ABSTRACT
As of 2024, Thailand has not incorporated the varicella-zoster virus (VZV) vaccine into the Expanded Program on Immunization (EPI). This study aimed to evaluate VZV seroprevalence across all age groups in Chonburi Province, Thailand, during the post-COVID-19 era, and to support the development of a vaccination plan against VZV. A total of 950 participants were enrolled from October 2022 to January 2023. VZV antibody levels were measured using ELISA kits (EUROIMMUN, Lübeck, Germany), with seropositivity set at ≥110 IU/L. The overall VZV seropositivity rate was 64.8%, similar to rates in 1994 and 2014. However, seropositivity rates for the 5–9, 10–14, and 15–19 age groups were significantly higher in the 1994 study, and for the 10–14 and 15–19 age groups in the 2014 study, indicating a declining trend among young Thai individuals. The seropositivity rate increased with age, with a seroprevalence exceeding 80% in individuals aged 30 years and older. Our study found a significant association between the history of varicella and seropositivity. Thus, a positive history may indicate immunity. In conclusion, a significant portion of Thai adolescents are still vulnerable to varicella, highlighting the crucial role of vaccination in averting serious illness.
Introduction
Varicella is a highly contagious disease caused by initial infection by the varicella-zoster virus (VZV). The primary VZV infection typically manifests with symptoms such as fever and pruritic erythematous macules, progressing to vesicular lesions on the scalp, face, and trunk. For the majority of children, varicella is self-limited, and management primarily involves relieving symptoms and treating minor complications, including primary and secondary skin infections.Citation1 Serious complications are rare. However, in adolescents, young adults, and immunocompromised individuals, varicella can be complicated and potentially life-threatening.
Previous studies showed that there is a difference between the epidemiology of VZV infections between temperate and tropical regions.Citation2 In temperate regions, approximately 90% of children had contracted varicella by the age of seven.Citation3–5 Most of the infections occurred in pre-school children (4–6 years), followed by the 6–9 years age group.Citation3 On the contrary, in tropical regions such as Singapore, the Philippines, Malaysia, and Thailand, VZV infections tend to occur during late adolescence and adulthood.Citation6–8 Temperate climates may facilitate the transmission of VZV, leading to a peak in incidence during cooler months and in regions with a cooler, more temperate climate.
As of 2024, Thailand has not included the varicella vaccine in the Expanded Program on Immunization (EPI). Nevertheless, the vaccine has been accessible for over two decades as an optional vaccine at both public hospitals and private medical practices. According to the National Disease Surveillance (Report 506) in Thailand, the overall incidence of varicella cases in 2023 was 26.48 per 100,000 population, encompassing all age groups from birth to 60 years. However, individuals in the age ranges of 0–4, 10–14, and 15–24 years were predominantly affected by varicella.Citation9 In 1994, a VZV seroprevalence study in Thailand showed that approximately 20–30% of adolescents and young adults were seronegative, and the seroprevalence rate surpassed 90% only in individuals aged over 30 years.Citation10 Twenty years later, in 2014, our group conducted a seroprevalence survey and demonstrated that only 46.2% of adolescents aged 10–14 years were found to be seropositive for VZV. This indicated that more than half of this population remained susceptible and at risk of VZV infection.Citation11
The COVID-19 pandemic had impacted the epidemiology of infectious diseases. For example, a previous study reported that the total number of notifications significantly decreased by 41% in the pandemic period compared to the pre-pandemic one, with very high reduction of certain disease notifications such as measles and varicella.Citation12 This could be attributed to the constant use of face masks and other personal protective equipment, the frequent hand-washing, more ventilation of the living locals, and less gathering, which reduced the occasions and the possibility to contract infectious diseases. The study aims to reevaluate the current seroprevalence of VZV across all age groups during the post-pandemic period and provide evidence supporting the development of a vaccination strategy against VZV in Thailand.
Methods
Study design and participants
The serosurvey was conducted in 11 districts in Chonburi province, Thailand between 1 October 2022 and 31 January 2023. The stratified clusters within the 11 districts were divided into urban and rural strata. From each district, each cluster was selected using the probability-proportionate-to-size sampling method. This study served as a sub-study within the larger research project aimed at determining the overall prevalence of SARS-CoV-2 infection in Thailand.Citation13 Chonburi province, located about 90 km from Bangkok on the eastern Gulf of Thailand, was chosen as a representative city due to its diverse landscape and economy. Chonburi features a mix of urban areas, tourist attractions like Pattaya City, and growing industrial estates alongside rural agricultural regions as previously described.Citation13 The large research project was a population-based, age-stratified, random sampling study that included 1459 individuals. A total of 950 from the 1459 specimens were randomly selected to evaluate anti-VZV IgG in this study. The inclusion criteria comprised individuals between 1 month to 80 years with no immunosuppressed status, malignancy, or severe hematologic disorders. The interviewer-administered questionnaires collected information on age, sex, history of varicella and varicella vaccination.
The protocol was reviewed and approved by the Institutional Review Board of Chonburi Provincial Public Health Office (IRB no. CBO Rec 65–0024) and the Institutional Review Board of the Faculty of Medicine, Chulalongkorn University (IRB no. 0706/65 CoA No. 0066/2023). This research study was conducted in accordance with the Declaration of Helsinki and the principles of good clinical practice. All participants or their parents were informed about the study’s objectives, and written consent was obtained prior to their enrollment in the study.
Sample collection
Blood samples (3–5 mL) were collected at the study site in Chonburi province between 1 October 2022 and 31 January 2023. The blood samples underwent centrifugation to obtain serum samples, which were then aliquoted and stored at −20°C until laboratory testing. Subsequently, the samples were transported to the virology laboratory at the Center of Excellence in Clinical Virology, Faculty of Medicine, Chulalongkorn University in Bangkok for serological testing.
Serological assays
Commercial enzyme-linked immunosorbent assay (ELISA) kits (EURROIMMUN, Lubeck, Germany) were utilized in accordance with the manufacturer’s instructions to measure the concentrations of IgG against the VZV. Serum samples were initially diluted 1:100 and then further diluted to obtain values within the detection range. Anti-VZV IgG concentrations are expressed in international units (IU/L). According to the manufacturer’s information, anti-VZV IgG concentration of ≥10 IU/L were defined as seropositive.Citation14 The serological testing was conducted at the Center of Excellence in Clinical Virology, Faculty of Medicine, Chulalongkorn University.
Statistical analysis
Data on the seropositivity rate of anti-VZV IgG are presented as numbers and percentages. The geometric mean titers (GMT) with their 95% confidence intervals were calculated. One-way analysis of variance was used to evaluate statistically significant differences in the GMT between different age groups. Associations between seropositivity status and self-reported medical history were performed using Person’s chi-square test. The comparison of seropositivity status and GMT between vaccinated and unvaccinated children was performed using Pearson’s chi-square test and the t-test, respectively. Furthermore, the seropositivity rate in this study was compared to the rates observed in two prior sero-epidemiological surveys conducted in 1994 and 2014 using the chi-square test.Citation10,Citation11 All statistical analyses were performed with SPSS v23.0 (IBM Corp., Chicago, IL). Figures were generated using GraphPad Prism v9.4.1 (GraphPad Software, San Diego, CA). A p-value < .05 was considered statistically significant.
Results
Demographic characteristics of study participants
presents the demographic characteristics of the participants enrolled in the study. The cohort comprised 471 male individuals (49.6%) and 479 female individuals (50.4%). To facilitate comparison with the seropositivity rates observed in the two earlier sero-epidemiological surveys conducted in 1994 and 2014, the age groups of participants were re-categorized as follows: <1, 1–4, 5–9, 10–14, 15–19, 20–29, 30–39, 40–49, 50–59, 60–69, and ≥70 years.Citation10, Citation11 The participants’ ages ranged from 4 months to 79 years. A total of 28.2% reported a past history of varicella, while 7.2% (68/950) indicated having received the varicella vaccination. Among individuals who reported having received the varicella vaccination, the majority (30/68; 44.1%) were children below 10 years of age, followed by the adolescents between 11–20 years of age (22/68; 32.3%).
Table 1. Demographic characteristics of the participants included in this study (N = 950).
Seropositivity rate and GMT of anti-VZV IgG
Out of the 950 serum specimens analyzed for anti-VZV IgG levels, the overall VZV seropositivity rate was 64.8%, with a GMT of 187.9 IU/L (95% CI: 162.7–217.0) (). The seropositivity rate increased with age. Specifically, it was 16.1% in the <4 years age group, increased to 30.0% in the 5–9-year-old age group, reached 33.6% in the 10–14-year-old age group, rose to 44.6% in the 15–19-year-old age group, increased to 74.2% in the 20–29-year-old age group, and consistently exceeded 80% in individuals aged 30 years and older (). No significant differences were observed in the VZV seropositivity rate between male and female participants.
Figure 1. Seropositivity rates and geometric mean titer (GMT) of anti-varicella zoster virus (anti-VZV) IgG in individuals across different age groups residing in Chonburi Province, Thailand (2022–2023).
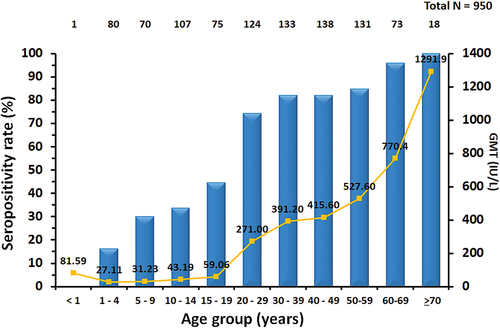
Table 2. Seroprevalence of anti-VZV IgG by age group and sex.
The GMT of anti-VZV IgG increased with age in parallel with the increase in seropositivity. Specifically, the GMT was 27.5 IU/L in the <4 years old age group, 31.2 IU/L in the 5–9-year-old age group, 43.2 IU/L in the 10–14-year-old age group, 59.1 IU/L in the 15–19-year-old age group and surged to 271.0 IU/L in the 20–29-year-old age group. The GMT of individuals aged 20 years and above was significantly higher compared to those in the <4 years old age group (p < .001). Likewise, there were no statistical differences in the GMT of anti-VZV IgG between male and female participants.
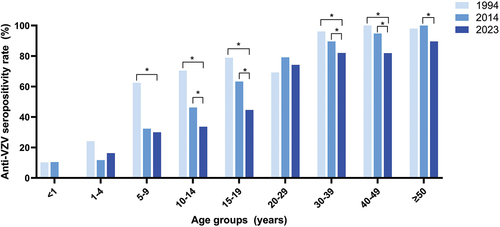
We conducted a comparison of the seropositivity rates in our study (2023) with those from previous studies conducted in Thailand in 1994 and 2014 ().Citation10,Citation11 The findings indicated that the overall seropositivity rate in our study (64.8%) was similar to the two previous studies (61.4% in 1994 and 54.1% in 2014). Nevertheless, the estimates of seropositivity rates among individuals aged 5–9, 10–14 and 15–19 years were significantly higher in the Thai 1994 study than in the current study (p < .001). Similarly, the estimates of the seropositivity rate among individuals aged 10–14 and 15–19 years of age were also significantly higher in the Thai 2014 study than in the current study (p < .001). Conversely, there were no significant differences in the seropositivity rate among individuals aged 20–29 years across the three studies.
Figure 2. Comparison of the VZV seroprevalence in the present study with the two previous studies conducted in Thailand in 1994 and 2014.
Associations between serological results and self-reported medical history
There was a significant association between the history of varicella and seropositivity rate (p < .001) as shown in supplement materials (Table S1). A total of 90.3% (242/268) of individuals with a history of varicella were seropositive. The positive predictive value and the negative predictive value for the history of varicella were 90.3% and 46.8%, respectively.
In contrast, no association was found between the history of varicella vaccination and seropositivity status (p = .714) in the studied population (Table S2). Nonetheless, data from children aged 1–10 years who were likely to have received varicella vaccination were analyzed separately as a subgroup analysis. Out of the 174 children aged 1–10 years, 38 could not provide their vaccination history, resulting in the inclusion of only the 136 children with available vaccination history in the analysis. The overall seropositivity rate within this group was 23.5% (32 out of 136), with 22.1% (30 out of 136) having a history of varicella vaccination. The Chi-square test indicated a significant association between vaccination history and seropositivity status (p < .001) in children aged 1–10 years (Table S3). Additionally, the independent t-test demonstrated that vaccinated children had significantly higher anti-VZV GMT (101.2 IU/L) compared to unvaccinated children (23.49 IU/L) (p < .001).
Discussion
The present study provides VZV sero-epidemiological data encompassing individuals of all age groups residing in Chonburi province, Thailand. The data collection period spans from October 2022 to January 2023, representing the early post-pandemic period. The findings revealed an overall seropositivity rate of 64.8% which is similar to the rates observed in earlier studies conducted in Thailand in 1994 (61.4%) and 2014 (54.1%).Citation10,Citation11 Nonetheless, the seropositivity rate estimates for young children to adolescents, specifically in the age groups of 5–9, 10–14, and 15–19 years, were significantly higher in the 1994 study. Additionally, the seropositivity rates for the age groups 10–14 and 15–19 years were higher in the 2014 study compared to the present study. This suggests a declining trend in VZV seropositivity among young Thai individuals. Prior epidemiological research demonstrated that the COVID-19 lockdown and associated measures markedly diminished the incidence of numerous communicable diseases, including varicella.Citation12,Citation15,Citation16 Thailand also experienced the first wave of COVID-19 outbreak in March 2020, prompting a nationwide lockdown and implementation of other public health measures aimed at controlling transmission between March and May 2020.Citation17 Following the first wave, public health and social measures such as the utilization of face masks, maintaining physical distance, and practicing hand hygiene remained in effect to combat subsequent waves between 2021 and 2022.Citation18 These COVID-19 preventive measures may contribute to the decreased transmission of varicella in the community resulting in a low seropositivity rate in the current study. Data from the national surveillance report also indicated that the incidence of varicella in 1994 and 2014 exceeded that reported in 2023, with rates of 54.8 per 100,000 population in 1994 and 128.7 per 100,000 population in 2014, compared to 26.48 per 100,000 population in 2023.Citation9 The reduced natural exposure to VZV and the lack of universal varicella vaccination campaign in Thailand contributed to the low seropositivity rate in young Thai individuals. Conversely, the seropositivity rate exceeding 80% in individuals over 30 years of age in this study suggests that they may have experienced natural infection in the past, potentially conferring lifelong immunity.
The VZV seropositivity rates observed in this study exhibited a similar pattern to those reported in other countries within tropical regions that have not implemented universal varicella vaccination. For instance, the VZV seropositivity rate was 16% in children aged 1–4 years in India,Citation19 11% in children aged 0–5 years in Congo,Citation20 28.4% in children aged 0–5 years in Pakistan,Citation21 30% of children under 5 years of age in the Philippines,Citation8 and 33.2% of children under 5 years in Singapore.Citation22 In addition to climatic factors, other elements such as population density and household size may influence VZV epidemiology.Citation23,Citation24 For example, the low VZV seroprevalence among children in Congo compared to other tropical regions might be attributable to its low population density.Citation20,Citation25 A previous study in Thailand showed that the VZV seronegativity rate among adolescents and adults was higher in rural areas (low population density) compared to urban areas (high population density), suggesting that population density is one of the determinants of VZV seroprevalence in tropical regions.Citation24 In contrast, a study from Mexico found that household size and population density were not associated with varicella incidence.Citation26 Overall trends in tropical regions suggest that the highest rate of seroconversion occurs during adolescence and adulthood, with seropositivity increasing with age.Citation2,Citation22 This is concerning as infection in older ages is associated with increased rates of severe disease and complications, including but not limited to skin infections, pneumonia, encephalitis, and increased mortality.Citation1
Our study showed a significant association between the history of varicella and seropositivity status. This aligns with the previous studies indicating a significant association between history of varicella and seropositive status.Citation10,Citation27 A previous review also found that a history of varicella had a high positive predictive value and a low negative predictive value for immunity.Citation28 Consequently, in populations with lower risk, considering a positive history of varicella as an indicator of immunity may be a reasonable approach. However, for older individuals with a negative or uncertain history of varicella, serologic testing is recommended before suggesting varicella vaccination, as a majority of these individuals are likely to be immune.
This study has some limitations. Firstly, the absence of verified medical records means that the reporting of varicella history and varicella vaccination relies on self-reporting. This could introduce the potential for recall bias, as well as increased levels of uncertainty and unavailable data. Besides, it is not possible to determine whether the antibodies of the study participants are due to infection or immunization. Secondly, the ELISA kits utilized in the two preceding studies conducted in Thailand differed from those employed in the current study. Previous studies reported variations in sensitivity across different ELISA kits.Citation14 Finally, the limited sample size in the <1 year-old age group posed challenges in conducting meaningful statistical comparisons.
In conclusion, this study revealed that over 50% of young children and adolescents were seronegative for anti-VZV IgG. Furthermore, approximately 25% of young adults aged 20–29 years were found to be susceptible to varicella. Consequently, it is recommended that vaccination programs in Thailand should consider including children and susceptible adolescents and adults at risk of severe complications from varicella. Future investigations, including cost-benefit analyses, should be conducted to facilitate the implementation of varicella vaccination in Thailand.
Author contributions statement
Conceptualization: TT, NW, YP
Data curation: JC, TT
Formal Analysis: TT, JC, NW
Acquisition: NW, YP
Investigation: RA, SK, TT
Supervision: NW, YP
Writing-Original draft: TT
Writing-Review and Editing: NW, YP
Ethics statement
The protocol was reviewed and approved by the Institutional Review Board of Chonburi Provincial Public Health Office (IRB numbers CBO Rec 65–0024) and the Institutional Review Board of the Faculty of Medicine, Chulalongkorn University (IRB no. 0706/65 CoA No. 0066/2023).
Informed consent statement
Informed consent was obtained before participant enrollment. The study was conducted according to the Declaration of Helsinki and the Good Clinical Practice Guidelines (ICH-GCP) principles.
Supplementary Materials_28052024.docx
Download MS Word (16.3 KB)Acknowledgments
We thank the staff from the hospitals and health care centers in Chonburi Province for enrolling participants. We thank the staff from the Chonburi Provincial Public Health Office and the Division of Communicable Diseases, Department of Disease Control, Ministry of Public Health, Thailand for their contribution in this project.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data will be made available upon request.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2024.2367283
Additional information
Funding
References
- Arvin AM. Varicella-zoster virus. Clin Microbiol Rev. 1996;9(3):361–6. doi:10.1128/CMR.9.3.361.
- Daulagala S, Noordeen F. Epidemiology and factors influencing varicella infections in tropical countries including Sri Lanka. VirusDis. 2018;29(3):277–284. doi:10.1007/s13337-018-0459-z.
- Cohen DI, Davidovici BB, Smetana Z, Balicer RD, Klement E, Mendelson E, Green MS. Seroepidemiology of varicella zoster in Israel prior to large-scale use of varicella vaccines. Infection. 2006;34(4):208–213. doi:10.1007/s15010-006-6604-4.
- Salleras L, Domínguez A, Vidal J, Plans P, Salleras M, Taberner JL. Seroepidemiology of varicella-zoster virus infection in Catalonia (Spain). Rationale for universal vaccination programmes. Vaccine. 2000;19(2–3):183–188. doi:10.1016/S0264-410X(00)00178-X.
- Wiese-Posselt M, Siedler A, Mankertz A, Sauerbrei A, Hengel H, Wichmann O, Poethko-Müller C. Varicella-zoster virus seroprevalence in children and adolescents in the pre-varicella vaccine era, Germany. BMC Infect Dis. 2017;17(1):356. doi:10.1186/s12879-017-2461-2.
- Ooi PL, Goh KT, Doraisingham S, Ling AE. Prevalence of varicella-zoster virus infection in Singapore. Southeast Asian J Trop Med Public Health. 1992;23(1):22–25.
- Lee BW. Review of varicella zoster seroepidemiology in India and Southeast Asia. Trop Med Int Health. 1998;3(11):886–890. doi:10.1046/j.1365-3156.1998.00316.x.
- Barzaga N, Roxas J, Florese R. Varicella zoster virus prevalence in metro Manila, Philippines. J Am Med Assoc. 1994;274:5–633.
- The Bureau of Epidemiology, Department of Disease Control, the Ministry of Public Health, Thailand. National Disease Surveillance Report 506: Chicken pox. [accessed 2024 Jan 13]. http://doe.moph.go.th/surdata/disease.php?ds=17.
- Migasena S, Simasathien S, Desakorn V, Phonrat B, Suntharasamai P, Pitisuttitham P, Aree C, Naksrisook S, Supeeranun L, Samakoses R, et al. Seroprevalence of varicella-zoster virus antibody in Thailand. Int J Infect Dis. 1997;2(1):26–30. doi:10.1016/S1201-9712(97)90007-2.
- Thantithaveewat T, Thongmee T, Vichaiwattana P, Posuwan N, Suntharn P, Yoocharoen P, Tharmaphornpilas P, Poovorawan Y. Seroprevalence of varicella-zoster antibodies in a Thai population. Southeast Asian J Trop Med Public Health. 2019;50:94–100.
- Facciolà A, Laganà A, Genovese G, Romeo B, Sidoti S, D’Andrea G, Raco C, Visalli G, DI Pietro A. Impact of the COVID-19 pandemic on the infectious disease epidemiology. J Prev Med Hyg. 2023;64(3):e82–e82. doi:10.15167/2421-4248/jpmh2023.64.3.2904.
- Chansaenroj J, Suntronwong N, Kanokudom S, Assawakosri S, Vichaiwattana P, Klinfueng S, Wongsrisang L, Thongmee T, Aeemjinda R, Khanarat N, et al. Seroprevalence of SARS-CoV-2 anti-nucleocapsid total Ig, anti-RBD IgG antibodies, and infection in Thailand: a cross-sectional survey from October 2022 to January 2023. Sci Rep. 2023;13(1):15595. doi:10.1038/s41598-023-42754-2.
- Sauerbrei A, Schäfler A, Hofmann J, Schacke M, Gruhn B, Wutzler P. Evaluation of three commercial varicella-zoster virus IgG enzyme-linked immunosorbent assays in comparison to the fluorescent-antibody-to-membrane-antigen test. Clin Vaccine Immunol. 2012;19(8):1261–8. doi:10.1128/CVI.00183-12.
- Launay T, Souty C, Vilcu AM, Turbelin C, Blanchon T, Guerrisi C, Hanslik T, Colizza V, Bardoulat I, Lemaître M, et al. Common communicable diseases in the general population in France during the COVID-19 pandemic. PLOS ONE. 2021;16(10):e0258391. doi:10.1371/journal.pone.0258391.
- Adegbija O, Walker J, Smoll N, Khan A, Graham J, Khandaker G. Notifiable diseases after implementation of COVID-19 public health prevention measures in Central Queensland, Australia. Commun Dis Intell. 2021;45. doi:10.33321/cdi.2021.45.11.
- Triukose S, Nitinawarat S, Satian P, Somboonsavatdee A, Chotikarn P, Thammasanya T, Wanlapakorn N, Sudhinaraset N, Boonyamalik P, Kakhong B, et al. Effects of public health interventions on the epidemiological spread during the first wave of the COVID-19 outbreak in Thailand. PLOS ONE. 2021;16(2):e0246274. doi:10.1371/journal.pone.0246274.
- Rajatanavin N, Tuangratananon T, Suphanchaimat R, Tangcharoensathien V. Responding to the COVID-19 second wave in Thailand by diversifying and adapting lessons from the first wave. BMJ Glob Health. 2021;6(7):e006178. doi:10.1136/bmjgh-2021-006178.
- Venkitaraman AR, Seigneurin JM, Lenoir GM, John TJ. Infections due to the human herpesviruses in southern India: a seroepidemiological survey. Int J Epidemiol. 1986;15(4):561–566. doi:10.1093/ije/15.4.561.
- Doshi RH, Alfonso VH, Mukadi P, Hoff NA, Gerber S, Bwaka A, Higgins SG, Sinai C, Cowell B, Ngoie Mwamba G, et al. Low varicella zoster virus seroprevalence among young children in the Democratic Republic of the Congo. Pediatr Infect Dis J. 2018;37(2):138–431. doi:10.1097/INF.0000000000001750.
- Akram DS, Qureshi H, Mahmud A, Khan AA, Kundi Z, Shafi S, Olowokure B, Weil J, Bock H, et al. Seroepidemiology of varicella-zoster in Pakistan. Southeast Asian J Trop Med Public Health. 2000;31(4):646–649.
- Fatha N, Ang LW, Goh KT. Changing seroprevalence of varicella zoster virus infection in a tropical city state, Singapore. Int J Infect Dis. 2014;22:73–77. doi:10.1016/j.ijid.2013.10.003.
- Nichols RA, Averbeck KT, Poulsen AG, Al Bassam MM, Cabral F, Aaby P, Breuer J. Household size is critical to varicella-zoster virus transmission in the tropics despite lower viral infectivity. Epidemics. 2011;3(1):12–18. doi:10.1016/j.epidem.2010.11.003.
- Sornchai S, Warachit W, Lolekha P, Kosuwan P, Sutra S, Tanthiphabha B, Sutra S, Chup-Upprakarn S, Bock HL, Kosuwan P, et al. Effect of climatic factors and population density on varicella zoster virus epidemiology within a tropical country. The Am J Trop Med And Hyg. 2001;64(3):131–136. doi:10.4269/ajtmh.2001.64.131.
- PopulationPyramid.net. Population density. [accessed 2024 May 28]. https://www.populationpyramid.net/population-density/democratic-republic-of-the-congo/2013/.
- Vergara-Castañeda A, Escobar-Gutiérrez A, Ruiz-Tovar K, Sotelo J, Ordoñez G, Cruz-Rivera MY, Fonseca-Coronado S, Martinez-Guarneros A, Carpio-Pedroza JC, Vaughan G, et al. Epidemiology of varicella in Mexico. J Clin Virol. 2012;55(1):51–57. doi:10.1016/j.jcv.2012.06.004.
- Bhattarakosol P, Chantarabul S, Pittayathikhun K, Mung-Mee V, Punnarugsa V. Prevalence of anti-varicella zoster IgG antibody in undergraduate students. Asian Pac J Allergy Immunol. 1996;14(2):31–129.
- Holmes CN. Predictive value of a history of varicella infection. Can Fam Phys. 2005;51:60–65.
