ABSTRACT
The outbreak of the COVID-19 has seriously affected the whole society, and vaccines were the most effective means to contain the epidemic. This paper aims to determine the top 100 articles cited most frequently in COVID-19 vaccines and to analyze the research status and hot spots in this field through bibliometrics, to provide a reference for future research. We conducted a comprehensive search of the Web of Science Core Collection database on November 29, 2023, and identified the top 100 articles by ranking them from highest to lowest citation frequency. In addition, we analyzed the year of publication, citation, author, country, institution, journal, and keywords with Microsoft Excel 2019 and VOSviewer 1.6.18. Research focused on vaccine immunogenicity and safety, vaccine hesitancy, and vaccination intention.
Introduction
The Severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2), also known as coronavirus disease 2019 (COVID-19), which is a disease characterized by respiratory distress, fever, cough, fatigue, pneumonia, and muscle pain.Citation1 Patients with severe infection may also develop neurological manifestations, such as acute cerebrovascular disease, skeletal muscle damage, and disturbance of consciousness.Citation2 The virus has also been reported to cause loss of smell and taste.Citation3 In general, fatigue, muscle aches, and headaches have a higher incidence in all subsequent stages.Citation4 And it carries a high risk of death in older people with cardiovascular disease (coronary artery disease, heart failure, and arrhythmias) and lung disease (chronic obstructive pulmonary disease).Citation5 SARS-CoV-2 is transmitted primarily from person to person through short-range airborne aerosols, respiratory droplets, and directly or indirectly contacted with infectious respiratory droplets.Citation6 The persistent COVID-19 pandemic has devastating consequences on populations, social structures, and global economic growth.Citation7 Moreover, studies have shown that control measures such as the use of masks, physical distancing, testing of exposed or symptomatic individuals, contact tracing, and quarantine are not sufficient to stop the transmission of COVID-19 caused by SARS-CoV-2.Citation8 Mass vaccination of at-risk populations and the subsequent general population is the single most effective public health measure to mitigate the coronavirus disease (COVID-19) pandemic.Citation9
Although there have been articles on COVID-19 vaccines from a bibliometric perspective, the top 100 most-cited articles on COVID-19 vaccines to date have not been studied.Citation10–14 Citation analysis was one of the bibliometric analysis methods that has been used to quantify the relative importance of scientific articles by examining citations attributed to the paper.Citation15 The total number of citations for published articles indicate the importance of published articles in that area of practice.Citation16 This study analyzes the bibliometric characteristics of the top 100 cited articles on COVID-19 vaccines since the outbreak of COVID-19, and discusses the research hotspots and trends in this field, as well as the collaboration between authors and institutions, so as to provide reference for subsequent research.
Methods
Data sources and search strategies
The Web of Science (WOS) is a comprehensive multidisciplinary database that includes all high-impact scientific journals and world-class indexesCitation17,Citation18 and has the title of “academic recognition as one of the most comprehensive bases for several areas of scientific knowledge.”Citation19 Compared with Scopus or MEDLINE/PubMed, the Web of Science (WOS) database can extract more complete information for bibliometric analysis. Therefore, we conduct a comprehensive search of the web of science (WOS) core collection database on November 29, 2022.The specific search strategy is explained in Appendix 1. Descriptors are defined from the Medical Subject Headings (MeSH) and Emtree directories.Citation19 The period is 2020-present (November 29, 2022) and there were no language restrictions. In addition, we include only articles and reviews. In addition, we also rank the articles according to their relevance and select the literature related to the COVID-19 vaccine online. A total of 6,957 publications are identified, and then we sort the 6,957 retrieved publications from highest to lowest in terms of total citation volume. The most recent article ranks higher if the total number of citations is the same.
Data extraction and bibliometric analysis
Two reviewers (LY and CX) extract all the data including title, country, institution, journal, author, etc, and discuss with the third reviewer (JT) in case of disagreement. Then we import the data into Microsoft Excel 2019 for statistical and descriptive analysis. Journal impact factors (IF) and journal category quartiles (Q) are from the 2022 Journal Citation Report (JCR).Citation20 In addition, it is used to construct evidence maps and this study is presented in the form of bubble charts, pie charts, and histograms.Citation21 We use VOSviewer1.6.18 (Van Eck and Waltman, Leiden University, Netherlands) for bibliometric analysis and mapping processes, and to construct and visualize bibliometric networks.Citation22–24 We use it to construct cooperative network maps of countries, authors, and institutions as well as co-emergence networks of author keywords, to better understand the framework and evolutionary track of scientific research and identify research frontiers and hot spots.Citation25 In the visual network diagram generated by VOSviewer1.6.18, each node represents a different element (author, country, organization, and keyword), the size of the node represents the frequency of occurrence of the element or the number of publications, the lines between the nodes represents cooperative or co-occurrence relationships, and the thickness of the lines between the nodes indicates the degree of connection.Citation16,Citation20,Citation26 The node colors indicate different clusters.
Results
Basic characteristics of the data
Table S 1 lists the top 100 most-cited articles in the field of COVID-19 vaccines, in descending order according to the number of citations in the WOS Core database. In the top 100 cited publications, 86 articles and 14 reviews were published in English. The number of citations ranged from 345 to 8,381. The total number of citations reached 78,881, with an average of 788.81 citations per article.
Publication year and citations
The 100 articles identified were published between 2020 and 2022. The relationship between the number of publications (x-axis), the average number of citations (y-axis), the total number of citations (bubble size), and the year of publication (different colors) was shown in Figure S1. In the data label, the former represented the annual number of publications, the middle was the average number of citations, and the latter represented the total number of citations. The total number of citations (46,878) and the number of publications (65) was the highest in 2021, the average number of citations (1004) was the highest in 2020, and 2022 was the lowest in both the number of publications (7) and the average number of citations (557).
WoS categories
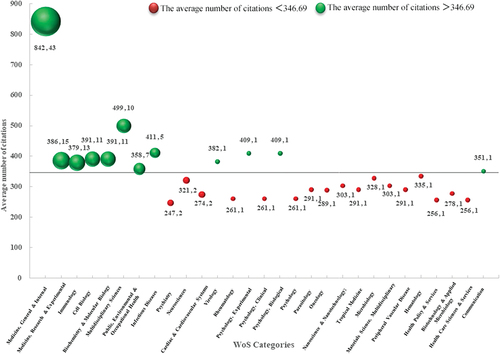
compares the average citations of 100 articles based on different WOS categories. The 100 publications were distributed across 29 WOS categories, with an average citation of 346.69 as the boundary. There were 12 species above the border. The top three by the number of articles were Medicine, General & Internal (n = 43), Medicine, Research & Experimental (n = 15), and Immunology(n = 13). In terms of the total number of citations and the average number of citations, the highest was Medicine, General & Internal (n = 36197, n = 842). Interestingly, Medicine, General & Internal were far ahead of the runners-up in terms of the number of articles, total citations, and average citations. Overall, there were fewer green bubbles than red bubbles, suggesting that only a few categories contributed to the overall increase in average citations.
Figure 1. Comparison of each WoS category’s average number of citations with average citations of the top 100 cited articles. Note: The X-axis referred to different categories, the Y-axis represented the average number of citations, and the bubble size stood for the corresponding number of articles. In the data label, the former value was the Y value, and the latter was the value of the bubble size.
Journal of publication
The top 100 cited publications were published in 37 different journals (Table S2). We can see that these journals were administered by the USA, the UK, the Netherlands, Switzerland, and China Mainland. 17 journals were run by the USA and 15 in the UK, accounting for more than 86%. In terms of the number of publications, the New England Journal of Medicine published the most (n = 24), followed by the Lancet (n = 15), Nature Medicine (n = 7), and Nature (n = 7), accounting for 53% of the top 100 articles. In terms of journal types, 53.7% of the journals published only one article. The highest impact factor was the Lancet (168.9), and the lowest was the Journal of Multidisciplinary Healthcare (3.3). Although the New England Journal of Medicine ranked first in both the number of publications and the number of citations, it did not have the highest IF. In addition, in terms of the category quartile of journals, there were 31 journals belonging to Q1, accounting for more than 84%.
Authorship impact on COVID-19 vaccine research
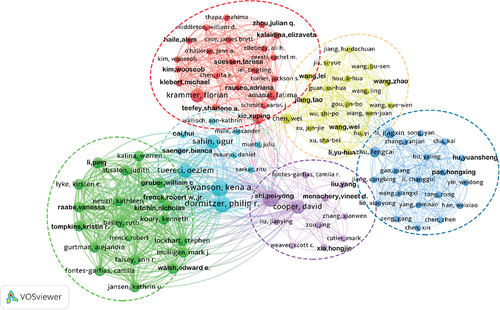
Of the 618 authors who contributed to the top 100 most-cited articles, 556 published just one article, about 90% of all authors. shows only 17 authors with ≥ 3 publications, of which 10 were from the USA, 3 from Israel, 2 from Germany, and 2 from the UK. In addition, we confirmed that Swanso Kena A, and Dormitzer Philip R from Pfizer in the USA, as well as Shi Pei-Yong from the University of Texas System were the most prolific authors in this field, with 4 publications. Meanwhile, Swanso Kena A and Dormitze Philip R have the highest citations (2708), suggesting that they may be the most influential authors in the top 100 most-cited COVID-19 vaccine studies.
Table 1. The most prolific authors in COVID-19 vaccine research.
As shown in , we constructed a cooperative cluster analysis for 618 authors who participated in the top 100 most-cited articles. Only 103 authors were associated with others and formed a total of 6 clusters.
Top productive institutions in COVID-19 vaccine
and show the institutions that were most active in the top 100 most cited COVID-19 vaccine studies. A total of 346 institutions and universities around the world have collaborated independently or in small groups in this area. lists only 18 institutions with publication volume ≥ 4, of which 9 were from the USA, 4 were from Israel, 3 were from the UK, and the remaining 2 were from Germany and China respectively. is a collaborative network composed of 67 institutions associated with other institutions with more than 2 publications. The network was composed of 67 nodes, 305 links, and 6 clusters. We found that these studies were mainly the result of cooperation between researchers from universities in Europe, Asia, and the USA. The UK’s University of Oxford and the University of London were the most prolific in the field with 12 publications, followed by Pfizer and Harvard University in the USA with 10. They have played a bigger role than other institutions in the publication of these studies, and we can also say that universities in the USA and the UK have played a prominent role in the publication of research on COVID-19 vaccines. In addition, Pfizer from the USA had the highest cited times of 10,999, followed by BioNTech from Germany with the cited times of 10,249. In other words, Pfizer and BioNTech have published papers that have the greatest impact on citations in the scientific community.
Figure 3. Collaborative network and cluster distribution of institutions in the top 100 articles on COVID-19 vaccines (the number of publications ≥ 3).
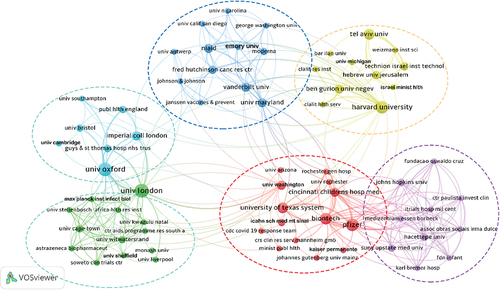
Table 2. The most prolific institutions in COVID-19 vaccine research.
National distribution and cooperation
shows the countries of the corresponding authors of the top 100 articles, who come from 25 different countries. The USA topped the top 100 most-cited COVID-19 vaccine studies with 33 articles, followed by the UK with 16 articles and Israel with 11 articles. In addition, authors from Russia, India, Turkey, Norway, Malaysia, and Canada did not collaborate with other countries, but only within their own countries. As shown in , although the USA was the country with the largest number of publications, its international cooperation rate is relatively low.
Figure 4. Country analysis. (a) Corresponding Author’s Country (Where MCP represents the number of coauthored papers with authors from other countries; SCP represents the number of papers coauthored by authors of the same nationality; MCP Ratio indicates the ratio of international cooperation.) (b) National collaborative networks and cluster distribution in the top 100 articles on COVID-19 vaccines.
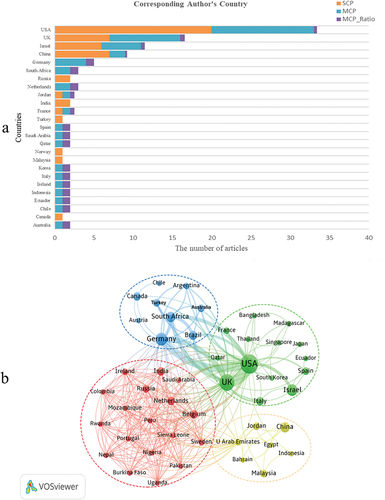
includes 48 countries that were involved in COVID-19 vaccine research in partnership with other countries, forming four clusters. Cluster 1(red) was made of six European countries represented by Belgium and Russia, four Asian countries represented by India, six African countries represented by Burkina Faso, and two South American countries represented by Colombia. Cluster2 (green) was consisted mainly of 14 countries with a core of the USA, UK, and Israel. Cluster3 (blue) was consisted mainly of nine countries, including Germany, South Africa, Brazil, Canada, Turkey, Australia, Argentina, Austria, and Chile. Cluster 4 (yellow) was consisted of six Asian countries represented by China and Egypt from Africa. Not only that but also reveals the cooperation between countries, with the USA and the UK (link strength = 18) having the closest cooperation, followed by the USA and Germany (link strength = 12) and the UK and Germany (link strength = 9).
Research hotspot and co-occurrence keyword clustering network analysis
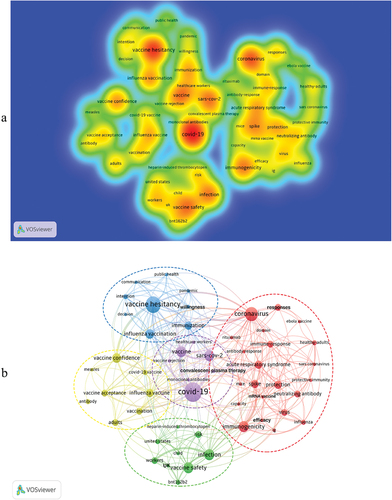
The top 100 most-cited articles involved 210 keywords. We showed the density of the main keywords in , and the frequency was greater than or equal to 2. The graph showed 55 nodes, with the brightest spot relating to COVID-19 and vaccine hesitancy, followed by vaccines, immunogenicity, vaccine confidence, and vaccine safety. In , 55 keywords that appear more than twice were included and classified into 5 clusters in the map. Cluster1 (red) mainly included 23 keywords such as coronavirus, immunogenicity, acute respiratory syndrome, spike, antibody-response, mRNA vaccine, immune response, neutralizing antibody, protective immunity, rituximab, etc, which were mainly related to immunological research of COVID-19 vaccine. Cluster 2 (green) mainly consisted of 9 keywords, such as infection, vaccine safety, risk, workers, children, and heparin-induced thrombocytopenia, which mainly studied the safety and risk after vaccination. Cluster 3 (blue) included vaccine hesitancy, influenza, vaccination, immunization, intention, communication, decision, pandemic, and public health willingness, which looked at people’s willingness to be immunized against vaccines during influenza pandemic. Cluster 4 (yellow) included vaccine confidence, influenza vaccine, adults, vaccination, vaccine acceptance, antibody, COVID-19 vaccine, and measles. It focused on adults’ attitudes toward COVID-19 vaccines. Cluster 5(purple) was composed of 7 keywords. They were COVID-19, SARS-Cov-2, vaccine, convalescent plasma therapy, healthcare workers, monoclonal antibodies, and vaccine rejection. The cluster focused on vaccine rejection and the use of novel therapeutic monoclonal antibodies. In conclusion, the research hotspots of the top 100 cited articles on the COVID-19 vaccine mainly concentrated on vaccine immunology, vaccine safety, vaccine hesitancy, people’s attitude toward vaccines, and vaccine rejection.
Discussion
The top 100 most-cited articles may be highly recognized articles in a certain field, and bibliometric analysis of these articles can quantitatively determine their main research concerns and dynamic change information.Citation15 Through the analysis of the years of these 100 articles, it is found that the average amount of citations in 2020 is the highest, the number of publications in 2021 is the highest, while the number of publications and the average number of citations in 2022 is both the lowest, which may be related to the publication time of the article.
A total of 37 journals publishes the top 100 articles cited on the COVID-19 vaccine, 95% of which are included by SCI. In addition, high-impact medical journals such as Nature, Science, JAMA, Nature Medicine, and Cell also participate in the publication of the top 100 articles on the COVID-19 vaccine, indicating that the vaccine has received global attention. In addition, we can also find that the Lancet, Natural Medicine, and Nature journals are not only the most productive journals but also the journals with the highest citation times and journal impact. Therefore, we can say how much attention the topic is getting. Focus not only on the number of articles but also on the quality of articles. Based on the research focus and the number of citations accumulated, the most cited articles provide more in terms of the general interest of the research.Citation27
A total of 346 institutions and 49 countries contribute to the publication of the top 100 cited articles on COVID-19 vaccines. After analyzing 18 institutions with more than 4 publications, we found that half of them is from the USA, and affiliate with universities. The cooperative network diagram of institutions show that different institutions are connected but not closely. From the perspective of national contribution, most of the countries participating in the top 100 published articles are developed countries, and the USA is the country with the largest number of published articles. And because the USA has been developing well in the medical field and even other fields, we can undoubtedly conclude that the USA still has the greatest influence in this field and is in a leading position in this field. In our analysis of cooperation networks in 48 countries, we found that these countries are not very well connected to the rest of the world. Of the 49 countries, 30 are from developing countries, and 20 published only one paper, which may be due to international differences in economic levels. Research has shown that funding is one of the potential mechanisms for encouraging and enhancing productivity, and developed countries have the most potential funding institutions for scientific research.Citation28 Consequently, we should be encouraged to study high productive country and less activity countries of international cooperation between the author, in order to promote the research in the future better.
Swanson Kena A and Dormitzer Philip R from Pfizer in the USA as well as Shi Pei-Yong from the University of Texas System in the USA are the authors of the top 100 cited articles on the COVID-19 vaccine with 4 articles published, and they are also the authors with the most cited articles. Pfizer is the world’s leading research-based biomedical and pharmaceutical company with its headquarters in New York, USA. The University of Texas system is the second largest state public university system in the USA after the University of California system. Some studies show that an excellent platform is the basis of the author’s research, and the author’s research results can represent and enhance the influence of the institution or even the country.Citation21
In bibliometrics, keyword co-occurrence can reflect different research hotspots and topics.Citation15 According to the analysis of 5 clusters consisting of 55 keywords with co-occurrence frequency greater than or equal to 2, we found that cluster 1 (red) consists of 23 keywords, which mainly study the immunology of the COVID-19 vaccine. Studies by Warsey M et al. demonstrated that the use of viral vectors to induce an immune response against spike proteins could protect humans from this disease.Citation29 It has also been shown that a single dose can cause humoral and cellular responses to SARS-CoV-2, and strengthening immunity can improve the neutralizing antibody titer and anti-VOC activity.Citation30,Citation31 Moreover, allogenic prime-booster vaccination increases the immunogenicity of either vaccine alone or the homologous prime-booster combination.Citation32 Cluster 2(green) consists of 9 keywords and focuses on the safety of the COVID-19 vaccine. Many articles on vaccine safety can be retrieved from the WOS Core collection database, and it can be found that most vaccine adverse reactions are mild and transient local or systemic, but a small number of serious adverse events can occur, such as myocarditis, thrombocytopenia, disseminated intravascular coagulation and thrombosis.Citation33–35 Cluster 3 include 9 keywords related to the intention and vaccine hesitancy for COVID-19 or influenza vaccination. Vaccine hesitancy, a global phenomenon that is a barrier to full vaccination against highly infectious diseases,Citation36,Citation37 dates back to the 1800s,Citation38 and many factors influence willingness to vaccinate. The most common reasons for vaccine hesitation or refusal include inadequate perception of disease risk, low confidence in vaccine safety and efficacy, previous experience, certain religious beliefs, education, and income levels, public health policies, social factors, and media communication.Citation36,Citation39 In addition, other studies have shown that vaccine hesitancy is associated with gender, race, age, economics (higher hesitancy among women, young people, black people, and economically disadvantaged groups), and fear of injection,Citation40,Citation41 and that hesitancy was higher among healthcare workers among nurses than among doctors.Citation37 Cluster 4 consists of 8 keywords, which mainly study vaccine confidence and acceptability. In Vaccines (Basel), 16 February 2021, Sallam M. entitled “COVID-19 Vaccine Hesitancy Worldwide: A concise systematic review of vaccine acceptance rates”Citation36 notes that there are significant differences in vaccine acceptance rates in different countries and regions of the world, ranging from 38% in the northeast to 49% in the west. Acceptance is higher in some Asian countries and lowers in the Middle East, Eastern Europe, and Russia. The rapid development, political interference, and misinformation that have dominated the vaccine discussion have undermined confidence in the rigor of the approval process and in the use of the vaccine itself. Emphasizing transparency and adherence to scientific standards throughout the process of vaccine development, approval and distribution can restore confidence. Cluster 5 (purple) consists of seven keywords that may be associated with vaccine rejection in healthcare workers. Hospital healthcare workers are at high risk during the outbreak. Studies have found that healthcare workers who believe they were immune to COVID-19 and those who are confident they will not get it have the highest rates of COVID-19 vaccine refusal, healthcare providers who do not care for COVID-19-positive patients have higher rates of vaccination refusal, and nurses are more likely to reject vaccines than doctors.Citation37,Citation42
In addition, an analysis of the top 100 cited studies of COVID-19 vaccines revealed the following problems in the development of COVID-19 vaccines. ① At present, there are few or no vaccines developed specifically for infants, the elderly and pregnant women, which will increase the risk of infection in these groups to a certain extent. ② Most of these literatures focus on the efficacy and safety of vaccines, but do not pay attention to the production cost of vaccines, which may add a certain economic burden to the society. ③ Due to the normalization of the COVID-19 epidemic, the demand for SARS-CoV-2 vaccines has declined sharply, which has led to the lack of subjects for SARS-CoV-2 vaccines and it is difficult to develop new vaccines.
Although the epidemic has become normalized, considering that coronavirus has become an epidemic pathogen that seriously threatens human beings for several times, it is recommended to pay attention to the improvement and establishment of public health defense system and emergency response system, Improve the construction of a universal coronavirus emergency vaccine research and development platform, a universal respiratory virus emergency vaccine research and development platform, and even a universal infectious disease pathogen emergency vaccine research and development platform to avoid the situation of delayed vaccines in the face of epidemic again. In the future, some vaccines should be developed specifically for special populations. In addition, the pandemic is a battle that seriously threatens human lives and affects social economy. In the future, we should not only pay attention to the efficacy and safety of vaccines, but also consider the production cost, and strive to develop low-cost, effective and safe vaccines.
Conclusion
The study provides a quantitative and qualitative analysis of the top 100 most-cited articles on COVID-19 vaccines. According to the analysis results of bibliometrics, developed countries, especially the USA, are the biggest contributors, and universities are the main body of research in this field. The number of publications on COVID-19 vaccines in the New England Journal, the Lancet, Natural Medicine, and Nature shows how much attention the topic is getting. In addition, the collaboration network map showed that the collaboration among countries, institutions, and authors is fragmented. We should be encouraged to study high productive country and less activity countries of international cooperation between the author, in order to promote the research in the future better. Research hotspots mainly focus on vaccine safety (adverse reactions), immunogenicity, vaccine hesitancy, vaccine vaccination intention, and medical staff’s attitude toward vaccination. To have an in-depth understanding of research hotspots and trends in this field, this study identified and analyzed the countries, institutions, authors, and keywords involved in the 100 most influential articles on the COVID-19 vaccine, which may provide a reference value for later research. However, this paper also has certain limitations. First of all, the data in this paper only came from the Web of science, which may cause data bias. Secondly, although we have standardized and unified the authors, institutions, and keywords involved in this study, we still cannot rule out the possible bias caused by our subjective factors.
List of Abbreviations
| SARS-CoV-2 | = | Severe acute respiratory syndrome coronavirus 2 |
| COVID-19 | = | Corona virus disease 2019 |
| USA | = | The United States of America |
| UK | = | The United Kingdom |
| IF | = | Impact Factor |
| JCR | = | Journal Citation Report |
| WOS | = | Web of Science |
supplementary materials.docx
Download MS Word (149.5 KB)Acknowledgments
LY and CX conducted data integration and mapping and wrote the manuscript. JT and XL were responsible for the study concept and design and supervised the study. MZ, YZ, ZH, LL, and YL were responsible for data collection and collation. All authors reviewed and approved the final submitted version.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2024.2370605
Additional information
Funding
References
- Fernandes Q, Inchakalody VP, Merhi M, Mestiri S, Taib N, Moustafa Abo El-Ella D, Bedhiafi T, Raza A, Al-Zaidan L, Mohsen MO, et al. Emerging COVID-19 variants and their impact on SARS-CoV-2 diagnosis, therapeutics and vaccines. Ann Med. 2022;54(1):524–10. doi:10.1080/07853890.2022.2031274.
- Meng X, Deng Y, Dai Z, Meng Z. COVID-19 and anosmia: a review based on up-to-date knowledge. Am J Otolaryngol. 2020;41(5):102581. doi:10.1016/j.amjoto.2020.102581.
- Tsang HF, Chan LWC, Cho WCS, Yu ACS, Yim AKY, Chan AKC, Ng LPW, Wong YKE, Pei XM, Li MJW, et al. An update on COVID-19 pandemic: the epidemiology, pathogenesis, prevention and treatment strategies. Expert Rev Anti Infect Ther. 2021;19(7):877–88. doi:10.1080/14787210.2021.1863146.
- Yang FW, Pang B, Jin XY, Chen Z, Pang W, Liu Q, Zhang J, Zhang B. Post COVID-19 burden: focus on the short-term condition. Acupunct Herb Med. 2022;2(3):139–42. doi:10.1097/HM9.0000000000000036.
- Campos DMO, Fulco UL, de Oliveira CBS, Oliveira JIN. SARS-CoV-2 virus infection: targets and antiviral pharmacological strategies. J Evid Based Med. 2020;13(4):255–60. doi:10.1111/jebm.12414.
- To KK, Sridhar S, Chiu KH, Hung DLL, Li X, Hung IFN, Tam AR, Chung TWH, Chan JFW, Zhang AJX, et al. Lessons learned 1 year after SARS-CoV-2 emergence leading to COVID-19 pandemic. Emerg Microbes Infect. 2021;10(1):507–35. doi:10.1080/22221751.2021.1898291.
- Sreepadmanabh M, Sahu AK, Chande A. COVID-19: advances in diagnostic tools, treatment strategies, and vaccine development. J Biosci. 2020;45(1):148. doi:10.1007/s12038-020-00114-6.
- Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–16. doi:10.1056/NEJMoa2035389.
- Li X, Wichai N, Wang JB, Liu X, Yan H, Wang Y, Luo M, Zhou S, Wang K, Li L, et al. Regulation of innate and adaptive immunity using herbal medicine: benefits for the COVID-19 vaccination. Acupunct Herb Med. 2022;2(3):196–206. doi:10.1097/HM9.0000000000000046.
- Ahmad T, Murad MA, Baig M, Hui J. Research trends in COVID-19 vaccine: a bibliometric analysis. Hum Vaccines Immunother. 2021;17(8):2367–72. doi:10.1080/21645515.2021.1886806.
- Chen Y, Cheng L, Lian R, Song Z, Tian J. COVID-19 vaccine research focusses on safety, efficacy, immunoinformatics, and vaccine production and delivery: a bibliometric analysis based on VOSviewer. Biosci Trends. 2021;15:64–73. doi:10.5582/bst.2021.01061.
- Wei WT, Wei CK, Wu CC. Trends in research about COVID-19 vaccine documented through bibliometric and visualization analysis. Healthcare (Basel). 2022;10(10):1942. doi:10.3390/healthcare10101942.
- Rosen B, Davidovitch N, Chodick G, Israeli A. The role of Israeli researchers in the scientific literature regarding COVID-19 vaccines. Isr J Health Policy Res. 2022;11(1):39. doi:10.1186/s13584-022-00548-3.
- Noruzi A, Gholampour B, Gholampour S, Jafari S, Farshid R, Stanek A, Saboury AA. Current and future perspectives on the COVID-19 vaccine: a scientometric review. J Clin Med. 2022;11(3):750. doi:10.3390/jcm11030750.
- Song Y, Zhang L, Yang Y, Sun J. The top 100 most cited articles in anaphylaxis: a bibliometric analysis. Clin Exp Med. 2022;23(5):1–17. doi:10.1007/s10238-022-00890-5.
- Zyoud SH, Waring WS, Al-Jabi SW, Sweileh WM, Awang R. The 100 most influential publications in paracetamol poisoning treatment: a bibliometric analysis of human studies. Springerplus. 2016;5(1):1534. doi:10.1186/s40064-016-3240-z.
- Xia Y, Yao RQ, Zhao PY, Tao Z-B, Zheng L-Y, Zhou H-T, Yao Y-M, Song X-M. Publication trends of research on COVID-19 and host immune response: a bibliometric analysis. Front Public Health. 2022;10:939053. doi:10.3389/fpubh.2022.939053.
- Ying J, Tan GMY, Zhang MW. Intellectual disability and COVID-19: a bibliometric review. Front Psychiatry. 2022;13:1052929. doi:10.3389/fpsyt.2022.1052929.
- Oliveira EMN, Carvalho ARB, Silva JSE, Sousa Neto AR, Moura MEB, Freitas DRJ. Analysis of scientific production on the new coronavirus (COVID-19): a bibliometric analysis. Sao Paulo Med J. 2021;139(1):3–9. doi:10.1590/1516-3180.2020.0449.R1.01102020.
- Ni XJ, Zhong H, Liu YX, Lin HW, Gu ZC. Current trends and hotspots in drug-resistant epilepsy research: Insights from a bibliometric analysis. Front Neurol. 2022;13:1023832. doi:10.3389/fneur.2022.1023832.
- Shi S, Gao Y, Liu M, Bu Y, Wu J, Tian J, Zhang J. Top 100 most-cited articles on exosomes in the field of cancer: a bibliometric analysis and evidence mapping. Clin Exp Med. 2021;21(2):181–94. doi:10.1007/s10238-020-00624-5.
- Pasin O, Pasin T. Bibliometric analysis of COVID-19 and the association with the number of total cases. Disaster Med Public Health Prep. 2022;16(5):1947–52. doi:10.1017/dmp.2021.177.
- Soytas RB. A bibliometric analysis of publications on COVID-19 and older adults. Ann Geriatr Med Res. 2021;25(3):197–203. doi:10.4235/agmr.21.0060.
- Cui Y, Ouyang S, Zhao Y, Tie L, Shao C, Duan H. Plant responses to high temperature and drought: a bibliometrics analysis. Front Plant Sci. 2022;13:1052660. doi:10.3389/fpls.2022.1052660.
- Wu T, Huang W, Qi J, Li Y, Zhang Y, Jiang H, Wang J, Zhang J, Jiang Z, Chen L, et al. Research trends and frontiers on antiphospholipid syndrome: a 10-year bibliometric analysis (2012–2021). Front Pharmacol. 2022;13:1035229. doi:10.3389/fphar.2022.1035229.
- Dai Y, Chen Y, Hu Y, Zhang L. Current knowledge and future perspectives on exosomes in the field of regenerative medicine: a bibliometric analysis. Regen Med. 2023;18(2):123–36. doi:10.2217/rme-2022-0141.
- Akintunde TY, Chen S, Musa TH, Amoo FO, Adedeji A, Ibrahim E, Tassang AE, Musa IH, Musa HH. Tracking the progress in COVID-19 and vaccine safety research – a comprehensive bibliometric analysis of publications indexed in Scopus database. Hum Vaccin Immunother. 2021;17(11):3887–97. doi:10.1080/21645515.2021.1969851.
- Musa HH, Musa TH. A systematic and thematic analysis of the top 100 cited articles on mRNA vaccine indexed in Scopus database. Hum Vaccin Immunother. 2022;18(6):2135927. doi:10.1080/21645515.2022.2135927.
- Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi:10.1016/S0140-6736(20)32661-1.
- Azamor T, Horbach IS, Brito E, Cunha D, Silva AMVD, Tubarão LN, Azevedo ADS, Santos RT, Alves NDS, Machado TL, et al. Protective immunity of COVID-19 vaccination with ChAdOx1 nCoV-19 following previous SARS-CoV-2 infection: a humoral and cellular investigation. Viruses. 2022;14(9):1916. doi:10.3390/v14091916.
- Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S, Bellamy D, Bibi S, Bittaye M, Clutterbuck EA, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396(10249):467–78. doi:10.1016/S0140-6736(20)31604-4.
- Cohen G, Jungsomsri P, Sangwongwanich J, Tawinprai K, Siripongboonsitti T, Porntharukchareon T, Wittayasak K, Thonwirak N, Soonklang K, Sornsamdang G, et al. Immunogenicity and reactogenicity after heterologous prime-boost vaccination with CoronaVac and ChAdox1 nCov-19 (AZD1222) vaccines. Hum Vaccin Immunother. 2022;18(5):2052525. doi:10.1080/21645515.2022.2052525.
- Bozkurt B, Kamat I, Hotez PJ. Myocarditis with COVID-19 mRNA Vaccines. Circulation. 2021;144(6):471–84. doi:10.1161/CIRCULATIONAHA.121.056135.
- Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384(22):2092–101. doi:10.1056/NEJMoa2104840.
- See I, Su JR, Lale A, Woo EJ, Guh AY, Shimabukuro TT, Streiff MB, Rao AK, Wheeler AP, Beavers SF, et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA. 2021;325(24):2448–56. doi:10.1001/jama.2021.7517.
- Sallam M. COVID-19 vaccine hesitancy worldwide: a concise systematic review of vaccine acceptance rates. Vaccines (Basel). 2021;9(2):160. doi:10.3390/vaccines9020160.
- Dror AA, Eisenbach N, Taiber S, Morozov NG, Mizrachi M, Zigron A, Srouji S, Sela E. Vaccine hesitancy: the next challenge in the fight against COVID-19. Eur J Epidemiol. 2020;35(8):775–9. doi:10.1007/s10654-020-00671-y.
- Lin C, Tu P, Beitsch LM. Confidence and receptivity for COVID-19 vaccines: a rapid systematic review. Vaccines (Basel). 2020;9(1):16. doi:10.3390/vaccines9010016.
- Soares P, Rocha JV, Moniz M, Gama A, Laires PA, Pedro AR, Dias S, Leite A, Nunes C. Factors associated with COVID-19 vaccine hesitancy. Vaccines (Basel). 2021;9(3):300. doi:10.3390/vaccines9030300.
- Borga LG, Clark AE, D’Ambrosio C, Lepinteur A. Characteristics associated with COVID-19 vaccine hesitancy. Sci Rep. 2022;12(1):12435. doi:10.1038/s41598-022-16572-x.
- Freeman D, Lambe S, Yu LM, Freeman J, Chadwick A, Vaccari C, Waite F, Rosebrock L, Petit A, Vanderslott S, et al. Injection fears and COVID-19 vaccine hesitancy. Psychol Med. 2021;53(4):1185–95. doi:10.1017/S0033291721002609.
- Shekhar R, Sheikh AB, Upadhyay S, Singh M, Kottewar S, Mir H, Barrett E, Pal S. COVID-19 vaccine acceptance among health care workers in the United States. Vaccines (Basel). 2021;9(2):119. doi:10.3390/vaccines9020119.