibodies aimed at lipoproteins or cytokines. PCSK9, a serine protease binding to LDL receptors and elevating plasma LDL levels, is a key focus.Citation32 Notably, two most widely used human monoclonal antibodies targeting PCSK9 are Alirocumab and Evolocumab, which exhibit anti-AS effects via lipid-lowering, anti-inflammatory, and antioxidant pathways, earning approval in multiple regions.Citation33 Cytokines can diminish plaque stability and advance AS, while antibodies against cytokines like tumor necrosis factor-α, interleukin-1β, interleukin-6 and vascular cell adhesion molecule-1 significantly reduce inflammatory factors levels, reducing AS.Citation34,Citation35 Furthermore, antibodies specific to malondialdehyde-oxLDL, B cell activating factor-receptor, and aldehyde dehydrogenase 4 family member A1 have proven effective in reduce plaque burden and halting disease progression.Citation36–38
Despite strides in AS protective antibody research, hurdles remain before widespread clinical application. Challenges include the high cost and short duration of action of PCSK9 inhibitors, posing financial burdens on patients.Citation39 Additionally, the therapeutic potential of cytokine-related antibodies warrants further validation, with targeting cytokine antibodies like interleukin-17a and interleukin-12/23 potentially triggering pathogenic effects, heightening the risk of major adverse cardiovascular events (MACEs).Citation34 Further investigation into antibody specificity and mechanisms across different AS scenarios is essential. Enhancing existing antibodies through suitable adjuvants and other strategies can extend their duration of action, reduce treatment costs, and bolster clinical viability while maintaining efficacy.
Emerging and booming topic: active immunotherapy – vaccines for as associated antigens
The crux of developing AS vaccines lies in pinpointing specific antigens closely linked to AS formation to trigger immune responses within the body. Currently, researchers have developed vaccines targeting antigens intricately tied to AS’s pathological mechanisms, encompassing antigens related to plaque, lipid metabolism, vascular intimal hyperplasia, and pathogen infection, yielding promising outcomes as follows.
Vaccines for intraplaque antigens: LDL, oxLDL, and apolipoprotein B (ApoB) stand as the primary antigens in active immunity efforts against AS. These antigens inhibit AS progression by stimulating specific immune cell or antibody responses. Palinski pioneered the immunization of high-cholesterol rabbits with oxLDL, leading to heightened oxLDL antibody levels.Citation40 Subsequent studies corroborated that using oxLDL or LDL as antigens increased corresponding antibody levels in experimental rabbits or mice, thereby mitigating AS lesions.Citation41–43 Moreover, Treg cells, a T cell subtype, produce diverse anti-inflammatory cytokines like interleukin-10 and transforming growth factor beta, exerting anti-AS effects.Citation44 Trials of ApoB peptide and heat shock protein (HSP)-related vaccines in animal models revealed that vaccination with human ApoB-100 derived peptide antigens like p2, p18, p143, and p210, as well as HSP60/65 protein, induced protective Treg cells, thereby reducing AS .Citation45–49
Vaccines for lipid metabolism antigens: cholesteryl ester transfer protein (CETP) is a plasma protein that promotes the transport of cholesterol esters and triglycerides between lipoproteins.Citation50 The paper titled “Vaccine-induced antibodies inhibit CETP activity in vivo and reduce aortic lesions in a rabbit model of atherosclerosis” by C W Rittershaus et al., ranking ninth in citation frequency, employed a peptide containing the CETP region as an antigen to synthesize the vaccine and immunize AS rabbits. Results manifested that the vaccine significantly reduced CETP activity in model rabbits, elevated plasma high density lipoprotein (HDL) ratio, decreased LDL ratio, and ultimately diminished AS lesion percentage on the aorta’s surface.Citation24
Vaccines for vascular endometrial hyperplasia antigens: the migration of vascular smooth muscle cells contributes to abnormal vascular tension and intimal thickening, accelerating AS development.Citation51 Zihan Ma et al. devised a novel vaccine (ATS7vac) utilizing B cell epitopic peptides derived from the metalloproteinase ADAMTS-7, which could significantly inhibit ADAMTS-7 mediated degradation of cartilage oligomeric matrix protein and platelet reactive protein-1, thereby restraining vascular smooth muscle cell migration and exerting anti-AS effects.Citation52
Vaccines for pathogen antigens: Chronic inflammation induced by pathogen infection correlates closely with AS formation.Citation5 “Association Between Influenza Vaccination and Cardiovascular Outcomes in High-Risk Patients A Meta-analysis,” ranked as the 7th most frequently cited article in AS vaccine research, systematically evaluated influenza vaccines’ impact on cardiovascular events across six randomized clinical trials involving 6735 patients.Citation22 Results indicated influenza vaccine administration reduced MACEs. The third most frequently cited article in this field, “Pneumococcal vaccination decreases atherosclerotic lesion formation: molecular mimicry between Streptococcus pneumoniae and oxidized LDL,” elucidated the mechanism of the pneumococcal vaccine against AS, that is, the pneumococcal vaccine induced sustained expansion of specific B cells in mice, leading to high circulating levels of oxLDL specific IgM and T15 IgM, effectively blocking oxLDL binding to macrophages and thereby reducing AS pathology.Citation19 Additionally, vaccines designed with antigens from pathogens like Porphyromonas gingivalis, intestinal worms, and cytomegalovirus offer potential for AS prevention and treatment.Citation53–56
Vaccines targeting multiple antigens: Long Jun et al. engineered a vaccine targeting both Hsp65 and CETP antigens, evaluating its impact on AS lesions in rabbits fed high-cholesterol diets.Citation57 Test results unveiled the vaccine’s ability to elicit specific antibodies in serum, nasal cavity and lung, exerting a combined effect of anti-inflammatory and lipid-regulating effect, significantly reducing atherosclerotic plaque size. Xinjie Lu et al. integrated multiple antigenic epitopes from ApoB100, HSP60, and Chlamydophila pneumoniae into a dendroaspin scaffold, immunizing mice with alum adjuvant.Citation58 Their study showcased that the multi-target vaccine induced robust Treg cell responses, markedly enhancing protective effect against AS.
Current passive immunotherapy strategies for AS, which are mediated through the administration of antibodies, targets the direct modulation of inflammation or lipid metabolism to elicit anti-AS effects. However, these approaches are hindered by the limited duration of the antibodies’ action, necessitating frequent administrations to sustain long-term effects.Citation59 Nevertheless, the transient efficacy of these antibodies necessitates repeated doses to achieve sustained therapeutic outcomes. In contrast, active immunization strategies utilizing vaccines to provoke an antigen-specific response, offer a prolonged and highly targeted anti-AS effect, alongside being cost-effective. Addressing current hurdles, including the refinement of immune cell selection processes, active immunotherapy is anticipated to ascend as the premier prophylactic approach against AS.
Moreover, recent studies underscore the pivotal role of gut microbiota composition and functional changes in immune regulation, implicating their influence on AS onset and progression.Citation60 Consequently, gut microbiota is expected to be a promising new target for CVD treatment.Citation60,Citation61 However, the development of AS vaccines leveraging gut microbiota as antigens or carriers remains nascent. The future prospects of AS vaccines utilizing gut microbiota as antigens or carriers hold vast potential.
Niche and well-developed topic: the efficacy and safety of as vaccines
Distinct subtypes of immune cells or antibodies exert varying effects on AS. For instance, CD4+T cells differentiate into diverse subtypes such as Th cells or Treg cells upon antigen presentation by antigen-presenting cells. Among these, Th1 cells exhibit a pro-AS effect, while Tregs serve as protective agents against AS.Citation62 B cells, likewise, produce two subtypes: B-1 and B-2. B-1 cells generate protective IgM antibodies, whereas B-2 cells produce class-switched antibodies (IgG, IgA, and IgE), potentially contributing to AS.Citation63 Nonetheless, recent findings indicate that B-2 cells also yield AS protective antibodies like IgG2b against ALDH4A1, complicating immune cell screening. To ensure the effectiveness and safety of vaccines, it is imperative to induce specific immune cells or antibodies with AS protective function through antigen selection targeting.Citation45,Citation64 However, the precise mechanism of immune cell transformation in AS remains largely unknown, posing challenges in antigenic epitope screening. Misdirected vaccines, inadvertently inducing immune cells or antibodies that promote AS, can inflict serious harm.
We propose three suggestions to enhance the accuracy, stability, and safety of AS vaccines in eliciting protective immune responses. (1) Continued Research: Investigate immune cell transformation and antibody class transformation mechanisms, delving into the effects of different immune cell and antibody subtypes on AS. (2) Advanced Technologies: Utilize bioinformatics methods and innovative technologies such as protein mass spectrometry, T cell sequencing, and antigenomics to predict and screen new antigens. (3) Establish a large-scale AS antigen epitope database through international collaboration, employing artificial intelligence algorithms like machine learning to develop epitope prediction tools, thereby furnishing new targets for AS vaccine development.
Adjuvants are pivotal components of vaccines, and selecting safe and effective adjuvants to bolster the host’s immune response to antigens holds great importance.Citation65 Classical adjuvants employed in clinical practice have demonstrated commendable biosafety; however, their immune-stimulating capacity is constrained. While the vaccine inoculated with complete and incomplete Freund’s adjuvant (CFA/IFA) have shown promise in protecting mice from atherosclerosis, the adverse reactions associated with CFA/IFA have impeded their clinical utility.Citation66 Presently, there has been notable progress in researching novel adjuvants. Kouji Kobiyama et al. developed a squalene-based oil-in-water adjuvant similar to MF59, exhibiting efficacy comparable to CFA/IFA while eliciting interleukin-10 production in peritoneal cells, paving the way for clinical translation.Citation66 Moreover, active ingredients derived from traditional Chinese medicine showcase the potential to modulate immune cell function in diverse ways to combat AS, highlighting promise as vaccine adjuvants.Citation67 Given the challenges in developing new vaccine adjuvants, enhancing the formulation and optimizing the performance of classic vaccine adjuvants like alum presents a feasible approach. This can be achieved through surface modification, combined utilization with other adjuvants, and integration with new technologies such as nanoliposomes and lasers to enhance the immune efficacy while upholding the safety profile of classic adjuvants.Citation68,Citation69
Developing personalized vaccination strategies proves beneficial in augmenting efficacy and safety, encompassing factors such as dosage, timing, frequency, and route of vaccine administration.Citation70 A study suggested that immunization had an anti-AS effect on 6–7-week-old mice but lacked efficacy on 20-week-old mice, underscoring the impact of vaccination timing on therapeutic outcomes.Citation71 Furthermore, many vaccines necessitate regular administration to sustain immune response, yet the necessity for repeat administration of anti-AS vaccines, along with specifics regarding frequency, timing, and dosage, remains unresolved. Additionally, the choice of vaccine administration route warrants attention. Although studies have explored subcutaneous, oral, and nasal administration routes for AS vaccines, a dearth of comparative analyses on the efficacy and safety of different administration routes obscures their specific applicability.Citation72,Citation73 Therefore, we suggest developing personalized vaccination plans based on biological attributes such as population, age, medical history, and genetic composition, as well as the severity of AS, to optimize vaccine efficacy and safety. Moreover, pre-vaccination screening for potential immune diseases in recipients aids in evaluating the efficacy and safety of the vaccination, thereby minimizing possible side effects.
Monitoring the therapeutic response of vaccines using biomarkers is essential for promptly addressing issues and intervening in adverse events, thereby enhancing vaccine safety. However, research on biomarkers suitable for monitoring AS vaccine treatment response remains limited. Amir Abbas Momtazi-Borojeni et al. utilized biomarkers such as blood lipid indices, urea, creatinine, glutamic-oxalacetic transaminase, glutamic-pyruvic transaminase, alkaline phosphatase, fasting blood glucose levels, and whole blood cell count as safety indicators for the anti-PCSK9 vaccine in healthy mice, providing valuable insights for studying biomarkers to monitor vaccine treatment response. In the future, with an understanding of the diverse types and mechanisms of action of AS vaccines, efforts can be directed toward identifying biomarkers for monitoring their therapeutic response. Additionally, the development of risk prediction models holds promise for improving the safety of clinical applications of AS vaccines.
Lastly, it is noteworthy that “immunity, inflammation, in vivo” falls within the third quadrant, encapsulating the pivotal theme associated with in vivo research into the pathogenesis of AS (). Utilizing the search strategy “TS = (atherosclerosis AND in-vivo AND vaccine)” in the WoSCC database, we identified a trend of moderately low annual publication volumes averaging 1 to 5 articles on this subject, indicating a tepid level of activity in this area. Upon closer analysis, we observed that over the past three decades, in vivo studies of AS vaccines have primarily been conducted in animal models, with a limited number of human trials employing monoclonal antibody vaccines. It is posited that with further advancements in research, in vivo trials of AS vaccines will shift from animal models to human subjects, leading to a subsequent rise in the focus and interest on in vivo studies of the AS pathogenesis.
Limitations
Firstly, the bibliometric analysis solely encompassed publications from the WoSCC database, thereby lacking publications from other databases such as PubMed and Embase, which may have impacted the comprehensiveness of the data analysis. Secondly, the study only included articles published before February 8, 2024, potentially overlooking the recent research findings. Thirdly, despite conducting a thorough review of the existing literature to formulate the search strategy and exerting concerted effort of three authors to screen for literature pertinent to AS vaccine research, the potential for subjective bias in the selection process could not be discounted. This bias posed a risk of overlooking relevant literature or including irrelevant ones, potentially compromising the integrity and precision of our analysis.
Conclusion
The research on AS vaccines is currently experiencing steady development. This study employed bibliometrics method to conduct a comprehensive visual analysis of publications, countries, journals, authors, and keyword information in AS vaccine research within the WoSCC database, identifying noteworthy research topics. Breakthroughs have been achieved in AS protective antibody vaccines, with future research focusing on enhancing antibody duration and reducing usage costs. The research on AS-related antigen vaccines is progressing rapidly, with the primary research goal being the discovery of efficient and safe antigen epitopes. Ultimately, enhancing the efficacy and safety of AS vaccines through various approaches is crucial for their widespread clinical application. This study serves as a guiding reference for researchers in the field of AS vaccines.
Contributor statements
All authors contributed to the study conception and design. Bochao Jia, Rui Wei, Chenlu Yuan: article design and writing. Tao Cheng, Shuai Shi and Yuguang Chu: data acquisition and analysis. Yuanhui Hu: article ideas and revision suggestions. All authors participated in the manuscript revision and approved the final manuscript for submission.
Acknowledgments
We highly appreciate the support from Beijing University of Chinese Medicine and Guang ‘anmen Hospital of China Academy of Chinese Medical Sciences.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data supporting the results of this study can be obtained from the corresponding authors upon reasonable request.
Additional information
Funding
References
- Kong P, Cui Z-Y, Huang X-F, Zhang D-D, Guo R-J, Han M. Inflammation and atherosclerosis: signaling pathways and therapeutic intervention. Sig Transduct Target Ther. 2022;7(1). doi:10.1038/s41392-022-00955-7.
- Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ, Benziger CP, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019. J Am Coll Cardiol. 2020;76(25):2982–14. doi:10.1016/j.jacc.2020.11.010.
- Song L, Zhang J, Ma D, Fan Y, Lai R, Tian W, Zhang Z, Ju J, Xu H. A bibliometric and knowledge-map analysis of macrophage polarization in atherosclerosis from 2001 to 2021. Front Immunol. 2022;13. doi:10.3389/fimmu.2022.910444.
- Zhang T, Pang C, Xu M, Zhao Q, Hu Z, Jiang X, Guo M. The role of immune system in atherosclerosis: molecular mechanisms, controversies, and future possibilities. Human Immunol. 2024;85(2):110765. doi:10.1016/j.humimm.2024.110765.
- Ley K. Inflammation and Atherosclerosis. Cells. 2021;10(5):1197. doi:10.3390/cells10051197.
- Zheng WC, Chan W, Dart A, Shaw JA. Novel therapeutic targets and emerging treatments for atherosclerotic cardiovascular disease. Eur Heart J - Cardiovascular Pharmacotherapy. 2024;10(1):53–67. doi:10.1093/ehjcvp/pvad074.
- Barale C, Melchionda E, Morotti A, Russo I. PCSK9 biology and its role in atherothrombosis. IJMS. 2021;22(11):5880. doi:10.3390/ijms22115880.
- Moreno-Gonzalez MA, Ortega-Rivera OA, Steinmetz NF. Two decades of vaccine development against atherosclerosis. Nano Today. 2023;50:101822. doi:10.1016/j.nantod.2023.101822.
- Yin H, Zhang F, Yang X, Meng X, Miao Y, Noor Hussain MS, Yang L, Li Z. Research trends of artificial intelligence in pancreatic cancer: a bibliometric analysis. Front Oncol. 2022;12. doi:10.3389/fonc.2022.973999.
- Pranckutė R. Web of Science (WoS) and Scopus: the titans of bibliographic information in today’s academic world. Publications. 2021;9(1):12. doi:10.3390/publications9010012.
- Arruda H, Silva ER, Lessa M, Proença Jr D, Bartholo R. VOSviewer and bibliometrix. J Med Libr Assoc. 2022;110(3):392–5. doi:10.5195/jmla.2022.1434.
- Hirsch JE. An index to quantify an individual’s scientific research output. Proc Natl Acad Sci USA. 2005;102(46):16569–72. doi:10.1073/pnas.0507655102.
- Wang S, Tian C, Gao Z, Zhang B, Zhao L. Research status and trends of the diabetic cardiomyopathy in the past 10 years (2012–2021): a bibliometric analysis. Front Cardiovasc Med. 2022;9. doi:10.3389/fcvm.2022.1018841.
- Wei N, Xu Y, Li Y, Shi J, Zhang X, You Y, Sun Q, Zhai H, Hu Y. A bibliometric analysis of T cell and atherosclerosis. Front Immunol. 2022;13. doi:10.3389/fimmu.2022.948314.
- Chen Z, Liu Z, Feng Y, Shi A, Wu L, Sang Y, Li C. Global research on RNA vaccines for COVID-19 from 2019 to 2023: a bibliometric analysis. Front Immunol. 2024;15. doi:10.3389/fimmu.2024.1259788.
- Wolf D, Ley K. Immunity and inflammation in atherosclerosis. Circ Res. 2019;124(2):315–27. doi:10.1161/CIRCRESAHA.118.313591.
- Crotty S. T follicular helper cell biology: a decade of discovery and diseases. Immunity. 2019;50(5):1132–48. doi:10.1016/j.immuni.2019.04.011.
- Shaw AC, Goldstein DR, Montgomery RR. Age-dependent dysregulation of innate immunity. Nat Rev Immunol. 2013;13(12):875–87. doi:10.1038/nri3547.
- Binder CJ, Hörkkö S, Dewan A, Chang M-K, Kieu EP, Goodyear CS, Shaw PX, Palinski W, Witztum JL, Silverman GJ, et al. Pneumococcal vaccination decreases atherosclerotic lesion formation: molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nat Med. 2003;9(6):736–43. doi:10.1038/nm876.
- Stoll G, Bendszus M. Inflammation and atherosclerosis: novel insights into plaque formation and destabilization. Stroke. 2006;37(7):1923–32. doi:10.1161/01.STR.0000226901.34927.10.
- Vlachopoulos C, Dima I, Aznaouridis K, Vasiliadou C, Ioakeimidis N, Aggeli C, Toutouza M, Stefanadis C. Acute systemic inflammation increases arterial stiffness and decreases wave reflections in healthy individuals. Circulation. 2005;112(14):2193–200. doi:10.1161/CIRCULATIONAHA.105.535435.
- Udell JA, Zawi R, Bhatt DL, Keshtkar-Jahromi M, Gaughran F, Phrommintikul A, Ciszewski A, Vakili H, Hoffman EB, Farkouh ME, et al. Association between influenza vaccination and cardiovascular outcomes in high-risk patients: a meta-analysis. JAMA. 2013;310(16):1711–20. doi:10.1001/jama.2013.279206.
- Libby P, Loscalzo J, Ridker PM, Farkouh ME, Hsue PY, Fuster V, Hasan AA, Amar S. Inflammation, immunity, and infection in atherothrombosis: JACC review topic of the week. J Am Coll Cardiol. 2018;72(17):2071–81. doi:10.1016/j.jacc.2018.08.1043.
- Rittershaus CW, Miller DP, Thomas LJ, Picard MD, Honan CM, Emmett CD, Pettey CL, Adari H, Hammond RA, Beattie DT, et al. Vaccine-induced antibodies inhibit CETP activity in vivo and reduce aortic lesions in a rabbit model of atherosclerosis. Arterioscler Thromb Vasc Biol. 2000;20(9):2106–12. doi:10.1161/01.ATV.20.9.2106.
- Castelo-Branco C, Soveral I. The immune system and aging: a review. Gynecol Endocrinol. 2014;30(1):16–22. doi:10.3109/09513590.2013.852531.
- Zhu X, Hu J, Deng S, Tan Y, Qiu C, Zhang M, Ni X, Lu H, Wang Z, Li L, et al. Comprehensive bibliometric analysis of the kynurenine pathway in mood disorders: focus on gut microbiota research. Front Pharmacol. 2021;12. doi:10.3389/fphar.2021.687757.
- Kobiyama K, Saigusa R, Ley K. Vaccination against atherosclerosis. Curr Opin Immunol. 2019;59:15–24. doi:10.1016/j.coi.2019.02.008.
- Gisterå A, Hermansson A, Strodthoff D, Klement ML, Hedin U, Fredrikson GN, Nilsson J, Hansson GK, Ketelhuth DFJ. Vaccination against T-cell epitopes of native ApoB100 reduces vascular inflammation and disease in a humanized mouse model of atherosclerosis. J Intern Med. 2017;281(4):383–97. doi:10.1111/joim.12589.
- Su J, Zhou H, Liu X, Nilsson J, Fredrikson GN, Zhao M. oxLDL antibody inhibits MCP-1 release in monocytes/macrophages by regulating Ca2+/K+ channel flow. J Cellular Molecular Medi. 2017;21(5):929–40. doi:10.1111/jcmm.13033.
- Fröbert O, Götberg M, Angerås O, Jonasson L, Erlinge D, Engstrøm T, Persson J, Jensen SE, Omerovic E, James SK, et al. Design and rationale for the influenza vaccination after myocardial infarction (IAMI) trial. A registry-based randomized clinical trial. Am Heart J. 2017;189:94–102. doi:10.1016/j.ahj.2017.04.003.
- Fan L, Liu J, Hu W, Chen Z, Lan J, Zhang T, Zhang Y, Wu X, Zhong Z, Zhang D, et al. Targeting pro-inflammatory T cells as a novel therapeutic approach to potentially resolve atherosclerosis in humans. Cell Res. 2024;34(6):407–27. doi:10.1038/s41422-024-00945-0.
- Sun H, Krauss RM, Chang JT, Teng BB. PCSK9 deficiency reduces atherosclerosis, apolipoprotein B secretion, and endothelial dysfunction. J Lipid Res. 2018;59(2):207–23. doi:10.1194/jlr.M078360.
- Yang J, Ma X, Niu D, Sun Y, Chai X, Deng Y, Wang J, Dong J. PCSK9 inhibitors suppress oxidative stress and inflammation in atherosclerotic development by promoting macrophage autophagy. Am J Transl Res. 2023;15(8):5129–44.
- Ji E, Lee S. Antibody-based therapeutics for atherosclerosis and cardiovascular diseases. Int J Mol Sci. 2021;22(11):5770. doi:10.3390/ijms22115770.
- Pickett JR, Wu Y, Zacchi LF, Ta HT. Targeting endothelial vascular cell adhesion molecule-1 in atherosclerosis: drug discovery and development of vascular cell adhesion molecule-1–directed novel therapeutics. Cardiovascular Research. 2023;119(13):2278–93. doi:10.1093/cvr/cvad130.
- Hutchinson MA, Park HS, Zanotti KJ, Alvarez-Gonzalez J, Zhang J, Zhang L, Telljohann R, Wang M, Lakatta EG, Gearhart PJ, et al. Auto-antibody production during experimental atherosclerosis in ApoE(-/-) mice. Front Immunol. 2021;12:695220. doi:10.3389/fimmu.2021.695220.
- Kyaw T, Cui P, Tay C, Kanellakis P, Hosseini H, Liu E, Rolink AG, Tipping P, Bobik A, Toh B-H, et al. BAFF receptor mAb treatment ameliorates development and progression of atherosclerosis in hyperlipidemic ApoE−/− mice. PLOS ONE. 2013;8(4):e60430. doi:10.1371/journal.pone.0060430.
- Lorenzo C, Delgado P, Busse CE, Sanz-Bravo A, Martos-Folgado I, Bonzon-Kulichenko E, Ferrarini A, Gonzalez-Valdes IB, Mur SM, Roldán-Montero R, et al. ALDH4A1 is an atherosclerosis auto-antigen targeted by protective antibodies. Nature. 2021;589(7841):287–92. doi:10.1038/s41586-020-2993-2.
- Hobbs HH, Cohen JC, Horton JD. PCSK9: from nature’s loss to patient’s gain. Circulation. 2024;149(3):171–3. doi:10.1161/CIRCULATIONAHA.123.064498.
- Palinski W, Miller E, Witztum JL. Immunization of low density lipoprotein (LDL) receptor-deficient rabbits with homologous malondialdehyde-modified LDL reduces atherogenesis. Proc Natl Acad Sci USA. 1995;92(3):821–5. doi:10.1073/pnas.92.3.821.
- Ameli S, Hultgårdh-Nilsson A, Regnström J, Calara F, Yano J, Cercek B, Shah PK, Nilsson J. Effect of immunization with homologous LDL and oxidized LDL on early atherosclerosis in hypercholesterolemic rabbits. ATVB. 1996;16(8):1074–9. doi:10.1161/01.ATV.16.8.1074.
- George J, Afek A, Gilburd B, Levkovitz H, Shaish A, Goldberg I, Kopolovic Y, Wick G, Shoenfeld Y, Harats D, et al. Hyperimmunization of apo-E-deficient mice with homologous malondialdehyde low-density lipoprotein suppresses early atherogenesis. Atherosclerosis. 1998;138(1):147–52. doi:10.1016/S0021-9150(98)00015-X.
- Freigang S, Hörkkö S, Miller E, Witztum JL, Palinski W. Immunization of LDL receptor–deficient mice with homologous malondialdehyde-modified and native LDL reduces progression of atherosclerosis by mechanisms other than induction of high titers of antibodies to oxidative neoepitopes. ATVB. 1998;18(12):1972–82. doi:10.1161/01.ATV.18.12.1972.
- Baardman J, Lutgens E. Regulatory T Cell Metabolism in Atherosclerosis. Metabolites. 2020;10(7):279. doi:10.3390/metabo10070279.
- Kimura T, Kobiyama K, Winkels H, Tse K, Miller J, Vassallo M, Wolf D, Ryden C, Orecchioni M, Dileepan T, et al. Regulatory CD4+ T cells recognize major histocompatibility complex class II molecule–restricted peptide epitopes of apolipoprotein B. Circulation. 2018;138(11):1130–43. doi:10.1161/CIRCULATIONAHA.117.031420.
- Chyu KY, Dimayuga PC, Shah PK. Vaccine against arteriosclerosis: an update. Ther Adv Vaccines. 2017;5(2):39–47. doi:10.1177/2051013617693753.
- Kimura T, Tse K, Sette A, Ley K. Vaccination to modulate atherosclerosis. Autoimmunity. 2015;48(3):152–60. doi:10.3109/08916934.2014.1003641.
- Zhong Y, Tang H, Wang X, Zeng Q, Liu Y, Zhao X, Yu K, Shi H, Zhu R, Mao X. Intranasal immunization with heat shock protein 60 induces CD4(+) CD25(+) GARP(+) and type 1 regulatory T cells and inhibits early atherosclerosis. Clinical And Experimental Immunology. 2016;183(3):452–68. doi:10.1111/cei.12726.
- Wick C, Onestingel E, Demetz E, Dietrich H, Wick G. Oral Tolerization with mycobacterial heat shock protein 65 reduces chronic experimental atherosclerosis in aged mice. Gerontology. 2018;64(1):36–48. doi:10.1159/000480436.
- de Grooth GJ, Klerkx AH, Stroes ES, Stalenhoef AF, Kastelein JJ, Kuivenhoven JA. A review of CETP and its relation to atherosclerosis. J Lipid Res. 2004;45(11):1967–74. doi:10.1194/jlr.R400007-JLR200.
- Riascos-Bernal DF, Ressa G, Korrapati A, Sibinga NES. The FAT1 cadherin drives vascular smooth muscle cell migration. Cells. 2023;12(12):1621. doi:10.3390/cells12121621.
- Ma Z, Mao C, Chen X, Yang S, Qiu Z, Yu B, Jia Y, Wu C, Wang Y, Wang Y, et al. Peptide vaccine against ADAMTS-7 ameliorates atherosclerosis and postinjury neointima hyperplasia. Circulation. 2023;147(9):728–42. doi:10.1161/CIRCULATIONAHA.122.061516.
- Ha HS, Kim TY, Han SJ, Sung H-J, Seo KY, Ha J-W. Anti-atherosclerotic vaccination against Porphyromonas gingivalis as a potential comparator of statin in mice. PeerJ. 2021;9:e11293. doi:10.7717/peerj.11293.
- Gurven MD, Finch CE, Wann LS. Are intestinal worms nature’s anti-atherosclerosis vaccine? Eur Heart J. 2018;39(18):1653. doi:10.1093/eurheartj/ehy129.
- Moseley P, Klenerman P, Kadambari S. Indirect effects of cytomegalovirus infection: implications for vaccine development. Rev Med Virol. 2023;33(1):e2405. doi:10.1002/rmv.2405.
- Saleki K, Alijanizade P, Moradi S, Rahmani A, Banazadeh M, Mohamadi MH, Shahabi F, Nouri HR. Engineering a novel immunogenic chimera protein utilizing bacterial infections associated with atherosclerosis to induce a deviation in adaptive immune responses via immunoinformatics approaches. Infect Genet Evol. 2022;102:105290. doi:10.1016/j.meegid.2022.105290.
- Jun L, Jie L, Dongping Y, Xin Y, Taiming L, Rongyue C, Jie W, Jingjing L. Effects of nasal immunization of multi-target preventive vaccines on atherosclerosis. Vaccine. 2012;30(6):1029–37. doi:10.1016/j.vaccine.2011.12.043.
- Lu X, Xia M, Endresz V, Faludi I, Szabo A, Gonczol E, Mundkur L, Chen D, Kakkar V. Impact of multiple antigenic epitopes from ApoB100, hHSP60 and Chlamydophila pneumoniae on atherosclerotic lesion development in Apob(tm2Sgy)Ldlr(tm1Her)J mice. Atherosclerosis. 2012;225(1):56–68. doi:10.1016/j.atherosclerosis.2012.07.021.
- Roy P, Ali AJ, Kobiyama K, Ghosheh Y, Ley K. Opportunities for an atherosclerosis vaccine: from mice to humans. Vaccine. 2020;38(28):4495–506. doi:10.1016/j.vaccine.2019.12.039.
- Witkowski M, Weeks TL, Hazen SL. Gut microbiota and cardiovascular disease. Circ Res. 2020;127(4):553–70. doi:10.1161/CIRCRESAHA.120.316242.
- Zou Y, Song X, Liu N, Sun W, Liu B. Intestinal flora: a potential new regulator of cardiovascular disease. Aging Dis. 2022;13(3):753–72. doi:10.14336/AD.2021.1022.
- Hinkley H, Counts DA, VonCanon E, Lacy M. T cells in atherosclerosis: key players in the pathogenesis of vascular disease. Cells. 2023;12(17):2152. doi:10.3390/cells12172152.
- Taylor JA, Hutchinson MA, Gearhart PJ, Maul RW. Antibodies in action: the role of humoral immunity in the fight against atherosclerosis. Immun Ageing. 2022;19(1):59. doi:10.1186/s12979-022-00316-6.
- Wigren M, Kolbus D, Dunér P, Ljungcrantz I, Söderberg I, Björkbacka H, Fredrikson GN, Nilsson J. Evidence for a role of regulatory T cells in mediating the atheroprotective effect of apolipoprotein B peptide vaccine. J Intern Med. 2011;269(5):546–56. doi:10.1111/j.1365-2796.2010.02311.x.
- Reed SG, Orr MT, Fox CB. Key roles of adjuvants in modern vaccines. Nat Med. 2013;19(12):1597–608. doi:10.1038/nm.3409.
- Kobiyama K, Vassallo M, Mitzi J, Winkels H, Pei H, Kimura T, Miller J, Wolf D, Ley K. A clinically applicable adjuvant for an atherosclerosis vaccine in mice. Eur J Immunol. 2018;48(9):1580–7. doi:10.1002/eji.201847584.
- Jing J, Zhu C, Gong R, Qi X, Zhang Y, Zhang Z. Research progress on the active ingredients of traditional Chinese medicine in the intervention of atherosclerosis: a promising natural immunotherapeutic adjuvant. Biomed Pharmacother= Biomedecine & Pharmacotherapie. 2023;159:114201. doi:10.1016/j.biopha.2022.114201.
- Mansoori B, Mohammadi A, Shajari N, Davudian S, Salehi S, Baradaran B. Nano-liposome-based target toxicity machine: an alternative/complementary approach in atopic diseases. Artif Cells, Nanomed Biotechnol. 2017;45(7):1292–7. doi:10.1080/21691401.2016.1261872.
- Kashiwagi S. Laser adjuvant for vaccination. Faseb J. 2020;34(3):3485–500. doi:10.1096/fj.201902164R.
- Nettersheim FS, De Vore L, Winkels H. Vaccination in atherosclerosis. Cells. 2020;9(12):2560. doi:10.3390/cells9122560.
- Chyu KY, Reyes OS, Zhao X, Yano J, Dimayuga P, Nilsson J, Cercek B, Shah PK. Timing affects the efficacy of LDL immunization on atherosclerotic lesions in apo E (−/−) mice. Atherosclerosis. 2004;176(1):27–35. doi:10.1016/j.atherosclerosis.2004.04.016.
- Hu Y, Chen Z, Jiang L, Chen F, Jin R, Cheng L. Effects of oral and subcutaneous administration of HSP60 on myeloid-derived suppressor cells and atherosclerosis in ApoE−/− mice. Biochem Bioph Res Co. 2018;498(4):701–6. doi:10.1016/j.bbrc.2017.10.150.
- Calixto-Tlacomulco S, Luna-Reyes I, Delgado-Coello B, Gutiérrez-Vidal R, Reyes-Grajeda JP, Mas-Oliva J. CETP-derived peptide seq-1, the key component of HB-ATV-8 vaccine prevents stress responses, and promotes downregulation of pro-fibrotic genes in hepatocytes and stellate cells. Arch Med Res. 2024;55(2):102937. doi:10.1016/j.arcmed.2023.102937.