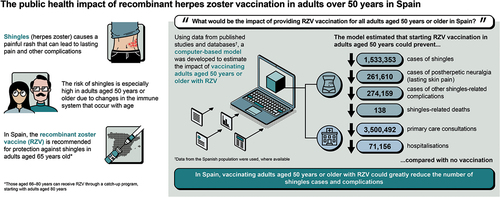
ABSTRACT
The recombinant zoster vaccine (RZV) is included in the Spanish National Immunisation Programme for adults 65 years of age (years), with a potential progressive catch-up program for adults 66–80 years, starting with 80 years. However, the risk of herpes zoster (HZ) increases significantly from 50 years. We estimated the public health impact (PHI) of vaccinating adults ≥50 years in Spain versus no vaccination, using a Markov model adapted to the Spanish setting. The model simulated a hypothetical ≥50 years cohort over a lifetime, with inputs from Spanish publications, databases, or publications from other countries where Spanish data were unavailable. Base case inputs included 67.7% RZV coverage and 61.1% second dose compliance. Outputs included clinical outcomes avoided, healthcare resource use avoided, and number-needed-to-vaccinate (NNV) to prevent one HZ case. Deterministic (DSA) and probabilistic sensitivity analyses (PSA) were also conducted. The model estimated that, compared with no vaccination, vaccinating adults ≥50 years in Spain (N = 19,850,213) with RZV could prevent 1,533,353 HZ cases, 261,610 postherpetic neuralgia episodes, 274,159 other complications, and 138 deaths through the cohorts’ remaining lifetime, mostly in the 50–59 years cohort. Furthermore, 3,500,492 primary care visits and 71,156 hospitalizations could be avoided, with NNV = 9 to prevent one HZ case. DSA predicted NNV = 7 to prevent one HZ case when second dose compliance was increased to 100%. PSA demonstrated ≥200,000 and ≥1,400,000 cases could be prevented in 86.9% and 18.4% of simulations, respectively. Starting RZV from 50 years could therefore prevent a substantial number of HZ cases and complications. Increasing RZV coverage and second dose compliance could further alleviate PHI of HZ.
Introduction
Herpes zoster (HZ), also known as shingles, culebrilla, or zona, is a disease elicited by the reactivation of the varicella-zoster virus (VZV) and is characterized by a painful vesicular rash.Citation1,Citation2 The incidence of HZ increases with age as cellular immunity against VZV declines.Citation2 Acute pain from HZ can last for weeks and evolve into postherpetic neuralgia (PHN), often defined as pain persisting for 90 days or more after rash onset.Citation3,Citation4 There are other complications associated with HZ, including ocular, neurological, cutaneous, and even cardiovascular manifestations.Citation5–8
In Spain, the incidence of HZ across all age groups has been estimated as 351.6 cases per 100,000 inhabitants for the period 2014–2018.Citation9 The incidence of HZ increases with age, with the highest incidence observed among adults in the 80–84 age cohort (877.1 cases per 100,000 inhabitants). However, a 41% increase in HZ incidence has been reported for the 50–54-year-old cohort compared with the 45–49-year-old cohort. Furthermore, between 1998 and 2017, 68.8% of HZ cases occurred in adults aged ≥50 years, accounting for 80.2% of total hospitalizations due to HZ.Citation9
The recombinant zoster vaccine (RZV) was first authorized in Spain on 16th October 2020 for the prevention of HZ and PHN in adults aged ≥50 years and in adults from 18 years who have an increased risk of HZ.Citation10,Citation11 RZV was subsequently introduced into the Spanish National Immunisation Programme (NIP) in 2023 for the prevention of HZ in higher-risk populations, including individuals with hematopoietic stem cell or solid organ transplantation, human immunodeficiency virus infection, Janus kinase inhibitor drug therapy, hematologic malignancies, and solid tumors receiving chemotherapy treatment.Citation12 RZV is also included for individuals aged 65 years, with a catch-up program in adults aged 66–80 years, starting with those aged 80 years.Citation13
Given that the epidemiology of HZ indicates a significant increase in the number of cases beyond 50 years of age, the objective of this study was to analyze the potential public health impact that could be expected by expanding the recommendation and funding of HZ vaccination to Spanish adults aged ≥50 years, compared with a non-vaccination strategy, in terms of HZ cases, HZ-related complications and deaths, and healthcare resource use (HCRU) avoided.
Materials and methods
Model structure
A multi-cohort Markov model, the ZOster ecoNomic Analysis (ZONA) model (Supplementary Figure S1), previously developed with Microsoft Excel was adapted to the Spanish setting.Citation14–16 ZONA includes up to five hypothetical cohorts split into various age groups and follows all adults within a cohort from the year of vaccination until their death, to capture the full benefits of HZ vaccination across their lifetime horizon.
In the present analysis, each age cohort included in the modeled population progresses under two different vaccination strategies: no vaccination and vaccination with RZV. Under the RZV strategy, individuals could be fully or partially compliant with the dosing schedule, depending on the assumed vaccine coverage and compliance rates. Individuals within the age cohorts in the model transition between the health states of HZ, natural death, HZ-related deaths, recovery, and recurrent HZ; the probability of transition is age-specific and determined using an annual time step.
Model inputs
Demographics
The base case population was all Spanish adults aged ≥50 years, stratified by different age cohorts, in 2022, based on estimations from the Spanish National Institute of Statistics (NIS).Citation17 Annual probability of all-cause mortality data was extracted from mortality tables from the NIS for 2019 to avoid confounding effects of the COVID-19 pandemic.Citation18
Epidemiology
Epidemiological inputs included the annual incidence of HZ, proportion of HZ resulting in PHN, non-PHN complications (ocular, neurological, cutaneous, and other non-pain complications), and HZ-related deaths. All inputs are detailed in Supplementary Table S1.
HZ incidence was extracted from the Base de Datos de Clínicos de Atención Primaria (BDCAP), a Spanish primary care database which collects annual anonymized and normalized clinical data from a sample of 4.9 million users assigned to Primary Care Teams from the Spanish National Health System.Citation19 Data from 2019 were considered since this is the latest year for which data were available.
Percentage of PHN cases among HZ patients was obtained from an earlier observational, population-based study that estimated the incidence of burden of PHN from 2009 to 2014 in Valencia by extracting data from a healthcare database from the same region.Citation20 Incidence of recurrent HZ and proportion of recurrent HZ cases with PHN were assumed equal to annual incidence and proportion of initial HZ with/without PHN.Citation7
HZ complications included in this analysis were PHN, ocular, neurological, cutaneous, and other non-pain complications. The proportion of HZ cases with such complications were derived from the Registro de Actividad de Atención Especializada – Conjunto Mínimo Básico de Datos database (RAE-CMBD).Citation21 HZ case fatality was extracted from the NIS mortality statistics, which reports the number of deaths by different causes.Citation22
HCRU
HCRU inputs included hospitalization and primary care physician visits per case due to HZ and HZ cases with PHN for the modeled population (Supplementary Table S1).
Hospitalization estimates were extracted from an analysis of the cost-effectiveness of RZV conducted by the Evaluation Services from the Canary Islands Health Service (SESCS), which presents the probability of hospitalization due to HZ, and were weighted by age group to fit ZONA model requirements.Citation23 An average of 2.28 primary care physician visits per patient with HZ was used according to a Spanish study.Citation20 The same value was assumed for all age groups as there are no data for the split of visits per age group.
Vaccine efficacy and waning
Vaccine efficacy and waning inputs were defined as the initial protection of RZV against HZ and PHN, the waning of vaccine protection and timing, and compliance for the second RZV dose (Supplementary Table S1). Efficacy and waning of vaccine protection were obtained from clinical trial values for one and two doses of the vaccine, as well as previously estimated vaccine waning rates over time.Citation14,Citation15
As there are no data for HZ vaccine coverage in Spain, this was assumed to be equal to the coverage for the adult flu vaccine for 2020–2021.Citation24 Second dose compliance was expected to be lower than in clinical trials since a real-world environment was considered in this model. In the absence of specific data on HZ and for the Spanish context, second dose compliance was extracted from the observed adherence to the second dose of a hepatitis A vaccine in adults from Germany.Citation25
Adverse events
Adverse event inputs describe the incidence of adverse events per dose of RZV. ZONA considers four vaccine-related adverse events: local/general reactions, general practitioner visits, emergency room visits, and hospitalizations (Supplementary Table S1).
Model outputs
The ZONA model calculates HZ cases, PHN cases, non-PHN complications, and HZ-related deaths that accumulate over the lifetime horizon of each age group under each intervention strategy. The number-needed-to-vaccinate (NNV) with RZV to avoid one case of HZ and PHN was also estimated. Outcomes were compared to calculate the incremental differences resulting from a RZV vaccination strategy versus no vaccination.
Scenario and sensitivity analyses
Deterministic sensitivity analyses (DSA)
The impact of uncertainty around base case inputs on the results was evaluated by DSA. Two one-way DSA were conducted by modifying the value of HZ cases and complications by ± 20% from the base case value reported in Supplementary Table S1.
Several scenario analyses were also performed by modifying model assumptions for coverage rate (50% and 100%), second dose compliance (0% and 100%), and age group (changing the population size in the analysis) to analyze cohorts of 50, 65, and 80 years. This enabled the impact of alternative scenarios to be seen, for example the impact on coverage rates by modifying the current vaccination strategy in Spain.
Probabilistic sensitivity analysis (PSA)
A PSA was also conducted to visualize the variation of result estimates for all inputs for which ranges are reported. A total of 1,000 simulations were conducted, using varying vaccine coverage inputs with a uniform distribution and all other variables with a beta distribution.
Results
Base case results
According to the ZONA model, for a base case population size of 19,850,213 individuals aged ≥50 years in Spain with a 67.7% vaccine coverage rate and 61.1% second dose compliance, RZV could prevent 1,533,353 cases of HZ, 261,610 PHN cases, 274,159 complications, and 138 HZ-related deaths over a lifetime horizon, compared with no vaccination (). NNV to prevent one HZ case was 9, while NNV to prevent 1 PHN case was 52. Most of the HZ cases averted with RZV were in the 50–59 age group, representing 40% of HZ cases averted in the base case population (). In terms of HCRU, vaccination with RZV in adults aged ≥50 years in Spain could prevent 3,500,492 primary care visits and 71,156 hospitalizations compared with no vaccination.
Figure 1: Number of HZ cases, cases of related complications, and deaths prevented by RZV versus no vaccination in Spain by age group.
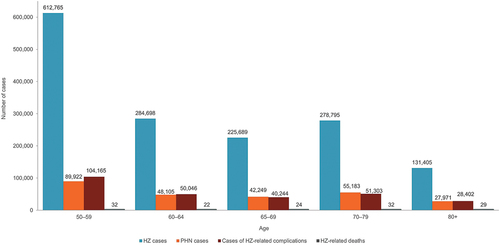
Table 1. Health outcomes and resource use prevented with RZV versus no vaccination with base case and alternative RZV coverage and compliance rates.
Scenario analysis
In the scenario analysis, an increase in second dose compliance to 100% could result in a 35–45% improvement in health outcomes and a reduction in HCRU, with 2,078,295 HZ cases, 260,989 PHN episodes, and 4,744,539 primary care visits avoided. An increase in coverage rate to 100% could also improve health outcomes and decrease HCRU by approximately 33%, with 2,038,431 HZ cases, 347,783 PHN cases, and 4,653,535 primary care visits prevented (). Results may be slightly higher when improving second dose compliance due to the direct impact of improving RZV efficacy by complying with the recommended vaccine schedule. Notably, base case coverage and compliance rates and a hypothetical first-dose coverage rate of 100% both resulted in a NNV of 9 to prevent one HZ case, while the NNV decreased to 7 with 100% second dose compliance.
Other target populations were evaluated in the model to determine the optimal age for vaccination with base case coverage rates. Immunization at an early age (50-year-old cohort) could result in 64,726 HZ cases avoided compared with 48,129 and 12,501 cases avoided by immunization in the 65- and 80-year-old cohorts, respectively. Improvements in other health outcomes and HCRU prevention were similarly higher in the 50-year-old cohort than the older cohorts (). This was expected given that the population covered by the vaccine is highest in the youngest cohort. Moreover, immunization at an early age would lead to a longer period of time during which individuals benefit from vaccine efficacy, thus enabling the health benefits of RZV and the number of prevented HZ cases to accumulate. Accordingly, NNV to prevent one HZ case was lower when vaccinating the younger 50- and 65-year-old cohorts (NNV: 8) compared with the 80-year-old cohort (NNV: 15) in the model.
Table 2. Health outcomes and resource use prevented with RZV versus no vaccination in different single age cohorts.
An additional scenario representing the current HZ immunization program in Spain for the general population (i.e., the single cohort of 65 years) was analyzed, but with a hypothetical coverage rate of 100% (). As expected, this scenario demonstrated the highest absolute number of HZ (71,091) and PHN cases (12,735), complications (12,677), deaths (7), and HCRU (primary care visits: 162,293; hospitalizations: 3,537) averted.
Sensitivity analyses
The one-way DSA demonstrated that changing rates of HZ incidence and complications to ± 20% of base case values showed a proportional result in comparison to base case results (Supplementary Figure S2), suggesting that RZV coverage and compliance had the greatest impact on results.
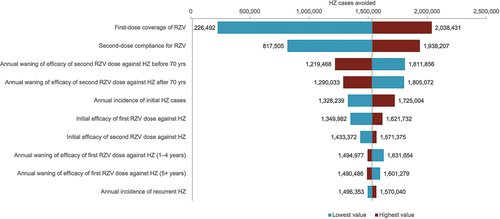
Results from the DSA of the scenario analyses further indicated that first dose coverage, compliance with the two-dose vaccine schedule, and annual waning of vaccine efficacy before 70 years of age were the most influential parameters on base case results (), highlighting the value of increasing vaccination coverage and compliance with the RZV schedule.
Figure 2: Deterministic sensitivity analysis: impact of alternative scenarios on base case HZ cases avoided in Spain.
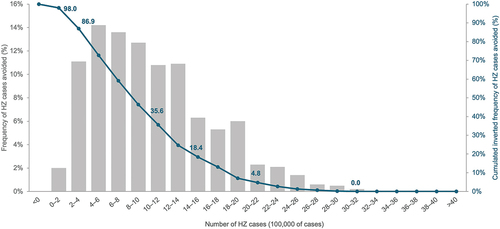
The PSA showed that >1,400,000 HZ cases could be avoided with RZV vaccination with an 18.4% probability and that >200,000 HZ cases could be avoided in 86.9% of the simulations ().
Figure 3: Probabilistic sensitivity analysis: variation in the number of HZ cases avoided in Spain.
Discussion
In the present analysis, the public health impact in Spain of an RZV vaccination strategy in adults aged ≥50 years in 2022 could result in 1,533,353 cases of HZ averted over a lifetime horizon. This strategy could also prevent 261,610 PHN cases, 274,159 complications (including ocular, neurological, cutaneous, and other non-pain complications), and 138 HZ-related deaths. These health outcomes could further result in the prevention of 3,500,492 primary care visits and 71,156 hospitalizations due to HZ, with a NNV to prevent one HZ case of 9.
The ≥50-year-old cohort was considered as the base case since the epidemiology of HZ indicates a significant increase in the number of cases beyond 50 years of age due to age-related decline in immunity (immunosenescence). Thus, starting vaccination at this age could highlight all the potential benefits of the RZV vaccine in high-risk populations. The current vaccination strategy recommended in Spain is vaccination of the 65-year-old cohort with a catch-up program for individuals aged 80 years old.Citation26 For that reason, several scenario analyses were evaluated in specific 50-, 65-, and 80-year-old cohorts in the general population, which also demonstrated HCRU reductions compared with a no vaccination strategy. Although these reductions were more limited compared with those seen in the base case model, this is to be expected given that the single age cohorts are of a much smaller population size than the base case cohort of all individuals aged ≥50 years. On the other hand, the NNV was comparable to base case, except for the 80-year-old cohort. These findings are in line with a 2018 report published by the SESCS, which demonstrated that RZV would be cost-effective in all age groups over 50 years in Spain.Citation23 This study therefore provides additional evidence supporting the expansion of the RZV vaccination program to more age groups, given the public health benefits.
The scenario analyses also assessed the effect of improving RZV second dose compliance rates and increasing vaccine coverage rates, revealing that higher rates could result in an improvement of at least 30% in the number of HZ cases prevented. This demonstrates the public health relevance of working toward achieving and maintaining high compliance and coverage to obtain all the potential benefits from the RZV vaccine. In order to evaluate the impact of the uncertainty surrounding base case input values, several one-way DSA were performed by modifying base case HZ incidence and rate of complications. This showed a limited impact of HZ incidence and related complications on base case results, given that vaccination with RZV would still have a significant public health impact for the Spanish population.
The public health impact of vaccinating against HZ with RZV in Spain has not been previously estimated. However, similar analyses have been conducted in other European countries, including Italy, Germany, and the United Kingdom, which consistently demonstrated a reduction in HZ cases and complications with HZ vaccination compared with no vaccination. Despite differences in the results due to the population sizes, the NNV to prevent one HZ case is aligned across all studies.Citation14,Citation15,Citation27,Citation28
The present analysis has been conducted using, whenever possible, inputs considered in a previous economic evaluation by the SESCS health technology agency.Citation23 Where possible, some values have been updated to values in 2022, the reference year for this analysis, using the same primary references and conservative estimates when necessary. In this regard, some limitations of the SESCS analysis would apply to the present analysis as well, firstly being the data inputs used for HZ incidence. These values were extracted from the BDCAP database, which does not specify if cases were reported in immunocompromised or general populations and could thus have overestimated HZ incidence in general populations.Citation19 On the other hand, using BDCAP as a source for the HZ incidence values may have also introduced bias as there could have been cases of HZ that were directly hospitalized without first going through primary care, and therefore not captured in the BDCAP database. Nevertheless, the BDCAP database is the only publicly available source of primary care cases to collect such data in Spain at a population level to date.
In addition, all complications were assumed to occur only in the hospital setting and, in the absence of data from primary care settings, data from the RAE-CMBD database have been used and assumed to be equal to primary care, potentially overestimating the incidence of HZ-related complications and their impact. Finally, there are intrinsic limitations present in all modeling studies that can only be addressed through the generation of real-world data. Therefore, future studies should aim to collect HZ epidemiological data from routine clinical practice in real-world settings, as these would serve as a key instrument to compare against results from public health impact models.
Conclusions
Results from this ZONA model demonstrated that vaccination against HZ with RZV in adults aged ≥50 years could have a relevant public health impact by preventing 1,533,353 HZ cases, 261,610 PHN episodes, 274,159 HZ-related complications, and 138 HZ-related deaths over the lifetime horizon, compared with a non-vaccination strategy. Furthermore, 71,156 hospitalizations and 3,500,492 primary care visits due to HZ could be avoided, resulting in a NNV of 9 to prevent one case of HZ.
These results may be useful to inform future public health policies in potentially expanding the current Spanish NIP for RZV in adults ≥65 years of age and risk group populations to include adults of ≥50 years of age. Additionally, this analysis highlighted the importance of achieving high coverage rates and second dose compliance with the current NIP for optimizing health outcomes in vaccinated individuals.
Authors’ contributions
Substantial contributions to study conception and design: AG, LAV-A, RCM; substantial contributions to analysis and interpretation of the data: AG, LAV-A, RCM; drafting the article or revising it critically for important intellectual content: AG, LAV-A, RCM; final approval of the version of the article to be published: AG, LAV-A, RCM.
Ethical approval
Not required as all model inputs were collected from public datasets or previously published data.
Suppl Figure 1.jpg
Download JPEG Image (320.1 KB)HZ PHI Spain Manuscript_Suppl Materials_Clean.docx
Download MS Word (178.1 KB)Suppl Figure 2_.jpg
Download JPEG Image (891.2 KB)Acknowledgments
The authors acknowledge Marie Nishimwe, GSK, France, Alen Marijam, GSK, Belgium, and Ahmed Ehab Salem, GSK, UAE, for contributions to the conception and adaptation of the model. The authors also thank Costello Medical for editorial assistance and publication coordination, on behalf of GSK, and acknowledge Ellie Fung, Costello Medical, UK, for medical writing and editorial assistance based on authors’ input and direction.
Disclosure statement
AG is an employee of the GSK group of companies; LAV-A and RCM are employees and hold shares of the GSK group of companies.
Data availability statement
GSK makes available anonymized individual participant data and associated documents from interventional clinical studies that evaluate medicines upon approval of proposals submitted to www.clinicalstudydatarequest.com. To access data for other types of GSK sponsored research, for study documents without patient-level data and for clinical studies not listed, please submit an inquiry via the website.
Supplemental material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2024.2366353
Additional information
Funding
References
- Kimberlin DW, Whitley RJ. Varicella–zoster vaccine for the prevention of herpes zoster. N Engl J Med. 2007;356(13):1338–7. doi:10.1056/NEJMct066061.
- Oxman MN. Herpes zoster pathogenesis and cell-mediated immunity and immunosenescence. J Am Osteopath Assoc. 2009;109(6 Suppl 2):S13–17.
- Kawai K, Preaud E, Baron-Papillon F, Largeron N, Acosta CJ. Cost-effectiveness of vaccination against herpes zoster and postherpetic neuralgia: a critical review. Vaccine. 2014;32(15):1645–53. doi:10.1016/j.vaccine.2014.01.058.
- Saguil A, Kane S, Mercado M, Lauters R. Herpes zoster and postherpetic neuralgia: prevention and management. Am Fam Physician. 2017;96:656–63.
- Dworkin RH, Johnson RW, Breuer J, Gnann JW, Levin MJ, Backonja M, Betts RF, Gershon AA, Haanpää ML, McKendrick MW. et al. Recommendations for the management of herpes zoster. Clin Infect Dis. 2007;44(Suppl 1):S1–26. doi:10.1086/510206.
- Mueller NH, Gilden DH, Cohrs RJ, Mahalingam R, Nagel MA. Varicella zoster virus infection: clinical features, molecular pathogenesis of disease, and latency. Neurol Clin. 2008;26(3):675–97, viii. doi:10.1016/j.ncl.2008.03.011.
- Yawn BP, Wollan PC, Kurland MJ, St Sauver JL, Saddier P. Herpes zoster recurrences more frequent than previously reported. Mayo Clin Proc. 2011;86(2):88–93. doi:10.4065/mcp.2010.0618.
- Erskine N, Tran H, Levin L, Ulbricht C, Fingeroth J, Kiefe C, Goldberg RJ, Singh S. A systematic review and meta-analysis on herpes zoster and the risk of cardiac and cerebrovascular events. PLOS ONE. 2017;12(7):e0181565. doi:10.1371/journal.pone.0181565.
- CIBERESP. CNdE. Informe epidemiológico sobre la situación del Herpes Zóster en España, 1998-2018. Madrid: Instituto de Salud Carlos III; 2020.
- Centro de Información de Medicamentos. Shingrix polvo y suspension para suspension inyectable. 2020 [accessed 2023 Jul 31]. https://cima.aemps.es/cima/publico/detalle.html?nregistro=1181272001#.
- Centro de Información de Medicamentos. Ficha tecnica Shingrix polvo y suspension para suspension inyectable. 2020 [accessed 2020 Jul 31]. https://cima.aemps.es/cima/dochtml/ft/1181272001/FT_1181272001.html.
- Consejo Interterritorial del Sistema Nacional de Salud. Calendario de vacunación en Grupos de Riesgo, población adulta. 2024 [accessed 2024 May 21]. https://www.sanidad.gob.es/areas/promocionPrevencion/vacunaciones/calendario/docs/CalendarioVacunacion_GRadultos.pdf.
- Consejo Interterritorial del Sistema Nacional de Salud. Calendario de vacunación a lo largo de toda la vida. 2024 [accessed 2024 May 21]. https://www.sanidad.gob.es/areas/promocionPrevencion/vacunaciones/calendario/docs/CalendarioVacunacion_Todalavida.pdf.
- Curran D, Van Oorschot D, Matthews S, Hain J, Salem AE, Schwarz M. Long-term efficacy data for the recombinant zoster vaccine: impact on public health and cost effectiveness in Germany. Hum Vaccin Immunother. 2021;17(12):5296–303. doi:10.1080/21645515.2021.2002085.
- Curran D, Van Oorschot D, Varghese L, Oostvogels L, Mrkvan T, Colindres R, von Krempelhuber A, Anastassopoulou A. Assessment of the potential public health impact of Herpes Zoster vaccination in Germany. Hum Vaccin Immunother. 2017;13(10):2213–21. doi:10.1080/21645515.2017.1345399.
- McGirr A, Van Oorschot D, Widenmaier R, Stokes M, Ganz ML, Jung H, Varghese L, Curran D. Public health impact and cost-effectiveness of non-live adjuvanted recombinant zoster vaccine in Canadian adults. Appl Health Econ Health Policy. 2019;17(5):723–32. doi:10.1007/s40258-019-00491-6.
- Instituto Nacional de Estadística. Cifras de Población. Población residente por fecha, sexo y edad. 2022 [accessed 2023 June 30]. https://www.ine.es/jaxiT3/Tabla.htm?t=59238&L=0.
- Instituto Nacional de Estadística. Resultados nacionales, por comunidades autónomas y provincias. Tablas de mortalidad por año, sexo, edad y funciones. 2021 [accessed 2023 June 30]. https://www.ine.es/jaxiT3/Datos.htm?t=27153.
- Ministerio de Sanidad. Base de Datos Clínicos de Atención Primaria - BDCAP. Portal Estadístico; 2021 [accessed 2023 June 30]. https://pestadistico.inteligenciadegestion.sanidad.gob.es/publicoSNS/S/base-de-datos-de-clinicos-de-atencion-primaria-bdcap.
- Muñoz-Quiles C, López-Lacort M, Orrico-Sánchez A, Díez-Domingo J. Impact of postherpetic neuralgia: a six year population-based analysis on people aged 50 years or older. J Infect. 2018;77(2):131–6. doi:10.1016/j.jinf.2018.04.004.
- Ministerio de Sanidad. Portal Estadístico. Registro de Actividad de Atención Especializada – RAE-CMBD. Desde 2016 en adelante. Diagnósticos Principales; 2021 [accessed 2023 June 30]. https://pestadistico.inteligenciadegestion.sanidad.gob.es/publicoSNS/C/rae-cmbd/rae-cmbd/diagnosticos-principales/diagnosticos-principales.
- Instituto Nacional de Estadística. Estadística de defunciones según la causa de muerte. Defunciones por causas (lista detallada), sexo y edad. 2020 [accessed 2022 June 30]. https://www.ine.es/jaxi/Tabla.htm?tpx=49913&L=0.
- Vallejo Torres L, Linertová R, Sanromá Ramos E, Ramos García V, Toledo Chávarri A, Herrera Ramos E, Pérez Martín JJ, Limia Sánchez A, Soler Soneira M, Castilla Catalán J. et al. Coste-efectividad de la vacunación frente a herpes zóster: Servicio Canario de la Salud. Ministerio de Sanidad, Consumo y Bienestar social. 2018.
- Ministerio de Sanidad. Vacunas y Programa de Vacunación. Histórico de coberturas de vacunación. Coberturas de vacunación anteriores: Año 2020. 2020 [accessed 2023 Aug 1]. https://www.sanidad.gob.es/areas/promocionPrevencion/vacunaciones/coberturas/docs/Todas_las_tablas2020.pdf.
- Jacob L, Kostev K. Compliance with vaccination against hepatitis a virus in Germany: a retrospective analysis. Int J Clin Pharmacol Ther. 2017;55(9):740–5. doi:10.5414/cp202990.
- Grupo de trabajo de vacunación frente a herpes zóster de la Ponencia de Programa y Registro de Vacunaciones. Recomendaciones de vacunacio n frente a herpes zoster. Ponencia de Programa y Registro de Vacunaciones 2020 Comisión de Salud Pública del Consejo Interterritorial del Sistema Nacional de Salud. Ministerio de Sanidad; 2021.
- van Oorschot DAM, Hunjan M, Bracke B, Lorenc S, Curran D, Starkie-Camejo H. Public health impact model estimating the impact of introducing an adjuvanted recombinant zoster vaccine into the UK universal mass vaccination programme. BMJ Open. 2019;9(5):e025553. doi:10.1136/bmjopen-2018-025553.
- Volpi A, Boccalini S, Dari S, Clarke C, Curran D, Loiacono I, Pitrelli A, Puggina A, Tosatto R, Van Oorschot D. et al. The potential public health impact of Herpes Zoster vaccination in the 65 years of age cohort in Italy. Hum Vaccin Immunother. 2020;16(2):327–34. doi:10.1080/21645515.2019.1657753.