 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Many pathogens enter the host through mucosal sites. Thus, interfering with pathogen entry through local neutralization at mucosal sites therefore is an effective strategy for preventing disease. Mucosally administered vaccines have the potential to induce protective immune responses at mucosal sites. This manuscript delves into some of the latest developments in mucosal vaccination, particularly focusing on advancements in adjuvant technologies and the role of these adjuvants in enhancing vaccine efficacy against respiratory pathogens. It highlights the anatomical and immunological complexities of the respiratory mucosal immune system, emphasizing the significance of mucosal secretory IgA and tissue-resident memory T cells in local immune responses. We further discuss the differences between immune responses induced through traditional parenteral vaccination approaches vs. mucosal administration strategies, and explore the protective advantages offered by immunization through mucosal routes.
Introduction
Mucosal surfaces, which line the respiratory, digestive, and genitourinary tracts, serve as primary barriers against environmental factors such as pathogens and allergens. These surfaces are continuously monitored by the immune system, balancing tolerance to benign factors and activation against harmful microbes. Traditional vaccines, administered subcutaneously or intramuscularly, primarily elicit systemic immune responses with elevated levels of antigen-specific T cells, memory B cells, and IgG antibodies, but limited mucosal immunity.Citation1 Conversely, mucosal vaccination more closely mimics the natural infection route, inducing both systemic and mucosal immunity, the latter characterized by mucosal secretory IgA (sIgA) and tissue-resident memory T cell (TRM) responses.Citation2 Additionally, mucosal vaccination provides superior immunity at pathogen entry sites, thus offering the potential to block pathogen transmission and eliminating the need for needles, making it practical for mass vaccination. However, mucosal vaccination presents specific challenges, including the need for immune stimulants like adjuvants to induce a protective immune response efficiently and safely, and the requirement for specialized devices for effective administration due to the compartmentalization of mucosal surfaces.Citation3
A thorough understanding of the immune response mechanisms at mucosal sites is essential for the development of more effective mucosal vaccines. This review first aims to evaluate the characteristics of the respiratory mucosal immune system in the context of mucosal infections and vaccination strategies. It will then discuss recent advancements in mucosal vaccines and adjuvants and highlight the potential of novel adjuvant formulations in addressing critical challenges in the development of respiratory mucosal vaccines.
Anatomy and characteristics of the respiratory mucosal immune system
The respiratory tract is divided into the upper respiratory tract (URT) and lower respiratory tract (LRT). The URT comprises the conducting airways, including the nasal cavity, pharynx, and larynx, which form a continuous passageway for air movement, along with the associated lymphoid tissue, including the nasal-associated lymphoid tissue (NALT), and the cervical lymph nodes, which drain the URT. The LRT contains the trachea, as well as the bronchi and bronchiole branches within the lungs. The anatomical structures of the upper and lower respiratory tracts vary between different age groups and species.Citation4 A good understanding of these differences is crucial for the development of safe and effective mucosal vaccines and adjuvant research. Notably, in humans, the nasal cavity is predominantly lined with respiratory epithelium, occupying approximately 90% of the epithelial surface, while the olfactory epithelium constitutes the remaining 10%.Citation5 However, in mice and rats, both the respiratory and olfactory epithelia each comprise about 50% of the nasal cavity. Specifically, the upper (larger-diameter) airway in humans primarily consists of ciliated cells and mucus-secreting goblet cells, while the lower (smaller-diameter) airway has fewer goblet cells but more secretory cells.Citation6 The mouse trachea has a similar cellular composition as the human bronchiole.
Lymphatic draining from the LRT goes to the bronchial-associated lymphoid tissue (BALT), along with the mediastinal lymph nodes, which together play critical roles in initiating local adaptive immune responses to pathogens infiltrating the lungs (). Within these structures of the lung, innate and adaptive immune mechanisms exist to maintain respiratory health and defend against pathogen infiltration.
Figure 1. Organization and cellular characteristics of the human respiratory airway.
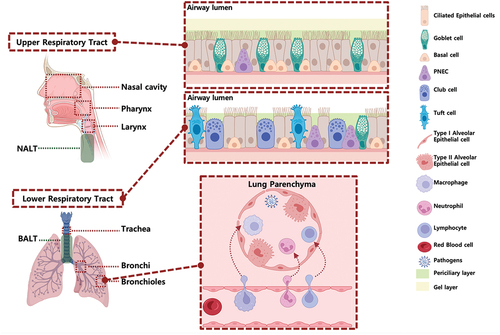
The mucosal immune system is classified into inductive sites, which recognize and process antigens (mucosa-associated lymphoid tissue (MALT), including NALT and BALT), and effector sites, which produce sIgA (nasal lamina propria and nasal mucosa).Citation5 Additionally, stimulation of specific inductive sites can induce similar immune responses at various effector sites, a critical concept for the design of mucosal vaccine delivery routes.Citation7 This unique interconnected circulation of lymphocytes within the mucosal immune system is termed the common mucosal immune system (CMIS). Microfold cells (M cells) within inductive sites capture antigens and facilitate their presentation to dendritic cells (DCs).Citation8 These DCs then migrate to the T cell region of the MALT and activate naïve T cells, which in turn stimulate the isotype switch of IgM+ B cells to IgA+ B cells, a process orchestrated by cytokines including TGF-β and IL-10.Citation7 Consequently, IgA+ B cells migrate to various mucosal effector sites through the lymphatic system, differentiate into plasma cells, and produce sIgA.Citation9 Mucosal tissue-resident IgA-producing plasma cells secrete IgA that polymerizes into polymeric IgA (pIgA) through J-chain linkage.Citation10 This pIgA, transported across epithelial cells by the polymeric Ig receptor (pIgR), undergoes cleavage to form sIgA, which can prevent microbial attachment to mucosal surfaces by neutralization and aggregation.Citation7,Citation11 Additionally, the upper and lower respiratory tracts exhibit distinct antibody compositions.Citation2,Citation11 sIgA predominates in the upper respiratory tract, where it plays a critical role in mucosal immunity by neutralizing pathogens and inhibiting their adherence to epithelial cells.Citation12 The immune response in the upper respiratory tract is characterized by the mucociliary clearance of sIgA-bound pathogens, effectively preventing colonization. However, both IgA and IgG in the lower respiratory tract are essential for maintaining homeostasis and providing protection against pathogens.Citation1,Citation13 IgG is critical for opsonization, pathogen neutralization, and activation of complement pathways, contributing to both lower respiratory tract and systemic protection.Citation14 Furthermore, the defense mechanisms in the lower respiratory tract involve robust IgG-mediated functions, supported by a strong cellular immune response involving alveolar macrophages and T lymphocytes.Citation1,Citation15
Innate immunity in the respiratory mucosa
Mucosal innate immunity encompasses mechanical, chemical, and cellular elements, which provide first-line defense in the respiratory mucosa. The mechanical elements include a physical barrier of tightly formed epithelium covered with a mucus coating. The ciliated epithelium, along with mucus secreted by goblet cells, expel debris, allergens, and potential pathogens in the URT.Citation11 The same cellular components are also present in the LRT, along with additional presence of club cells, non-ciliated epithelial cells that represent the major secretory cell in the small airway epithelium. These cellular components express various pattern recognition receptors (PRRs) and cytokine receptors, enabling them to continuously survey for pathogens and coordinate immune responses with both local and recruited immune cells.Citation16–18 Pulmonary endothelial cells (ECs) primarily function as a barrier between the blood and interstitium.Citation19 ECs also actively participate in immune surveillance, phagocytosis, immune cell recruitment, and antigen presentation, with some classified as immunomodulatory endothelial cells (IMECs) that modulate the balance between immune tolerance and inflammation.
Respiratory mucus consists of two defined layers: an upper viscous, gel-like mucus layer and an underlying periciliary layer (PCL) (). These mucus layers are characterized by a bottlebrush-like structure in which mucins, O-glycosylated proteins, are tethered to the airway surface.Citation20 Mucins are the primary components of both layers of mucus and are crucial in safeguarding the respiratory tract against pathogenic infections.Citation21 Aside from merely passive blocking of pathogens, dense glycosylation, and sialylation of mucins trap glycan-receptor-binding viruses, aiding in their mucociliary clearance. The mucus layer can play a factor in mucosal vaccine delivery. Retention times of vaccine formulations at mucosal sites are important factors for consideration in mucosal vaccine design, and formulation efforts that can promote mucoadhesion and mucopenetration are intense areas of research.Citation22
The main innate immune cellular elements include neutrophils, NK cells, macrophages, and dendritic cells.Citation11,Citation12 These cell types either directly engulf pathogens via phagocytosis or initiate signaling pathways that promote killing upon recognition of conserved structures on the pathogens.Citation12 Upon infection, neutrophils, being the first responders, are recruited to the lungs and airways.Citation23,Citation24 Within the respiratory tissue, neutrophils play a critical role in clearing pathogens and cellular debris from the airspaces through degranulation, phagocytosis, and the formation of neutrophil extracellular traps (NETs). NETs are web-like structures composed of cytosolic and granule proteins on decondensed chromatin, which trap and neutralize bacterial cells and sequester excess cytokines, thereby aiding in the regulation of pulmonary inflammation.Citation25
NK cells and macrophages are the frontline innate effector cells that mount the initial response against pathogens.Citation26–29 NK cells are innate lymphocytes that recognize and kill cancerous or virally infected cells, primarily through secretion of perforin, and granzymes, and mediation of antibody-dependent cellular cytotoxicity, as well as through their significant contribution to the production of interferon-gamma (IFN-γ).Citation11,Citation30 Macrophages, constituting approximately 70% of the lung’s leukocyte population,Citation31 function as phagocytes and sensors of respiratory infections. They express high levels of pattern recognition receptors (PRRs) for detecting infections and have a high propensity for surface Fc receptors, crucial for pathogen clearance. The presence of PRRs makes them good targets for adjuvants, which often are PRR agonists (see below). Vaccine induced non-neutralizing antibodies that engage Fc receptors have been correlated with protection against influenza viruses of different subtypes and provide an extra mechanism for protection beyond virus neutralization.Citation32 Respiratory or airway macrophages can be classified into two groups based on their localization and function: alveolar macrophages (AMs) (long-lived tissue-resident (TR-AMs) and monocyte-derived AMs), and interstitial macrophages (IMs). AMs, the primary phagocytes in the lung, are a distinct subset of self-renewing cells, originating from fetal liver precursors.Citation11,Citation29,Citation33,Citation34 TR-AMs engulf foreign material and apoptotic cells in the alveolar space and participate in constant crosstalk with epithelial cells within the lung to coordinate immune activation or suppression. TR-AMs provide the first-line defense in the LRT, releasing cytokines and chemokines upon infection to initiate inflammatory responses and recruit other effector cells to the alveolar space. However, AMs can also be exploited by various viruses for dissemination, persistence, and replication. Interstitial macrophages (IMs) are predominantly located between the microvascular endothelium and alveolar epithelium, where they perform phagocytosis and antigen presentation like AMs, and can also modulate tissue repair.Citation11,Citation29,Citation33,Citation35
DCs are long-lived, and can capture antigens from the airway lumen and migrate to the draining lymph nodes in the NALT and BALT, where they present or cross-present antigens to naïve CD4+ and CD8+ T cells.Citation11 Dendritic cells are classified into three main groups in the lungs: plasmacytoid DCs (pDCs) and the monocyte-derived conventional subsets, conventional cDC1 and cDC2. pDCs are dispersed throughout the lung tissue, including the airway, lung parenchyma, and lung stroma.Citation36 pDCs are vital in innate immunity due to their substantial production of type I interferons, especially in response to sensing of viral PAMPs, thereby triggering a cascade of antiviral pathways. pDCs typically respond rapidly upon sensing of bacterial or viral nucleic acids at the early stages of infection, and as such, play an important role during the initiation of the host response.Citation37 cDC1s are found in vascular and mucosal areas, initiating Th1 responses and cross-presenting to CD8+ T cells to promote cytotoxic functions for the killing of intracellular pathogens. In contrast, cDC2s, located in the lamina propria, prime CD4+ T cells and produce pro-inflammatory chemokines, which recruit various inflammatory cells to sites of lesions. Furthermore, cDC2s have been suggested to also help in the establishment of immune tolerance.Citation36 Given their role in antigen presentation, efficient DC activation and antigen delivery to DCs is a crucial step during mucosal vaccination.
Innate immune cells have been increasingly recognized for their ability to develop ‘trained immunity,’ a nonspecific memory-like function.Citation38–40 Trained immunity is exemplified by a ‘priming’ phase of innate immune cells in response to exposure to specific stimuli, in which epigenetic and metabolic reprogramming is induced in innate immune cells. Trained innate immunity has been ascribed to various innate immune cells such as monocytes, dendritic cells (DCs), natural killer (NK) cells, fibroblasts, and macrophages.Citation33–42 Since the mucosa of the respiratory tract is continuously exposed to a variety of pathogens, including viruses and bacteria,Citation43 it is clear that trained immunity has an important role in shaping mucosal innate immunity and subsequently downstream adaptive immune responses to both infection and vaccination. The impact of trained immunity on induction of mucosal vaccine responses and the outcome during reinfection is currently not fully understood, but a high priority research area with potential implications for the innate immune response during mucosal vaccination.
Components of the mucosal adaptive respiratory immune response
The respiratory adaptive immune system involves several key players. This includes CD8+ cytotoxic T lymphocytes (CTLs), which kill infected cells upon recognition of class I MHC-bound peptides.Citation44,Citation45 CTLs exert such activity through the secretion of cytokines, the release of cytotoxic granules, and through the induction of apoptotic pathways in target cells. CTLs are critical for eliminating viral and intracellular bacterial infections in the lung as well as in eliminating tumor cells; however, they can also cause collateral lung damage if dysregulated. Another key player is the CD4+ T cells, traditionally known as ‘helper’ cells, which enhance cytotoxic responses or stimulate B cell antibody production.Citation12 While multiple less abundant CD4+ T cell subsets have been recently defined, the five major CD4+ T cell subsets include Th1, Th2, Th17, regulatory T (Treg), and T follicular helper (TFH) cells. These subsets are classified based on the signature cytokines they produce and their lineage-specific transcription factors. Th1 cells, which respond to intracellular lung infections through enhancing both innate and adaptive cytotoxic responses, produce IFN-γ, IL-2, and TNF- and express T-bet. Th2 cells regulate airway inflammation and influence B-cell differentiation, antibody production, and eosinophil recruitment through secretion of IL-4, IL-5, and IL-13, and express GATA-3. The Th17 subset secretes IL-17A, IL-17F, IL-21 and IL-22, and express RoRγt. Th17 cells are especially important to host defense and homeostasis at mucosal sites, and their secreted cytokines lead to the recruitment of neutrophils as well as B cells to sites of infection, and can enhance the efficiency of APCs and the CTL response.Citation46 TFH cells, which produce IL-21 and express Bcl6, are essential to driving antigen-specific antibody responses through their function in germinal centers (GCs) formation and B cell maturation within these GCs. Finally, Treg express FoxP3 and CD25 and act to regulate immune responses and mediate airway tolerance and tissue repair to avoid immunopathology arising from overexuberant inflammatory responses.Citation45
Following an infection or vaccination, long-lived adaptive responses form through the establishment of memory T and B cells.Citation11 Of particular interest is the differentiation of subsets of T and B lymphocytes in the mucosae into tissue-resident memory (RM) lymphocytes, which are non-circulating and long-lived.Citation47 Unlike their circulating counterparts, these lymphocytes are specifically adapted to provide rapid and localized immune responses to repeated pathogen exposure. In the context of respiratory infections and mucosal vaccination, CD8+ TRM are particularly significant. These cells are identified by their expression of CD69, CD103, CXCR3 and reduced expression of the chemokine receptors, CCR7 and CD62L, enabling them to remain within peripheral tissues.Citation48 Functionally, CD8+ TRM aid in rapid viral clearance through the production of various cytokines (IFN-γ, TNFα, IL-2) and cytolytic molecules like granzyme B.Citation49 CD4+ TRM cells are more abundant than CD8+ TRM and play a crucial role in pulmonary defense.Citation50 These cells, often found around airways and within special lymphoid structures in the lungs, help in the development of CD8+ TRM and support B cells in antibody production. After lung infections, CD4+ TRM cells localize around the airways and within inducible bronchus-associated lymphoid tissues (iBALT), forming clusters with other immune cells such as follicular dendritic cells and other APCs.Citation51 iBALT, a tertiary lymphoid organ distinct from BALT, is formed in response to antigenic stimuli including infections, self-antigens, and other sources.Citation52 Similarly, tissue-resident memory B cells (BRM) are persistent cells located in mucosal tissues after infection that can quickly respond upon re-encountering antigen.Citation53 Notably, the localization of BRM varies among species. For instance, in the upper respiratory tract, BRM cell is situated in the tonsils and adenoids in humans, and in NALT in mice.Citation5 In the lower respiratory tract, BRM cells are found within iBALT or dispersed near alveoli, independently of iBALT.Citation54 Unlike circulating B cells, lung BRM engage in tissue scanning and transform into plasma cells upon encountering an antigen, enhancing local antibody production for quicker pathogen elimination. Studies involving the transfer of these cells have demonstrated that lung BRM effectively reduce viral levels in the lower respiratory tract, more so than memory B cells from the spleen.Citation55
Mucosal vaccination
Intramuscular vaccination often results in a suboptimal mucosal immune response, leading to the proposition that protective mucosal immunity may require mucosal vaccination routes.Citation56 Mucosal immunity would not only prevent establishment of respiratory infection but may also reduce transmission to new hosts by reducing virus shedding from mucosal sites.Citation57 Nasal and pulmonary delivery of vaccines is particularly effective in eliciting both a local and a systemic immune response. Mucosal immunization generates antigen-specific IgA antibodies at the site of vaccine administration and systemic IgG antibodies, and stimulates cell-mediated responses as well, including helper CD4+ T cells and CD8+ cytotoxic T lymphocytes.Citation4 Furthermore, mucosal vaccination can efficiently induce long-lasting B- and T-cell memory.Citation14 However, in mucosal vaccine design, the route of administration and vaccine formulation affecting immunogenicity must be considered.Citation18,Citation58
As mentioned before, localized immunization at mucosal induction sites tends to enhance antigen-specific IgA responses in effector sites that are immunologically and physiologically adjacent or related. For example, nasal, oral, or intratracheal immunization preferentially boosts specific IgA levels in the corresponding mucosal tissues. Therefore, targeted vaccine delivery through specific mucosal routes may optimize antigen-specific immunity in the desired location ().
Table 2. Licensed mucosal vaccines.
The intrinsic properties of each mucosal site must also be considered when assessing the proper route of vaccine administration. Oral vaccination, in which the vaccine is swallowed, provides easy access and less discomfort which allows large-scale vaccine rollouts and higher compliance, and also has a better safety profile compared to intranasal administration as it avoids the potential risk of antigen migration to the brain through olfactory epithelium and bulbs.Citation59 However, as the harsher environment of the GI tract and the presence of specialized oral tolerance mechanisms remain major challenges for this route, and sublingual or buccal vaccination, which deliver antigen into non-keratinized epithelium and thinner cell layers, may be more promising. Sublingual/buccal vaccination can generate mucosal immune responses in the respiratory, digestive, and reproductive tracts, whereas immunity induced by oral immunization has been mostly restricted to the digestive tract.Citation60 Intranasal vaccine administration presents several advantages such as the lack of acidity, and secreted vaccine-degrading enzymes.Citation60 When rationally designing a mucosal vaccine formulation, one has to keep in mind that factors such as particle size, surface charge, and hydrophobicity affect vaccine immunogenicity. Particle size between 1–5 µm are considered ideal for lung deposition.Citation61,Citation62 Furthermore, distinct particle sizes are favored for uptake by different cell types within the lung and draining lymph nodes. Larger particles are typically taken up by DCs at injection site and transported to the draining lymph nodes, whereas smaller particles may drain directly to the lymph nodes before being taken up by lymph node-resident DCs and macrophages.Citation63 Surface charge also affects the cellular uptake of particles.Citation64 Fromen et al. suggested that cationic nanoparticles were mostly associated with DCs, whereas anionic particles were mostly internalized by alveolar macrophages.Citation64 Additionally, hydrophobic particles easily associate within the lipid bilayer of cells, promoting uptake for further immune processing. In contrast, hydrophilic polymers such as poly ethylene glycol, and poly carboxybetaine were shown to be efficient in decreasing production of TNF- and IL6 in BALF of mice.Citation65 Although, particle size, surface charge, and hydrophobicity of vaccines are known to influence immune responses in parenteral administration routes, the specific role they play in mucosal vaccines is yet to be fully explored.
Current status of mucosal vaccines
Over the past several decades, there has been a significant shift in traditional injectable vaccines – from inactivated or attenuated vaccines – toward adjuvanted subunit, viral vectored, DNA, and mRNA vaccines.Citation66 Many of these newer generation vaccines are currently tested for their feasibility as mucosal vaccines. However, several obstacles exist in the field of mucosal vaccination, including tolerance toward orally administered inactivated antigens, susceptibility of unprotected subunit antigens to degradation and clearance, as well as the absence of established mucosal adjuvants to overcome these issues.Citation12,Citation13 Multiple vector and formulation methods are currently being tested to optimize mucosal vaccine delivery and effectiveness.Citation67,Citation68 Given the limited efficacy associated with mucosal immunization methods, few vaccines have been licensed for mucosal administration. Currently, licensed mucosal vaccines are limited to gastrointestinal diseases or and mucosal vaccines that target respiratory mucosae are only available for influenza. They typically consist of the inactivated whole or live attenuated pathogens (). FluMist, which was approved in 2003, and consists of trivalent cold-adapted influenza viruses is an intranasally administered live attenuated vaccine.Citation5 The vaccine has been approved for use in non-immunocompromised individuals aged 2–49. Treanor et al. showed that FluMist-immunized subjects demonstrated a slightly higher protective efficacy against wild-type influenza virus infection compared to the trivalent inactivated influenza virus vaccine (TIV) in a human study. In the studies, 45% (14/31) of the placebo group showed laboratory-confirmed influenza following exposure to the wild-type influenza virus, compared to 13% (4/32) of those receiving the TIV group and 7% (2/29) of FluMist group.Citation69 Interestingly, in this study FluMist was more effective in preventing respiratory illness upon experimental challenge than TIV despite the low antibody response rates observed in serum.
Table 1. Expression of mucosal IgA immune responses after different routes of vaccination.
Current licensed vaccine adjuvants
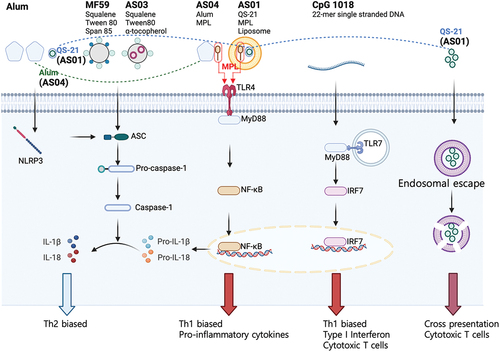
Adjuvants are critical components to enhance the uptake and/or immunogenicity of antigens.Citation70–72 Adjuvants primarily enhance immunogenicity by stimulating antigen-presenting cells (APCs) to enhance antigen presentation and co-stimulatory signals.Citation71 Despite numerous attempts to develop novel adjuvants, only a limited number have been licensed (all for parenteral administration), such as aluminum adjuvants,Citation73 MF59,Citation74 AS01,Citation75 AS03,Citation76 AS04,Citation77 CpG 1018,Citation78 and Matrix-MCitation79 (). Since these adjuvants have been reviewed in depth elsewhere, we will only focus on them when relevant for mucosal vaccination strategies.
Figure 2. Simplified diagram of the major signaling pathways of classical adjuvants.
Mucosal vaccine adjuvants
No adjuvants specifically for nasal vaccinations have been licensed for human use.Citation80 Antigen-only mucosal vaccinations often fail to elicit a robust immune response that provides enduring protection against infections.Citation15 One strategy for developing adjuvants for mucosal vaccines is to employ the above-mentioned adjuvants that have been approved for parenteral human vaccine use.Citation13,Citation80–82 Aluminum salt, the first adjuvant to receive licensing, has undergone extensive study in various nasal vaccination formulations, in both dry and liquid vaccines.Citation81 Thakkar et al. demonstrated the efficacy of an intranasal dry powder vaccine comprising ovalbumin and aluminum salt adjuvants.Citation83 The intranasal immunization in rats with this dry powder vaccine with aluminum salt adjuvant induced specific antibody responses in both serum and mucosal secretions. Furthermore, a recent study proposed using porous aluminum-based metal-organic framework nanoparticles (MOF NPs) as inhalable adjuvants.Citation81 When compared to traditional alum, these MOF NPs had superior capacity to activate APCs, stimulate higher IgG2a titer, and elevate Th1 responses, when assessed using ovalbumin as a model antigen.Citation81
AS04 has also shown potential in enhancing mucosal and systemic immunity, when utilized in intranasal vaccines.Citation82 Intranasal administration of AS04-adjuvanted vaccines in mice elicited both mucosal (IgA in nasal and lung mucosae) and systemic immune (IgG in the serum) responses, in contrast to the solely systemic response observed with subcutaneous administration. AS04 demonstrated superior antigen deposition in the nasal cavity in mouse models.Citation84
An intranasal H5N1 influenza vaccine containing a virosomal formulation adjuvanted with Matrix-M exhibited substantial protection in mice against viral challenge with 100 LD50.Citation84 This vaccine stimulated a well-balanced immune response, generating both Th1 and Th2 cytokines.
As an alternative to repurposing parenteral vaccine adjuvants for mucosal administration, there is a focus on pioneering novel mucosal adjuvants, including those based on pattern recognition receptor (PRR) agonists, toxoids, and muco-adhesive systems.
Agonists of PRRs
Mechanistically, many adjuvants activate PRRs on innate immune cells, including Toll-like receptors (TLRs), STING agonists, C-type lectin receptors (CLRs), retinoic acid-inducible gene I (RIG-I)-like receptors (RLRs), and NOD-like receptors (NLRs). Activation of these receptors triggers signaling cascades that lead to dendritic cell maturation, cytokine secretion, and T helper cell stimulation.Citation80 TLR agonists, widely recognized as potential adjuvants for parenteral vaccines, have also been extensively researched as key mucosal adjuvants, including Pam3CsK4 (TLR2), Polyinosinic-polycytidylic acid (Poly I:C, TLR3), Monophosphoryl Lipid A (MPL, TLR4), flagellin (TLR5), gardiquimod (TLR7/8), and CpG-oligodeoxynucleotide (CpG-ODN, TLR9).Citation80
Pam3CSK4, a TLR2 agonist, has demonstrated efficacy in intranasal RSV virosomes vaccines, inducing serum IgG, mucosal antibodies and safe Th1-skewed cellular immune responses, without priming for enhanced disease in mice.Citation85 Additionally, utilization of synthetic macrophage-activating lipopeptide-2 (MALP-2), a TLR2/6 agonist, as an adjuvant has demonstrated enhancement of systemic and mucosal antibody responses, as well as robust cell-mediated immunity in animal models.Citation86
Poly I:C, a synthetic double-stranded RNA analogue that activates TLR3, has been studied extensively as a nasal vaccine adjuvant. Sabbaghi et al. explored the immunological effects of intranasal immunization in mice using a gamma-irradiated H1N1 vaccine augmented with Poly I:C and/or chemokine (C–C motif) ligand 21 (CCL21).Citation87 This was evidenced by an increase in the ratio of IgG2a, IFN-γ, and IL-12, which are markers of Th1 immune responses. Additionally, there was a notable elevation in both IgA- and IgG-mediated humoral immunity. The study also observed increased lymphocytic proliferation and enhanced cytotoxic activity in spleen cells. The potential of flagellin (a TLR5 agonist)Citation88,Citation89 and CpG-ODN TLR9 agonist)Citation90,Citation91 in mucosal adjuvant applications has been investigated in various studies. These investigations have revealed that both flagellin and CpG-ODN demonstrate significant promise as mucosal vaccine adjuvants in preclinical studies.
Among the PRRs, RIG-I agonists have been proposed as novel adjuvants for mucosal vaccines.Citation92,Citation93 RIG-I receptors are in the cytosol and recognize short double-stranded RNA (dsRNA), a replication intermediate for RNA viruses, that exhibit viral motifs such as an uncapped 5′ diphosphate or triphosphate end. We have innovatively developed an intranasal adjuvant, combining an RNA-based RIG-I agonist (IVT DI) with a nanoemulsion (NE) that activates TLRs and NLRP3, to enhance immune responses.Citation94–96 The NE formulation of this intranasal adjuvant can also serve as a delivery vehicle for IVT DI and antigen as well, enhancing the retention time of the nasal vaccine at mucosal sites and improving cellular uptake of the antigen and agonist cargo. The NE/IVT adjuvant, when paired with the recombinant SARS-CoV-2 receptor binding domain (RBD) protein, has been shown to induce strong and lasting humoral, mucosal, and cellular immunity.Citation94 Furthermore, NE/IVT with the ancestral SARS-CoV-2 spike protein antigen elicited enhanced Th1-biased cellular responses and high titers of virus-neutralizing antibodies effective against a broader range of divergent SARS-CoV-2 variants, including those of the Omicron lineage.Citation95 Notably, it elicits the production of antigen-specific IFN-γ/IL-2/TNF-α, which is crucial for protective immunity, especially in the elderly.Citation94 In contrast to the licensed MF59-like adjuvant, Addavax, our NE/IVT formulation maintains its immunogenic effectiveness in preclinical experiments, regardless of age. Our studies and those of others suggest that optimal mucosal adjuvant development will likely benefit from the integration of multiple classes of adjuvants and delivery systems.
Double-mutant heat-labile toxin and multiple mutated cholera toxin
E. coli heat-labile toxin and Cholera toxin are considered among the top mucosal adjuvants.Citation97 However, due to their toxicity, there has been a push to improve safety while maintaining their adjuvant properties. This effort has led to the creation of modified versions such as the E. coli double-mutant heat-labile toxin (dmLT) and the multiple-mutated cholera toxin (mmCT). The inclusion of dmLT has notably enhanced responses to whole-cell antigens in vaccines like ETVAX, which is an oral, inactivated, enterotoxigenic E. coli vaccine.Citation97 Novel adjuvants including mmCT show potential in boosting Th1 and Th17 cellular responses.Citation98 CTA1-DD, a detoxified derivative of cholera toxin, has been effective in stimulating immune responses in (neonatal) mice and shows promise in influenza vaccination, although its clinical efficacy and safety are yet to be fully established.Citation99 However, the use of some toxoid adjuvants in nasal delivery, the most well-documented type of mucosal adjuvants, has been linked to adverse effects like Bell’s palsy, leading to the exploration of alternative methods like sublingual administration.Citation100 Recent clinical trials with another genetically detoxified heat-labile enterotoxin (LT)-derived from E. coli (LTh(αK)), have shown promising results in nasal vaccination without adverse effects.Citation101,Citation102 Overall, toxoid adjuvants represent a significant advancement in mucosal adjuvant technology, particularly for oral vaccine formulations with whole-cell killed pathogens.
Chitosan
Finally we want to mention chitosan as an example for natural polysaccharides, which have been increasingly recognized for their roles as mucosal adjuvants due to their capacity to activate a range of immune cells and augment the secretion of cytokines such as, IL-4 and IFN-γ as well as their muco-adhesive properties.Citation103 Chitosan has garnered interest in the field of mucosal adjuvants owing to its low toxicity, excellent biocompatibility, and biodegradability.Citation104 Additionally, chitosan has unique cationic properties, allowing it to adhere well to the mucosal surface, which not only prolongs the retention time of antigens but also opens tight junctions between mucosal epithelial cells so antigens can be more readily accessed by mucosal APCs.Citation105 A variety of chitosan derivatives have been explored as innovative mucosal adjuvants for different vaccine types.Citation106–108
Conclusions
Despite the many unique challenges, mucosal vaccination strategies have much potential in controlling respiratory pathogens. Mucosal vaccination strategies have potential in preventing both disease and transmission of pathogens, two crucial factors during infectious disease outbreaks with epidemic or pandemic potential. Therefore, there is great interest in mucosal vaccine development. We have highlighted some of the hurdles that need to be addressed during that process. It is likely that better adjuvant candidates, as well as novel formulation methods and vaccine vectors for mucosal vaccination than those currently licensed for parenteral administration, will move forward to the clinic in the coming years. Safety will remain a main driver of mucosal vaccine research, alongside effectiveness. A better understanding of the mucosal immune system will allow for the development of more targeted strategies. Here, we mainly focused on the mucosal sites of the respiratory tract, which comprises only part of the mucosal immune system. Interestingly, it became clear in recent years that significant immune cross talk between distant mucosal sites exists.Citation109 Therefore, mucosal vaccination at inductive sites that are easily accessed (nasal, oral) can also be targeted to induce mucosal immunity against pathogens that, for example, colonize the gut or vaginal mucosae. Novel vaccine technologies that focus on mucosal vaccine delivery, stability, and immunogenicity along with our rapidly expanding understanding of the mucosal immune system and the mucosal immune correlates of protection gained through more powerful systems immunological tools will together enable development of the next generation of mucosal vaccines.
Acknowledgments
Research on mucosal immunology and vaccination in the Schotsaert and Wong laboratories is supported by NIAID grants R21AI176069 and R01AI160706. S.C.P. has received support from the Korea Health Industry Development Institute (KHIDI) for research under the Biomedical Global Talent Nurturing Program (HI22C2101).
Disclosure statement
The M.S. laboratory received unrelated research support as sponsored research agreements from ArgenX BV, Phio Pharmaceuticals, 7Hills Pharma LLC and Moderna. The remaining authors declare no conflict of interest.
Additional information
Funding
References
- Neutra MR, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nat Rev Immunol. 2006;6(2):148–12. doi:10.1038/nri1777.
- Xu H, Cai L, Hufnagel S, Cui Z. Intranasal vaccine: factors to consider in research and development. Int J Pharm. 2021;609:121180. doi:10.1016/j.ijpharm.2021.121180.
- Bennett JV, Fernandez de Castro J, Valdespino-Gomez JL, MdL G-G, Islas-Romero R, Echaniz-Aviles G, Jimenez-Corona A, Sepulveda-Amor J. Aerosolized measles and measles-rubella vaccines induce better measles antibody booster responses than injected vaccines: randomized trials in Mexican schoolchildren. Bull World Health Organ. 2002;80(10):806–812.
- Hellfritzsch M, Scherließ R. Mucosal vaccination via the respiratory tract. Pharmaceutics. 2019;11(8). Epub 20190801. doi: 10.3390/pharmaceutics11080375. PubMed PMID: 31374959; PubMed Central PMCID: PMC6723941.
- Nakahashi-Ouchida R, Fujihashi K, Kurashima Y, Yuki Y, Kiyono H. Nasal vaccines: solutions for respiratory infectious diseases. Trends Mol Med. 2023;29(2):124–140. doi:10.1016/j.molmed.2022.10.009.
- Iwasaki A, Foxman EF, Molony RD. Early local immune defences in the respiratory tract. Nat Rev Immunol. 2017;17(1):7–20. Epub 20161128. doi: 10.1038/nri.2016.117. PubMed PMID: 27890913; PubMed Central PMCID: PMC5480291.
- Kurono Y. The mucosal immune system of the upper respiratory tract and recent progress in mucosal vaccines. Auris Nasus Larynx. 2022;49(1):1–10. Epub 20210723. doi:10.1016/j.anl.2021.07.003. PubMed PMID: 34304944.
- Dillon A, LO DD. M cells: intelligent engineering of mucosal immune surveillance. Front Immunol. 2019;10. doi:10.3389/fimmu.2019.01499.
- Steffen U, Koeleman CA, Sokolova MV, Bang H, Kleyer A, Rech J, Unterweger H, Schicht M, Garreis F, Hahn J, et al. IgA subclasses have different effector functions associated with distinct glycosylation profiles. Nat Commun. 2020;11(1):120. doi:10.1038/s41467-019-13992-8.
- Woof JM, Russell MW. Structure and function relationships in IgA. Mucosal Immunol. 2011;4(6):590–597. doi:10.1038/mi.2011.39.
- Mettelman RC, Allen EK, Thomas PG. Mucosal immune responses to infection and vaccination in the respiratory tract. Immunity. 2022;55(5):749–780. doi:10.1016/j.immuni.2022.04.013. PubMed PMID: 35545027; PubMed Central PMCID: PMC9087965.
- Tsai CJY, Loh JMS, Fujihashi K, Kiyono H. Mucosal vaccination: onward and upward. Expert Rev Vaccines. 2023;22(1):885–899. Epub 20231017. doi: 10.1080/14760584.2023.2268724. PubMed PMID: 37817433.
- Lavelle EC, Ward RW. Mucosal vaccines—fortifying the frontiers. Nat Rev Immunol. 2022;22(4):236–250. doi:10.1038/s41577-021-00583-2.
- Sallusto F, Lanzavecchia A, Araki K, Ahmed R. From vaccines to memory and back. Immunity. 2010;33(4):451–463. doi:10.1016/j.immuni.2010.10.008. PubMed PMID: 21029957; PubMed Central PMCID: PMC3760154.
- Rhee JH, Lee SE, Kim SY. Mucosal vaccine adjuvants update. Clin Exp Vaccine Res. 2012;1(1):50–63. doi:10.7774/cevr.2012.1.1.50.
- Lehmann R, Müller MM, Klassert TE, Driesch D, Stock M, Heinrich A, Conrad T, Moore C, Schier UK, Guthke R, et al. Differential regulation of the transcriptomic and secretomic landscape of sensor and effector functions of human airway epithelial cells. Mucosal Immunol. 2018;11(3):627–642. doi:10.1038/mi.2017.100.
- Takamura S, Kato S, Motozono C, Shimaoka T, Ueha S, Matsuo K, Miyauchi K, Masumoto T, Katsushima A, Nakayama T, et al. Interstitial-resident memory CD8+ T cells sustain frontline epithelial memory in the lung. J Exp Med. 2019;216(12):2736–2747. doi:10.1084/jem.20190557.
- Whitsett JA, Alenghat T. Respiratory epithelial cells orchestrate pulmonary innate immunity. Nat Immunol. 2015;16(1):27–35. doi:10.1038/ni.3045. PubMed PMID: 25521682; PubMed Central PMCID: PMC4318521.
- Amersfoort J, Eelen G, Carmeliet P. Immunomodulation by endothelial cells—partnering up with the immune system? Nat Rev Immunol. 2022;22(9):576–588. doi:10.1038/s41577-022-00694-4.
- Button B, Cai L-H, Ehre C, Kesimer M, Hill DB, Sheehan JK, Boucher RC, Rubinstein M. A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science. 2012;337(6097):937–941. doi:10.1126/science.1223012.
- Wallace LE, Liu M, van Kuppeveld Fjm, de Vries E, de Haan Cam, van Kuppeveld FJM, de Haan CAM. Respiratory mucus as a virus-host range determinant. Trends Microbiol. 2021;29(11):983–992. doi:10.1016/j.tim.2021.03.014.
- Vasquez-Martínez N, Guillen D, Moreno-Mendieta SA, Sanchez S, Rodríguez-Sanoja R. The role of mucoadhesion and mucopenetration in the immune response induced by polymer-based mucosal adjuvants. Polymers. 2023;15(7):1615. doi:10.3390/polym15071615.
- Konrad FM, Wohlert J, Gamper-Tsigaras J, Ngamsri K-C, Reutershan J. How adhesion molecule patterns change while neutrophils traffic through the lung during inflammation. Mediators Inflamm. 2019;2019:1208086. doi:10.1155/2019/1208086.
- Castanheira FV, Kubes P. Neutrophils during SARS‐CoV‐2 infection: Friend or foe? Immunol Rev. 2023;314(1):399–412. doi:10.1111/imr.13175.
- Capucetti A, Albano F, Bonecchi R. Multiple roles for chemokines in neutrophil biology. Front Immunol. 2020;11:11. doi:10.3389/fimmu.2020.01259.
- Chan L, Morovati S, Karimi N, Alizadeh K, Vanderkamp S, Kakish JE, Bridle BW, Karimi K. Neutrophil functional heterogeneity and implications for viral infections and treatments. Cells. 2022;11(8). Epub 20220413. doi: 10.3390/cells11081322. PubMed PMID: 35456003; PubMed Central PMCID: PMC9025666.
- Zhang R, Sun C, Han Y, Huang L, Sheng H, Wang J, Zhang Y, Lai J, Yuan J, Chen X, et al. Neutrophil autophagy and NETosis in COVID-19: perspectives. Autophagy. 2023;19(3):758–767. doi:10.1080/15548627.2022.2099206.
- Huot N, Planchais C, Rosenbaum P, Contreras V, Jacquelin B, Petitdemange C, Lazzerini M, Beaumont E, Orta-Resendiz A, Rey FA, et al. SARS-CoV-2 viral persistence in lung alveolar macrophages is controlled by IFN-γ and NK cells. Nat Immunol. 2023;24(12):2068–79. doi:10.1038/s41590-023-01661-4.
- Evren E, Ringqvist E, Doisne J-M, Thaller A, Sleiers N, Flavell RA, Di Santo JP, Willinger T. CD116+ fetal precursors migrate to the perinatal lung and give rise to human alveolar macrophages. J Exp Med. 2022;219(2):e20210987. doi:10.1084/jem.20210987.
- Lee MJ, Blish CA. Defining the role of natural killer cells in COVID-19. Nat Immunol. 2023;24(10):1628–1638. doi:10.1038/s41590-023-01560-8.
- Hou F, Xiao K, Tang L, Xie L. Diversity of macrophages in lung homeostasis and diseases. Front Immunol. 2021;12:753940. doi:10.3389/fimmu.2021.753940.
- Nachbagauer R, Feser J, Naficy A, Bernstein DI, Guptill J, Walter EB, Berlanda-Scorza F, Stadlbauer D, Wilson PC, Aydillo T, et al. A chimeric hemagglutinin-based universal influenza virus vaccine approach induces broad and long-lasting immunity in a randomized, placebo-controlled phase I trial. Nat Med. 2021;27(1):106–114. Epub 20201207. doi: 10.1038/s41591-020-1118-7. PubMed PMID: 33288923.
- Wang T, Zhang J, Wang Y, Li Y, Wang L, Yu Y, Yao Y. Influenza-trained mucosal-resident alveolar macrophages confer long-term antitumor immunity in the lungs. Nat Immunol. 2023;24(3):423–438. doi:10.1038/s41590-023-01428-x.
- Ding C, Shrestha R, Zhu X, Geller AE, Wu S, Woeste MR, Li W, Wang H, Yuan F, Xu R, et al. Inducing trained immunity in pro-metastatic macrophages to control tumor metastasis. Nat Immunol. 2023;24(2):239–254. doi:10.1038/s41590-022-01388-8.
- Abassi Z, Knaney Y, Karram T, Heyman SN. The lung macrophage in SARS-CoV-2 infection: a friend or a foe? Front Immunol. 2020;11:1312. doi:10.3389/fimmu.2020.01312.
- Wang X, Guan F, Miller H, Byazrova MG, Candotti F, Benlagha K, Camara NOS, Lei J, Filatov A, Liu C, et al. The role of dendritic cells in COVID-19 infection. Emerging Microbes Infect. 2023;12(1):2195019. doi:10.1080/22221751.2023.2195019.
- Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol. 2008;8(8):594–606. doi:10.1038/nri2358. PubMed PMID: 18641647.
- Netea MG, Domínguez-Andrés J, Barreiro LB, Chavakis T, Divangahi M, Fuchs E, Joosten LAB, van der Meer JWM, Mhlanga MM, Mulder WJM. et al. Defining trained immunity and its role in health and disease. Nat Rev Immunol. 2020;20(6):375–388. Epub 20200304. doi: 10.1038/s41577-020-0285-6. PubMed PMID: 32132681; PubMed Central PMCID: PMC7186935.
- Netea MG, Quintin J, Van Der Meer JW. Trained immunity: a memory for innate host defense. Cell Host Microbe. 2011;9(5):355–361. doi:10.1016/j.chom.2011.04.006.
- Netea MG, Joosten LAB, Latz E, Mills KHG, Natoli G, Stunnenberg HG, O’Neill LAJ, Xavier RJ. Trained immunity: a program of innate immune memory in health and disease. Science. 2016;352(6284):aaf1098. doi:10.1126/science.aaf1098.
- Ochando J, Mulder WJM, Madsen JC, Netea MG, Duivenvoorden R. Trained immunity—basic concepts and contributions to immunopathology. Nat Rev Nephrol. 2023;19(1):23–37. doi:10.1038/s41581-022-00633-5.
- Yu D, Zhang J, Wang S. Trained immunity in the mucosal diseases. WIREs Mech Dis. 2022;14(2):e1543. doi:10.1002/wsbm.1543.
- Momtazmanesh S, Moghaddam SS, Ghamari S-H, Rad EM, Rezaei N, Shobeiri P, Aali A, Abbasi-Kangevari M, Abbasi-Kangevari Z, Abdelmasseh M, et al. Global burden of chronic respiratory diseases and risk factors, 1990–2019: an update from the global burden of disease study 2019. eClinicalmedicine. 2023;59:101936. doi:10.1016/j.eclinm.2023.101936.
- Kingstad-Bakke B, Lee W, Chandrasekar SS, Gasper DJ, Salas-Quinchucua C, Cleven T, Sullivan JA, Talaat A, Osorio JE, Suresh M, et al. Vaccine-induced systemic and mucosal T cell immunity to SARS-CoV-2 viral variants. Proc Natl Acad Sci USA. 2022;119(20):e2118312119. doi:10.1073/pnas.2118312119.
- Winstead CJ. Follicular helper T cell-mediated mucosal barrier maintenance. Immunol Lett. 2014;162(2, Part A):39–47. doi:10.1016/j.imlet.2014.07.015.
- Paiva IA, Badolato-Corrêa J, Familiar-Macedo D, de-Oliveira-Pinto LM. Th17 cells in viral infections—friend or foe? Cells. 2021;10(5). Epub 20210511. doi: 10.3390/cells10051159. PubMed PMID: 34064728; PubMed Central PMCID: PMC8151546.
- Skon CN, Lee J-Y, Anderson KG, Masopust D, Hogquist KA, Jameson SC. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat Immunol. 2013;14(12):1285–1293. doi:10.1038/ni.2745.
- Lim JME, Tan AT, Le Bert N, Hang SK, Low JGH, Bertoletti A. SARS-CoV-2 breakthrough infection in vaccinees induces virus-specific nasal-resident CD8+ and CD4+ T cells of broad specificity. J Exp Med. 2022;219(10). doi:10.1084/jem.20220780.
- Schenkel JM, Fraser KA, Beura LK, Pauken KE, Vezys V, Masopust D. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science. 2014;346(6205):98–101. doi:10.1126/science.1254536.
- Schreiner D, King CG. CD4+ memory T cells at home in the tissue: mechanisms for health and disease. Front Immunol. 2018;9:2394. Epub 20181016. doi: 10.3389/fimmu.2018.02394. PubMed PMID: 30386342; PubMed Central PMCID: PMC6198086.
- Teijaro JR, Turner D, Pham Q, Wherry EJ, Lefrançois L, Farber DL. Cutting edge: tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J Immunol. 2011;187(11):5510–5514. doi:10.4049/jimmunol.1102243.
- Moyron-Quiroz JE, Rangel-Moreno J, Kusser K, Hartson L, Sprague F, Goodrich S, Woodland DL, Lund FE, Randall TD. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat Med. 2004;10(9):927–934. Epub 20040815. doi: 10.1038/nm1091. PubMed PMID: 15311275.
- Allie SR, Bradley JE, Mudunuru U, Schultz MD, Graf BA, Lund FE, Randall TD. The establishment of resident memory B cells in the lung requires local antigen encounter. Nat Immunol. 2019;20(1):97–108. doi:10.1038/s41590-018-0260-6.
- Tan HX, Juno JA, Esterbauer R, Kelly HG, Wragg KM, Konstandopoulos P, Alcantara S, Alvarado C, Jones R, Starkey G. et al. Lung-resident memory B cells established after pulmonary influenza infection display distinct transcriptional and phenotypic profiles. Sci Immunol. 2022;7(67):eabf5314. Epub 20220128. doi: 10.1126/sciimmunol.abf5314. PubMed PMID: 35089815.
- Onodera T, Takahashi Y, Yokoi Y, Ato M, Kodama Y, Hachimura S, Kurosaki T, Kobayashi K. Memory B cells in the lung participate in protective humoral immune responses to pulmonary influenza virus reinfection. Proc Natl Acad Sci USA. 2012;109(7):2485–2490. doi:10.1073/pnas.1115369109.
- Vujanic A, Sutton P, Snibson KJ, Yen HH, Scheerlinck JP. Mucosal vaccination: lung versus nose. Vet Immunol Immunopathol. 2012;148(1–2):172–177. Epub 20110323. doi: 10.1016/j.vetimm.2011.03.004. PubMed PMID: 21492942.
- Pilapitiya D, Wheatley AK, Tan H-X. Mucosal vaccines for SARS-CoV-2: triumph of hope over experience. EBioMedicine. 2023;92:104585. doi:10.1016/j.ebiom.2023.104585.
- Paris AL, Colomb E, Verrier B, Anjuère F, Monge C. Sublingual vaccination and delivery systems. J Control Release. 2021;332:553–562. Epub 20210315. doi: 10.1016/j.jconrel.2021.03.017. PubMed PMID: 33737202.
- van Ginkel Fw, Jackson RJ, Yuki Y, McGhee JR, van Ginkel FW. Cutting edge: the mucosal adjuvant cholera toxin redirects vaccine proteins into olfactory tissues. J Immunol. 2000;165(9):4778–482. doi:10.4049/jimmunol.165.9.4778.
- Kraan H, Vrieling H, Czerkinsky C, Jiskoot W, Kersten G, Amorij J-P. Buccal and sublingual vaccine delivery. J Controlled Release. 2014;190:580–592. doi:10.1016/j.jconrel.2014.05.060.
- El-Sherbiny IM, El-Baz NM, Yacoub MH. Inhaled nano- and microparticles for drug delivery. Glob Cardiol Sci Pract. 2015;2015(1):2. Epub 20150331. doi: 10.5339/gcsp.2015.2. PubMed PMID: 26779496; PubMed Central PMCID: PMC4386009.
- Jabbal S, Poli G, Lipworth B. Does size really matter?: relationship of particle size to lung deposition and exhaled fraction. J Allergy Clin Immunol. 2017;139(6):2013–2014. e1. doi:10.1016/j.jaci.2016.11.036.
- Marasini N, Kaminskas LM. Subunit-based mucosal vaccine delivery systems for pulmonary delivery-are they feasible? Drug Dev Ind Pharm. 2019;45(6):882–894. doi:10.1080/03639045.2019.1583758.
- Fromen CA, Rahhal TB, Robbins GR, Kai MP, Shen TW, Luft JC, DeSimone JM. Nanoparticle surface charge impacts distribution, uptake and lymph node trafficking by pulmonary antigen-presenting cells. Nanomed Nanotechnol Biol Med. 2016;12(3):677–687. Epub 20151201. doi:10.1016/j.nano.2015.11.002. PubMed PMID: 26656533; PubMed Central PMCID: PMC4839472.
- Moyano DF, Goldsmith M, Solfiell DJ, Landesman-Milo D, Miranda OR, Peer D, Rotello VM. Nanoparticle hydrophobicity dictates immune response. J Am Chem Soc. 2012;134(9):3965–3967. doi:10.1021/ja2108905.
- Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines—a new era in vaccinology. Nat Rev Drug Discov. 2018;17(4):261–279. doi:10.1038/nrd.2017.243.
- Wendorf J, Chesko J, Kazzaz J, Ugozzoli M, Vajdy M, O’Hagan D, Singh M. A comparison of anionic nanoparticles and microparticles as vaccine delivery systems. Hum Vaccin. 2008;4(1):44–49. Epub 20070815. doi:10.4161/hv.4.1.4886. PubMed PMID: 18438105.
- da Silva Aj, Zangirolami TC, Novo-Mansur MT, Giordano Rde C, Martins EA, Silva AJD. Live bacterial vaccine vectors: an overview. Braz J Microbiol. 2014;45(4):1117–11129. Epub 20150304. doi: 10.1590/s1517-83822014000400001. PubMed PMID: 25763014; PubMed Central PMCID: PMC4323283.
- Treanor JJ, Kotloff K, Betts RF, Belshe R, Newman F, Iacuzio D, Wittes J, Bryant M. Evaluation of trivalent, live, cold-adapted (CAIV-T) and inactivated (TIV) influenza vaccines in prevention of virus infection and illness following challenge of adults with wild-type influenza a (H1N1), a (H3N2), and B viruses. Vaccine. 1999;18(9–10):899–906. doi:10.1016/s0264-410x(99)00334-5. PubMed PMID: 10580204.
- Pulendran B, Arunachalam P, O’Hagan DT. Emerging concepts in the science of vaccine adjuvants. Nat Rev Drug Discov. 2021;20(6):454–475. doi:10.1038/s41573-021-00163-y.
- Zhao T, Cai Y, Jiang Y, He X, Wei Y, Yu Y, Tian X. Vaccine adjuvants: mechanisms and platforms. Signal Transduct Target Ther. 2023;8(1):283. doi:10.1038/s41392-023-01557-7.
- Guy B. The perfect mix: recent progress in adjuvant research. Nat Rev Microbiol. 2007;5(7):396–397. doi:10.1038/nrmicro1681.
- Li H, Nookala S, Re F. Aluminum hydroxide adjuvants activate caspase-1 and induce IL-1beta and IL-18 release. J Immunol. 2007;178(8):5271–5276. doi:10.4049/jimmunol.178.8.5271. PubMed PMID: 17404311.
- Seubert A, Calabro S, Santini L, Galli B, Genovese A, Valentini S, Aprea S, Colaprico A, D’Oro U, Giuliani MM. et al. Adjuvanticity of the oil-in-water emulsion MF59 is independent of Nlrp3 inflammasome but requires the adaptor protein MyD88. Proc Natl Acad Sci USA. 2011;108(27):11169–11174. doi:10.1073/pnas.1107941108.
- Didierlaurent AM, Laupèze B, Di Pasquale A, Hergli N, Collignon C, Garçon N. Adjuvant system AS01: helping to overcome the challenges of modern vaccines. Expert Rev Vaccines. 2017;16(1):55–63. doi:10.1080/14760584.2016.1213632.
- Cohet C, van der Most R, Bauchau V, Bekkat-Berkani R, Doherty TM, Schuind A, Tavares Da Silva F, Rappuoli R, Garçon N, Innis BL, et al. Safety of AS03-adjuvanted influenza vaccines: A review of the evidence. Vaccine. 2019;37(23):3006–3021. doi:10.1016/j.vaccine.2019.04.048.
- Garçon N, Morel S, Didierlaurent A, Descamps D, Wettendorff M, Van Mechelen M. Development of an AS04-adjuvanted HPV vaccine with the adjuvant system approach. BioDrugs. 2011;25(4):217–26. doi:10.2165/11591760-000000000-00000.
- Campbell JD, Cho Y, Foster ML, Kanzler H, Kachura MA, Lum JA, Ratcliffe MJ, Sathe A, Leishman AJ, Bahl A. et al. CpG-containing immunostimulatory DNA sequences elicit TNF-α–dependent toxicity in rodents but not in humans. J Clin Invest. 2009;119(9):2564–2576. doi:10.1172/JCI38294.
- Stertman L, Palm AE, Zarnegar B, Carow B, Lunderius Andersson C, Magnusson SE, Carnrot C, Shinde V, Smith G, Glenn G. et al. The matrix-M™ adjuvant: A critical component of vaccines for the 21 st century. Hum Vaccin Immunother. 2023;19(1):2189885. Epub 20230427. doi:10.1080/21645515.2023.2189885. PubMed PMID: 37113023; PubMed Central PMCID: PMC10158541.
- He X, Chen X, Wang H, Du G, Sun X. Recent advances in respiratory immunization: a focus on COVID-19 vaccines. J Controlled Release. 2023;355:655–674. doi:10.1016/j.jconrel.2023.02.011.
- Stillman ZS, Decker GE, Dworzak MR, Bloch ED, Fromen CA. Aluminum-based metal–organic framework nanoparticles as pulmonary vaccine adjuvants. J Nanobiotechnol. 2023;21(1):39. doi:10.1186/s12951-023-01782-w.
- Xu H, Alzhrani RF, Warnken ZN, Thakkar SG, Zeng M, Smyth HDC, Williams RO, Cui Z. Immunogenicity of antigen adjuvanted with AS04 and its deposition in the upper respiratory tract after intranasal administration. Mol Pharm. 2020;17(9):3259–3269. doi:10.1021/acs.molpharmaceut.0c00372.
- Thakkar SG, Warnken ZN, Alzhrani RF, Valdes SA, Aldayel AM, Xu H, Williams RO, Cui Z. Intranasal immunization with aluminum salt-adjuvanted dry powder vaccine. J Controlled Release. 2018;292:111–118. doi:10.1016/j.jconrel.2018.10.020.
- Pedersen G, Major D, Roseby S, Wood J, Madhun AS, Cox RJ. Matrix‐M adjuvanted virosomal H5N1 vaccine confers protection against lethal viral challenge in a murine model. Influenza Other Respi Viruses. 2011;5(6):426–437. doi:10.1111/j.1750-2659.2011.00256.x.
- Shafique M, Meijerhof T, Wilschut J, de Haan A, Renukaradhya GJ. Evaluation of an intranasal virosomal vaccine against respiratory syncytial virus in mice: effect of TLR2 and NOD2 ligands on induction of systemic and mucosal immune responses. PLOS ONE. 2013;8(4):e61287. Epub 20130408. doi:10.1371/journal.pone.0061287. PubMed PMID: 23593453; PubMed Central PMCID: PMC3620164.
- Cataldi A, Yevsa T, Vilte DA, Schulze K, Castro-Parodi M, Larzábal M, Ibarra C, Mercado EC, Guzmán CA. Efficient immune responses against Intimin and EspB of enterohaemorragic Escherichia coli after intranasal vaccination using the TLR2/6 agonist MALP-2 as adjuvant. Vaccine. 2008;26(44):5662–5667. doi:10.1016/j.vaccine.2008.07.027.
- Sabbaghi A, Malek M, Abdolahi S, Miri SM, Alizadeh L, Samadi M, Mohebbi SR, Ghaemi A. A formulated poly (I: C)/CCL21 as an effective mucosal adjuvant for gamma-irradiated influenza vaccine. Virol J. 2021;18(1):201. Epub 20211009. doi:10.1186/s12985-021-01672-3. PubMed PMID: 34627297; PubMed Central PMCID: PMC8501930.
- Strindelius L, Filler M, Sjöholm I. Mucosal immunization with purified flagellin from Salmonella induces systemic and mucosal immune responses in C3H/HeJ mice. Vaccine. 2004;22(27):3797–3808. doi:10.1016/j.vaccine.2003.12.035.
- Puth S, Hong SH, Park MJ, Lee HH, Lee YS, Jeong K, Kang I-C, Koh JT, Moon B, Park SC. et al. Mucosal immunization with a flagellin-adjuvanted Hgp44 vaccine enhances protective immune responses in a murine porphyromonas gingivalis infection model. Hum Vaccines Immunother. 2017;13(12):2794–2803. doi:10.1080/21645515.2017.1327109.
- Kawahara E, Yamamoto S, Shibata T, Hirai T, Yoshioka Y. CpG ODN enhances the efficacy of F protein vaccine against respiratory syncytial virus infection in the upper respiratory tract via CD4+ T cells. Biochem Bioph Res Co. 2023;686:149143. doi:10.1016/j.bbrc.2023.149143.
- McCluskie MJ, Davis HL. CpG DNA as mucosal adjuvant. Vaccine. 1999;18(3):231–237. doi:10.1016/S0264-410X(99)00194-2.
- Atalis A, Keenum MC, Pandey B, Beach A, Pradhan P, Vantucci C, O’Farrell L, Noel R, Jain R, Hosten J. et al. Nanoparticle-delivered TLR4 and RIG-I agonists enhance immune response to SARS-CoV-2 subunit vaccine. J Controlled Release. 2022;347:476–488. doi:10.1016/j.jconrel.2022.05.023.
- Wong PT, Goff PH, Sun RJ, Ruge MJ, Ermler ME, Sebring A, O’Konek JJ, Landers JJ, Janczak KW, Sun W. et al. Combined intranasal nanoemulsion and RIG-I Activating RNA adjuvants enhance mucosal, humoral, and cellular immunity to influenza virus. Mol Pharm. 2021;18(2):679–698. Epub 20200615. doi: 10.1021/acs.molpharmaceut.0c00315. PubMed PMID: 32491861.
- Jangra S, Landers JJ, Laghlali G, Rathnasinghe R, Warang P, Park SC, O’Konek JJ, Singh G, Janczak KW, García-Sastre A. et al. Multicomponent intranasal adjuvant for mucosal and durable systemic SARS-CoV-2 immunity in young and aged mice. NPJ Vaccines. 2023;8(1):96. Epub 20230629. doi:10.1038/s41541-023-00691-1. PubMed PMID: 37386041; PubMed Central PMCID: PMC10310740.
- Jangra S, Landers JJ, Rathnasinghe R, O’Konek JJ, Janczak KW, Cascalho M, Kennedy AA, Tai AW, Baker JR, Schotsaert M. et al. A combination adjuvant for the induction of potent antiviral immune responses for a recombinant SARS-CoV-2 protein vaccine. Front Immunol. 2021;12:729189. Epub 20210916. doi:10.3389/fimmu.2021.729189. PubMed PMID: 34603303; PubMed Central PMCID: PMC8481386.
- Jangra S, De Vrieze J, Choi A, Rathnasinghe R, Laghlali G, Uvyn A, Van Herck S, Nuhn L, Deswarte K, Zhong Z. et al. Sterilizing Immunity against SARS-CoV-2 Infection in mice by a single-shot and lipid amphiphile imidazoquinoline TLR7/8 agonist-adjuvanted recombinant spike protein vaccine*. Angew Chem Int Ed Engl. 2021;60(17):9467–9473. Epub 20210311. doi: 10.1002/anie.202015362. PubMed PMID: 33464672; PubMed Central PMCID: PMC8014308.
- Qadri F, Akhtar M, Bhuiyan TR, Chowdhury MI, Ahmed T, Rafique TA, Khan A, Rahman SIA, Khanam F, Lundgren A. et al. Safety and immunogenicity of the oral, inactivated, enterotoxigenic Escherichia coli vaccine ETVAX in Bangladeshi children and infants: a double-blind, randomised, placebo-controlled phase 1/2 trial. Lancet Infect Dis. 2020;20(2):208–219. Epub 20191119. doi:10.1016/s1473-3099(19)30571-7. PubMed PMID: 31757774; PubMed Central PMCID: PMC6990395.
- Lebens M, Terrinoni M, Karlsson SL, Larena M, Gustafsson-Hedberg T, Källgård S, Nygren E, Holmgren J. Construction and preclinical evaluation of mmCT, a novel mutant cholera toxin adjuvant that can be efficiently produced in genetically manipulated Vibrio cholerae. Vaccine. 2016;34(18):2121–2128. doi:10.1016/j.vaccine.2016.03.002.
- Agren LC, Ekman L, Löwenadler B, Lycke NY. Genetically engineered nontoxic vaccine adjuvant that combines B cell targeting with immunomodulation by cholera toxin A1 subunit. J Immunol (Baltimore, Md: 1950). 1997;158(8):3936–3946. doi:10.4049/jimmunol.158.8.3936.
- Schussek S, Bernasconi V, Mattsson J, Wenzel UA, Strömberg A, Gribonika I, Schön K, Lycke NY. The CTA1-DD adjuvant strongly potentiates follicular dendritic cell function and germinal center formation, which results in improved neonatal immunization. Mucosal Immunol. 2020;13(3):545–557. doi:10.1038/s41385-020-0253-2.
- Pan S-C, Hsu W-T, Lee W-S, Wang N-C, Chen T-J, Liu M-C, Pai H-C, Hsu Y-S, Chang M, Hsieh S-M, et al. A double-blind, randomized controlled trial to evaluate the safety and immunogenicity of an intranasally administered trivalent inactivated influenza vaccine with the adjuvant LTh(αK): a phase II study. Vaccine. 2020;38(5):1048–1056. doi:10.1016/j.vaccine.2019.11.047.
- Pan SC, Hsieh SM, Lin CF, Hsu YS, Chang M, Chang SC. A randomized, double-blind, controlled clinical trial to evaluate the safety and immunogenicity of an intranasally administered trivalent inactivated influenza vaccine with adjuvant LTh(αK): a phase I study. Vaccine. 2019;37(14):1994–2003. Epub 20190302. doi:10.1016/j.vaccine.2019.02.006. PubMed PMID: 30837170.
- Gao Y, Guo Y. Research progress in the development of natural-product-based mucosal vaccine adjuvants. Front Immunol. 2023;14:1152855. Epub 20230405. doi:10.3389/fimmu.2023.1152855. PubMed PMID: 37090704; PubMed Central PMCID: PMC10113501.
- Sun B, Yu S, Zhao D, Guo S, Wang X, Zhao K. Polysaccharides as vaccine adjuvants. Vaccine. 2018;36(35):5226–5234. doi:10.1016/j.vaccine.2018.07.040.
- Yu M, Yang Y, Zhu C, Guo S, Gan Y. Advances in the transepithelial transport of nanoparticles. Drug Discov Today. 2016;21(7):1155–1161. doi:10.1016/j.drudis.2016.05.007.
- Kumar US, Afjei R, Ferrara K, Massoud TF, Paulmurugan R. Gold-nanostar-chitosan-mediated delivery of SARS-CoV-2 DNA vaccine for respiratory mucosal immunization: development and proof-of-principle. ACS Nano. 2021;15(11):17582–17601. doi:10.1021/acsnano.1c05002.
- Svindland SC, Jul-Larsen Å, Pathirana R, Andersen S, Madhun A, Montomoli E, Jabbal‐Gill I, Cox RJ. The mucosal and systemic immune responses elicited by a chitosan-adjuvanted intranasal influenza H5N1 vaccine. Influenza Resp Viruses. 2012;6(2):90–100. Epub 20110712. doi:10.1111/j.1750-2659.2011.00271.x. PubMed PMID: 21749672; PubMed Central PMCID: PMC4942079.
- Gao Y, Gong X, Yu S, Jin Z, Ruan Q, Zhang C, Zhao K. Immune enhancement of N-2-hydroxypropyl trimethyl ammonium chloride chitosan/carboxymethyl chitosan nanoparticles vaccine. Int J Biol Macromol. 2022;220:183–192. doi:10.1016/j.ijbiomac.2022.08.073.
- Johansson E-L, Wassen L, Holmgren J, Jertborn M, Rudin A, Clements JD. Nasal and vaginal vaccinations have differential effects on antibody responses in vaginal and cervical secretions in humans. Infect Immun. 2001;69(12):7481–746. doi:10.1128/iai.69.12.7481-7486.2001.
