ABSTRACT
Recently, CAR-T cell therapy in hematological malignancies has received extensive attention. The objective of this study is to gain a comprehensive understanding of the current research status, development trends, research hotspots, and emerging topics pertaining to CAR-T cells in the treatment of hematological malignancies. Articles pertaining to CAR-T cell therapy for hematological malignancies from the years 2012 to 2023 were obtained and assessed from the Web of Science Core Collection (WoSCC). A bibliometric approach was employed to conduct a scientific, comprehensive, and objective quantitative analysis, as well as a visual analysis, of this particular research domain. A comprehensive analysis was conducted on a corpus of 3643 articles, which were collaboratively authored by 72 countries and various research institutions. CAR-T cell research in treating hematological malignancies shows an increasing trend each year. Notably, the study identified the countries and institutions displaying the highest level of activity, the journals with the most citations and output, as well as the authors who garnered the highest frequency of citations and co-citations. Furthermore, the analysis successfully identified the research hotspots and highlighted six emerging topics within this domain. This study conducted a comprehensive exploration and analysis of the research status, development trends, research hotspots, and emerging topics about CAR-T cells in the treatment of hematological malignancies from 2012 to 2023. The findings of this study will serve as a valuable reference and guide for researchers seeking to delve deeper into this field and determine the future direction of their research.
Introduction
Hematological malignancies refer to malignant tumors that arise from the abnormal proliferation of white blood cells or lymphocytes. Conventional treatment approaches have demonstrated limited effectiveness, necessitating the exploration of novel therapeutic strategies. One such emerging approach is CAR-T cell therapy, which involves the genetic modification of the patient’s own T cells to generate CAR-T cells possessing specific antigen receptors (CAR). The CAR protein comprises an external antigen recognition structure known as a single chain variable fragment (scFv), a transmembrane domain, and an internal signal transduction structure. CAR-T cell therapy has demonstrated significant advancements in the management of hematological malignancies, with notable success observed in the treatment of B-cell acute lymphoblastic leukemia (ALL) and B-cell lymphomas through targeting the CD19 antigen. In addition to CD19 and BCMA, other tumor antigens have garnered attention in the context of CAR-T cell therapy. For instance, the therapeutic potential of CD20 in non-Hodgkin lymphoma using CAR-T cells has been extensively investigated.Citation1 Similarly, the efficacy of CD22 in the treatment of B-ALL with CAR-T cells has been widely explored.Citation2,Citation3 Furthermore, novel targets including CD30,Citation4 CD33,Citation5 CD38,Citation6 CD133,Citation7 and Siglec-6Citation8 have garnered significant interest in recent research.
Despite the promising outcomes of CAR-T cell therapy in the management of hematological malignancies, several obstacles and constraints persist. Notably, treatment-related complications, such as cytokine release syndrome (CRS) and neurotoxicity, pose significant challenges. Additionally, the durability and resistance of CAR-T cells necessitate further enhancement. To surmount these hurdles, researchers are diligently endeavoring to refine CAR-T cell therapy. Several strategies can address these challenges, encompassing the optimization of CAR design to enhance the anti-tumor activity and longevity of CAR-T cells,Citation9 the development of novel antigen targets to broaden the applicability of CAR-T cell therapy,Citation10 and the exploration of combined treatment approaches involving immune checkpoint inhibitors,Citation11 radiotherapy,Citation12 or chemotherapyCitation13 to augment the therapeutic efficacy.
Currently, a substantial quantity of global clinical trials pertaining to CAR-T cell therapy have been conducted. As of December 2023, the American Clinical Trials Database (ClinicalTrials.gov) has documented the registration of 1,509 clinical trials involving CAR-T cell therapy. In recent times, the utilization of CAR-T cell therapy has assumed a significant role in the treatment of hematological malignancies. Bibliometric analysis is a quantitative research methodology that entails the utilization of statistical techniques and analytical tools to examine various aspects of scientific literature. This approach can identify trends in research fields, draw network maps, evaluate research impacts, and extract other relevant information. Furthermore, by conducting extensive statistical and quantitative analyses on a substantial corpus of literature data, bibliometric analysis facilitates the attainment of a comprehensive understanding and an objective evaluation of the research field under investigation.Citation14 This study aims to employ this research method to carry out scientific, comprehensive, and objective metrological analysis and visual analysis in this field.
Materials and methods
Data collection
Pertinent data were retrieved and downloaded from WoSCC. The search strategy, inclusion, and exclusion criteria for this study are explicated in Annexes 1. A total of 3643 eligible articles were obtained.
Data analysis and visualization
CiteSpace and VOSviewer are the most common bibliometrics software. The former offers an experimental platform conducive to the exploration of novel concepts and the comparison of diverse methodologies.Citation15 The latter primarily emphasizes the visualization of scientific knowledge and exhibits advantages in handling extensive maps.Citation16 In data management and analysis, Microsoft Excel 2019 is employed. Additionally, for the purposes of bibliometric analysis and visualized analysis, GraphPad Prism 8, CiteSpace, and VOSviewer are utilized.
Results
Analysis of annual publication volume
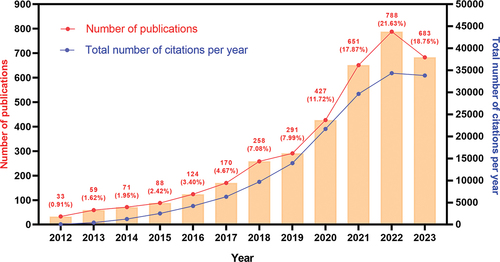
Based on the annual publishing volume within this particular field, a growth trend chart has been constructed. illustrates a consistent increase in the annual publishing volume over time. By analyzing the data, the growth trend can be categorized into three distinct stages: a slow stage spanning from 2012 to 2017, a high-speed growth stage from 2018 to 2021, and a stable growth stage from 2022 to 2023. During the second stage, a total of 1627 articles (accounting for 44.7% of the total) were published, while the third stage saw the publication of 1471 articles (representing 40.1% of the total). Currently, only 10 months’ worth of articles from 2023 have been acquired, resulting in a slightly lower publication volume compared to 2022. In conjunction with the developed annual publication quantity fitting equation (y = 70.12*x − 141168, correlation coefficient R2 = 0.8784, p ≤.0001) (Figure S1), it is anticipated that the publication volume in 2023 will marginally surpass that of 2022. Despite the current sustained high level of annual publications in this field, growth has decelerated and is projected to reach its peak in the forthcoming years. Furthermore, the 3643 articles garnered a total of 157,925 citations, resulting in an average of 39.74 citations per article. These publications possess an H-index of 172.
Countries and institutions
The study yielded a total of 3643 articles, which were collaboratively authored by numerous countries and institutions. As depicted in , the United States emerged as the most prolific publisher in this domain, with a substantial count of 1942 articles, surpassing all other nations by a significant margin. China followed suit with 902 articles, while Germany trailed behind with 446 articles. Interestingly, the number of publications between the first and fourth places shows a multiple relationship, that is, the number of publications from the United States is twice that of China, and so forth. Centrality serves as an index employed for the quantification of node significance within a network. A higher centrality value signifies a more pronounced role of the node in terms of connectivity. At the national level, Finland exhibited the highest centrality measure of 0.84, trailed by Austria at 0.67 and Venezuela at 0.57. The University of Pennsylvania emerged as the most prolific research institution, having published 279 articles, followed by Pennsylvania Medicine (n = 250) and the University of Texas System (n = 250). It is noteworthy that all of the top 10 research institutions are from the United States. In addition, the institutions with the highest centrality were UTMD Anderson Cancer Center (0.47) and Washington University (0.47). The national cooperation network map () indicates that while there has been some level of cooperation among countries in the preceding two years, the top ten countries primarily engage in cooperation within their own borders and exhibit a lack of collaboration with other nations. Conversely, the collaborative network diagram of institutions () reveals the presence of robust collaborative relationships among institutions, predominantly observed prior to 2021, with a notable decline in cooperation during the recent two-year period.
Figure 2. The co-occurrence map of countries (a) and institutions (b) about this research field. The order of countries and institutions is arranged clockwise according to the number of articles from large to small.
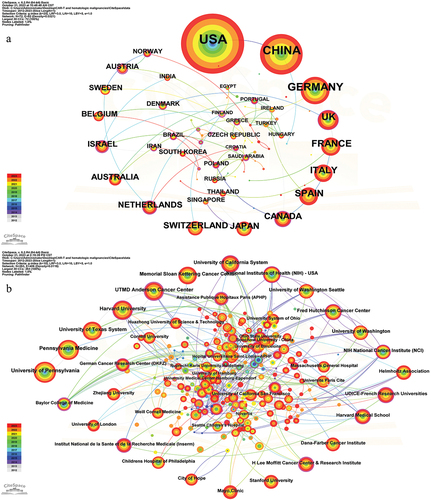
Table 1. The top 10 countries and institutions about t this research field.
Journals
A total of 3643 articles were published across 652 academic journals. The publication volume, citation frequency, impact factor, and JCR partition of these journals were subjected to scientific statistical analysis (). The concept of cited journals pertains to the occurrence where a journal article is referenced by other journal articles, serving as a metric for gauging a journal’s influence within a specific field. According to , the New England Journal of Medicine exhibited the highest citation frequency (n = 223,56) within this research domain, followed by Blood (n = 14,317) and Nature Medicine (n = 8,471). The New England Journal of Medicine, despite publishing a relatively small number of 22 articles in this research field, exhibits a significantly higher citation frequency compared to other journals, thus signifying its substantial academic influence. Notably, Frontiers in Immunology takes the lead with 162 articles, followed by Blood with 147 articles and Blood Advances with 27 articles. The density map depicted in presents journals that have published more than 15 articles, providing researchers with a tool for journal selection. Furthermore, showcases the results of a cluster analysis conducted in these journals. Periodical clustering, as an analytical approach, facilitates the grouping of related periodicals, thereby unveiling the interconnections between discipline structure and specific fields of study.
Figure 3. The density map of journals (a) and cluster analysis of journals (b) about this research field.
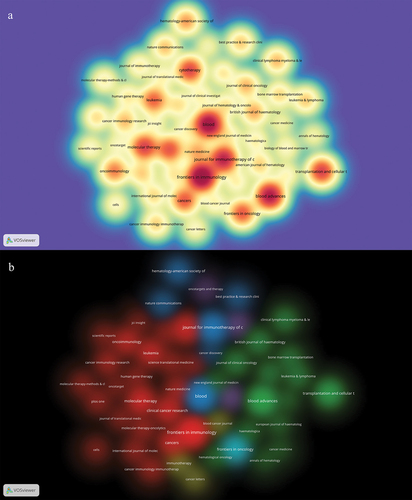
Table 2. The top 10 journals and co-cited journals about this research field.
Authors
A total of 23,852 authors coauthored these articles. To further explore the cooperative network between influential researchers and authors in this research field, we analyzed the cited authors and co-cited authors. The findings, presented in , reveal that Carl H. June is the most frequently cited researcher with a count of 17,177, followed by Stephan A. Grupp (n = 12,858), Bruce L. Levine (n = 12,396), and David L. Porter (n = 10,305). The inclusion criteria for this study involved researchers who have published a minimum of 10 articles. Subsequently, a cooperative network diagram was constructed to visualize the relationships among these researchers, resulting in the identification of approximately 16 major research groups (). These research teams hold significant academic influence within the field under investigation. Furthermore, also shows that there is cooperation among different research groups. Notably, the top 5 most frequently cited authors are all affiliated with the same research group (orange). The co-cited author analysis indicates that numerous authors are cited together within the same document. presents the findings that Shannon L. Maude is the most frequently cited author (n = 2013), followed by Daniel W. Lee (n = 1676) and James N. Kochenderfer (n = 1434). To construct a co-cited network diagram (), only researchers with a minimum of 150 co-cited frequencies were included. This diagram illustrates the citation relationship among co-cited authors. Furthermore, based on the correlation and similarity between co-cited authors, four distinct clusters were identified, with Shannon L. Maude, Daniel W. Lee, James N. Kochenderfer, and Sattva S. Neelapu serving as representatives in each cluster.
Figure 4. The visualization map of authors (a) co-cited authors (b) about this research field.
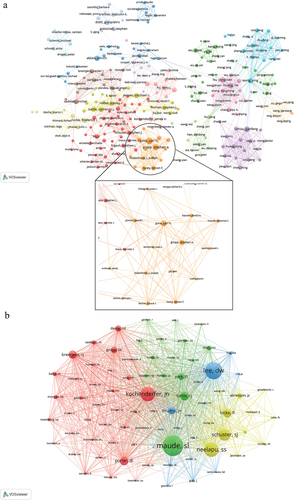
Table 3. The top 10 authors and co-cited authors about this research field.
Cluster and evolution of keywords
The analysis of keywords holds considerable importance in bibliometric analysis as it unveils the research emphasis within a particular field of study. After the process of cleaning and merging the original keyword data, a total of 8099 keywords were obtained. The top 20 keywords are presented in , with CAR-T (n = 1028) being the most frequently occurring, followed by immunotherapy (n = 986) and CAR (n = 894). To provide a more visual representation of keywords with varying frequencies, a density map () was constructed, incorporating keywords that appeared at least 50 times. The keyword network diagram () incorporates the keywords (130 in total) that occur at least 30 times. Subsequently, cluster analysis yielded three distinct clusters, each representing a primary research area. The most substantial cluster, denoted as cluster 1 (red), encompasses keywords such as CAR, immunotherapy, adoptive cell therapy, cancer, leukemia, solid tumors, efficacy, persistence, exhaustion, resistance, expansion, cytotoxicity, checkpoint inhibitors, and TME. Cluster 2 (green) includes CAR-T, ALL, NHL, MM, BCMA, bone marrow transplantation (BMW), survival, safety, term-follow-up, cytokine release syndrome (CRS), immune effector cell-associated neurotoxicity syndrome (ICANS), diagnosis, tocilizumab, and management. Cluster 3 (blue) includes CD19, lymphoma, hematological malignancies, CLL, clinical trials, and adverse events.
Figure 5. The co-occurrence density map (a) and network (b) of keywords about this research field.
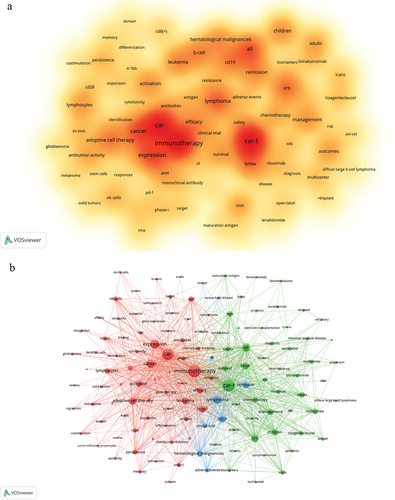
Table 4. Top 20 keywords about this research field.
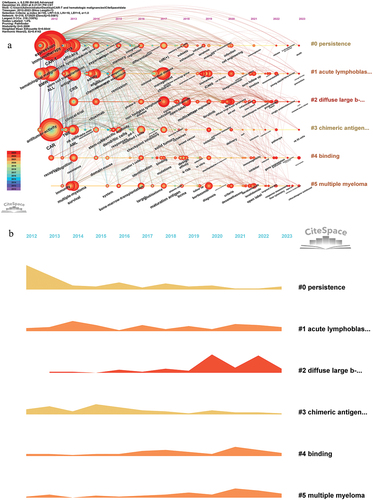
By utilizing the Timeline viewer of keywords, researchers can discern the dynamic trends and evolutionary patterns of keywords within the research field over time. This can help researchers understand the development of research fields, changes in research hotspots, and the evolution of disciplines. Utilizing the aforementioned keywords, we have built a timeline viewer () that provides a comprehensive depiction of the progression of keywords within this research domain since 2012, along with the primary research themes associated with each phase. Furthermore, this viewer has successfully generated 6 distinct clusters, which will be thoroughly elucidated in the subsequent discussion section. Additionally, visually presents the temporal dynamics of this field by employing a scientific approach to quantify the various keyword categories.
Co-cited articles and reference burst
When multiple articles reference the same article, a co-citation relationship emerges among these articles. Such co-cited articles can be considered influential and significant, as they have been cited by numerous independent researchers or research teams. To investigate the prominent areas of interest and significant research topics within this field, we conducted a co-citation analysis of these articles. presents a compilation of the ten articles exhibiting the highest citation frequency, encompassing the period from 2014 to 2020. Among these articles, the one that has garnered the most frequent citations (n = 781) is “Tisagenlecleucel in children and young adults with B-cell lymphotropic Leukemia,” authored by Maude et al. and published in 2018.Citation17
Table 5. The top 10 co-cited articles r about this research field.
To delve deeper into the burgeoning research areas within this particular field of study, we conducted a comprehensive citation burst analysis on the relevant scholarly papers. A total of 493 articles exhibiting citation bursts were identified, with 92 of them being in an urgent state, constituting 18.7% of the overall corpus (as depicted in Annexes 2). This finding implies that the field in question will continue to garner significant attention in the foreseeable future. The publication timeline of these papers predominantly spans from 2017 to 2021. Subsequently, the 92 papers were meticulously examined, and 23 articles published post-2019 were meticulously selected, summarized, and subjected to further analysis ().
Table 6. The references (in the state of citation burstiness) about this research field.
Discussion
With the breakthrough of CAR-T cell therapy in the treatment of hematological malignancies, this therapy has occupied an important position in the treatment of hematological malignancies. Currently, a large number of articles about CAR-T cells in the treatment of hematological malignancies are published every year. The related data and knowledge in this research field are increasing year by year. To help researchers better grasp the general situation of this research field, determine the research direction, focus on research hotspots, and improve research efficiency, this study carried out bibliometrics analysis and knowledge-map analysis on related research fields in the field of CAR-T cell therapy for hematological malignancies. It aims to provide scientific and objective references for researchers to explore the general situation and development trend of this research field, focus on research hotspots, and explore emerging research topics.
General information
CAR-T cell therapy has achieved remarkable success in the treatment of hematological malignancies. The advancement of CAR-T cell technology has been rapid, leading to the approval of six CAR-T cell therapies by the US Food and Drug Administration (FDA). This development has instilled newfound hope among numerous patients afflicted with hematological malignancies. Notably, the mature CAR-T cell therapies primarily target CD19 (2/3) and subsequently BCMA (1/3). Furthermore, China has also authorized two domestic CAR-T cell therapies specifically designed for CD19. Since 2018, there has been a significant surge in the registration of clinical trials within this particular research domain.Citation50 To date, a total of 1509 clinical trials about CAR-T cells have been registered on ClinicalTrials.gov. Our investigation reveals a consistent upward trajectory in the exploration of CAR-T cells for the treatment of hematological malignancies. While it is anticipated that the number of articles published in 2023 may marginally surpass that of 2022, amounting to a total of 788 articles, the overall trend is expected to gradually stabilize and reach a plateau. Currently, a substantial number of clinical trials continue to be registered annually in this domain. As pertinent clinical experimental data is obtained, a significant volume of associated scholarly articles will persistently emerge in the forthcoming period.
Through the analysis of countries, institutions, periodicals, and authors, important information related to this field has been obtained. Notably, the United States has emerged as the unequivocal frontrunner in this realm, both in terms of national contributions and research establishments. Our research showed that the United States and the University of Pennsylvania contributed the most publications. First, the United States has strong scientific research capabilities and financial support in the fields of scientific research and biomedicine. The American government, private foundations, and biotechnology companies have invested heavily in biomedical research, which provides sufficient resources and support for the advancement of CAR-T cell therapy. Secondly, the University of Pennsylvania has a first-class medical research team and facilities. The Abramson Cancer Center of the school has been devoted to the research of cancer immunotherapy, with a wealth of clinical expertise and scientific achievements. Notably, the center has made an important breakthrough in CAR-T cell therapy, and its research team is in an international leading position in clinical trials and technical improvement of CAR-T cell therapy. Moreover, the scientific research environment and legal system in the United States are relatively open, which is conducive to scientists and research institutions to carry out cutting-edge scientific research and clinical practice. This environment provides favorable conditions for the research and application of CAR-T cell therapy. In conclusion, the United States and the University of Pennsylvania are in a leading position in the field of CAR-T cell therapy due to their strong scientific research capabilities, financial support, first-class research team, and research environment, as well as rich clinical experience and scientific achievements. These factors make them the leaders in the field of CAR-T cell therapy.
Presently, China has registered a total of 749 clinical trials associated with CAR-T cells, constituting 49.6% of the overall count, whereas the United States accounts for 541 trials, representing 35.9% of the total (ClinicalTrials.gov). However, the United States surpasses China in terms of sample size, experimental scale, and experimental time span. Additionally, our findings indicate that the collaboration among the top ten countries in this research domain is primarily limited to their respective nations, with minimal engagement with other countries. Over the past two years, these countries have exhibited a dearth of new collaborative efforts with external counterparts. This phenomenon can potentially be attributed to factors such as technical conservatism, patent protection, and economic considerations. Consequently, the absence of inter-country cooperation may impede the progress and advancement of this field to a certain extent. Furthermore, we obtained a list of important journals and some authors in this field (see ). For example, our research indicates that the New England Journal of Medicine is the most frequently cited journal within this particular research field. By reviewing the related articles of the journal, we found that this journal published some important clinical studies related to this research field. These studies have been widely referenced by researchers due to their significant guidance and reference value. Through the analysis of journals and cited journals, we can better understand the influence, characteristics, and development trends of journals and better evaluate the influence of research. By searching the literature of these authors, we can quickly understand the development trend, research direction, and research hotspots in this research field.Citation51
Research hotspot analysis
To delve deeper into the prevailing areas of research and significant research subjects within this domain, we undertook a co-citation analysis of these articles, ultimately yielding the articles with the most notable co-citation frequency () ,Citation17–20 This collection of 10 studies primarily focuses on the application of CD19-CAR-T cells in the treatment of hematological malignancies (ALL and large B-cell lymphoma). Out of these, 6 studies specifically address the use of CD19-CAR-T cells in ALL,Citation17–26 while 4 studies explore their efficacy in large B-cell lymphoma.Citation18,Citation19,Citation21,Citation25 Additionally, 2 studies investigate the long-term follow-up and safety of CD19-CAR-T cells in the treatment of hematological malignancies,Citation20,Citation21 while another study examines the management of toxic reactions associated with CD19-CAR-T cell therapy.Citation26 The CD19-CAR-T cells employed in the aforementioned ten studies are all FDA-approved CAR-T cell products, namely tisagenlecleucel, axicabtagene ciloleucel, and lisocabtagene maraleucel. These studies mainly focus on the efficacy, safety, long-term follow-up, and toxic reaction management of CD19-CAR-T cells in hematological malignancies. These are research hotspots and important research topics in this field.
It has been proved that CAR-T cell therapy has a significant remission rate for hematological malignancies. Several meta-analysis studies have reported that a) For patients with relapsed or refractory B-cell acute lymphoblastic leukemia (R/R B-ALL), the total remission rate is found to be 76% (95% CI, 0.71–0.81), accompanied by a median survival time of 36.2 monthsCitation52; b) CD19-CAR-T cells demonstrate a favorable curative effect on B-cell lymphoma, with an overall remission rate of 63% (95% CI, 0.41–0.85)Citation53; c) In patients with relapsed or refractory acute myeloid leukemia (RR-AML), the total remission rate achieved with CD19-CAR-T cell therapy reaches 65.2% (95% CI, 0.36–0.91)Citation54; d)BCMA-CAR-T cells exhibit significant efficacy in patients with multiple myeloma (MM), resulting in a remarkable total remission rate of 80.5% (95% CI, 0.74–0.86).Citation55 Nonetheless, the persistent issue of high recurrence rate remains a significant concern that demands attention. A meta-analysis explored this area.Citation56 The findings revealed that following the administration of CAR-T cell therapy, the overall recurrence rate within one year was approximately 61% (95% CI, 43%-78%). Furthermore, the overall recurrence rate after one year was determined to be 24% (95% CI, 11%-42%). Multiple factors, including drug resistance, CAR-T cell failure, immune evasion, and the loss or down-regulation of tumor cell surface antigen, may contribute to this phenomenon,Citation57–59 Finding and identifying predictive biomarkers associated with long-term relapse-free survival following CAR-T cell infusion (e.g., CAR-T cell copy number and minimal residual disease) is important for early intervention in patients with poor prognosis.Citation60 Furthermore, several challenges persist in the application of CAR-T cells in hematological malignancies, including tumor heterogeneity, absence of tumor-specific antigens (TSA), and CAR-T cell exhaustion.Citation54
The assessment of efficacy and safety has consistently served as the primary metrics for evaluating the caliber of CAR-T cell therapy. During the administration of CD19-CAR-T cell therapy, patients typically experience varying degrees of toxic responses, including CRS, neurotoxicity, B cell dysplasia, CAR-T cell infusion-related infection (CTI), and hematological toxicity.Citation61,Citation62 Enhancing the safety of CAR-T cells while concurrently enhancing their therapeutic efficacy represents the principal avenue for optimizing this therapeutic approach. Researchers have concentrated their efforts on refining the structure of CAR to enhance the efficacy and/or safety of CAR-T cells, which has remained a focal point within this realm of research.Citation51 Since its proposal in 1989, the concept of CAR has developed into the fifth generation CAR. The first generation of CARs lacked the necessary second signal for cell proliferation within their intracellular domain, resulting in limited in vivo persistence and proliferation capabilities. However, the subsequent second generation CARs incorporated an active domain of costimulatory signaling within their intracellular domain, thereby enhancing the proliferation and persistence of CAR-T cells. Building upon this progress, the third generation CARs added two costimulatory signaling active domains. The inclusion of an anti-tumor cytokine receptor gene or suicide gene in the intracellular domain of the fourth generation CAR enhanced the anti-tumor efficacy and safety of CAR-T cells. The fifth generation of CARs (universal CARs) was to further transform its extracellular domain to enable it to recognize different tumor antigens. Despite the current global approval of CAR-T cells primarily based on second-generation CAR-T technology, researchers continue to actively optimize and enhance the structure of CARs. With the further development of CAR-T cell therapy, the third, fourth, and fifth generation CAR-T cells, and other innovative therapeutic strategies are expected to achieve more obvious advantages. Some CARs with special structures or systems can also improve the efficacy and safety of CAR-T cells, such as serial CAR, AND-gate CAR, ON/OFF-switch CAR, and inhibitory CAR.Citation9 Furthermore, the advent of CRISPR/Cas9 technology offers a convenient and efficient method for designing and optimizing CAR-T cell therapy,Citation63–65 In recent years, the concomitant utilization of CAR-T cell therapy in conjunction with other therapeutic modalities has demonstrated favorable therapeutic outcomes.Citation66,Citation67 These modalities encompass radiotherapy,Citation68 immune checkpoint inhibitors,Citation11,Citation69 small molecule drugs (e.g., lenalidomide), and nanotechnology.Citation70 Nevertheless, additional research and extensive clinical experimentation are imperative to ascertain the efficacy and safety of these CAR structures’ design and the approach to combined therapy.
Hotspot evolution and emerging topics
By analyzing keywords, we can understand the scope and characteristics of the research topics and fields. The application of keyword network analysis yielded three distinct clusters. The first cluster primarily encompasses investigations into adoptive cell therapy for cancer, specifically focusing on CAR-T cell therapy. The second cluster centers around CAR-T cell research in hematological malignancies, encompassing inquiries into therapeutic efficacy, long-term prognostic outcomes, safety considerations, and toxic reactions. The third cluster pertains predominantly to research concerning CD19-CAR-T cells in lymphoma and chronic lymphocytic leukemia (CLL).
The keyword timeline viewer () offers insights into the progression of research hotspots within this field and highlights the focal points of investigation during specific periods. This tool yields a wealth of valuable information. Notably, clusters #1, #2, #4, and #5 have exhibited a heightened generation of novel keywords in the past three years, signifying these research fields have high activity. Additionally, the evolution of thematic trends can also be discerned through this analysis. For example, in cluster#2, it is evident that the emergence of concerns regarding cytokine release syndrome (CRS) in the context of CAR-T cell therapy for acute lymphoblastic leukemia (ALL) occurred around 2013, followed by an increased focus on the safety aspects of CAR-T cell therapy around 2014. Since 2015, there has been a notable emphasis on investigating strategies to alleviate and manage the toxic reactions induced by CAR-T cells, which has become a significant area of research. Furthermore, in approximately 2019, immune effector cell-associated neurotoxicity syndrome (ICANS) also gained attention as an independent research topic. Presently, the long-term follow-up of CAR-T cell therapy in 2021 has emerged as a prominent research area.
This study has acquired a total of 23 references with strong citation bursts (), and through our inductive analysis, we have identified six emerging topics about this research domain, as outlined below:
Study on drug resistance related to CAR-T cellsCitation28,Citation45;
CD19-CAR-T cell transfusion-related infections (CTI)Citation34;
Combination of CD19-CAR-T cells and Ibrutinib in the treatment of CLLCitation42;
Application of CRISPR/Cas9 technology in CAR-T cellsCitation36,Citation47;
Application of chimeric antigen receptor macrophages (CAR-M) and CAR-NK cells in the treatment of hematological malignanciesCitation44,Citation48;
The application of tandem CAR-T cells in hematological malignancies, such as CD19-CD20-CAR-T for NHL and CLL,Citation29,Citation37 CD19-CD22-CAR-T for ALL,Citation40 and BCMA-GPRC5D-CAR-T for MM.Citation38
Based on scientific statistics and analysis, six emerging topics have been obtained, offering researchers a valuable point of reference. Despite the notable remission rates of CAR-T cells in treating hematological malignancies, their propensity for recurrence remains considerably elevated. The drug resistance of CAR-T cells is a significant factor that hinders their efficacy. Enhancing the curative potential of CAR-T cells necessitates addressing this drug resistance. Approaches to overcome drug resistance include optimizing the structure of CAR, enhancing the durability of CAR-T cells, mitigating the inhibitory impact of TME, and combining with other therapies.Citation71 CTI refers to an infection event that occurs in patients receiving CAR-T cell therapy. The incidence of CTI may be influenced by various factors, such as pre-treatment immunosuppression, lymphocyte depletion during treatment, post-treatment CRS, and neurotoxicity. These factors have the potential to compromise the patient’s immune system, thereby increasing the susceptibility to infections.Citation62,Citation72 One study indicated that the overall occurrence of CTI was as high as 33.8%, with severe infections accounting for 16.2% of cases, predominantly bacterial or viral in nature.Citation73 Consequently, it is imperative to diligently monitor infection-related complications, implement effective preventive measures, and gain further insights into the underlying mechanisms. Ibrutinib, a small molecule BTK (Bruton tyrosine kinase) inhibitor, functions by obstructing the B cell receptor signaling pathway, thereby impeding the proliferation and survival of malignant cells. Several clinical trials have demonstrated that the concurrent administration of CD19-CAR-T cells and ibrutinib exhibits a synergistic effect, leading to a reduction in adverse events and partially overcoming ibrutinib drug resistance in the treatment of CLL, small lymphocytic lymphoma (SLL), and mantle cell lymphoma.Citation42–76 Consequently, the combination of these two therapeutic approaches can further augment efficacy and safety, ultimately improving tumor remission rates.
The CRISPR/Cas9 technology is a highly efficient and accurate gene editing tool that offers cost-effective advantages. Currently, this technology has gained widespread application in cancer treatment. The utilization of CRISPR/Cas9 editing technology to enhance and refine CAR-T cells represents a highly promising area of research.Citation63 CRISPR/Cas9 technology has unique advantages in improving the function of CAR-T cells, including knocking out immune checkpoint genes to eliminate the inhibitory effect of immune checkpoints, enhancing the anti-exhaustion ability of CAR-T cells and assisting in the construction of universal CAR-T cells.Citation77 Dötsch et al.Citation78 found that the knockout of the PD-1 gene did not damage the function and life span of CAR-T cells. Following PD-1 knockout in CAR-T cells, the presence of CAR-T cells was consistently observed in vivo for a period exceeding 390 days. These findings suggest that PD-1 gene knockout can be applied to engineered T cell-based immunotherapies to overcome immune checkpoint suppression. Good et al.Citation79 discovered a novel mechanism leading to CAR-T cell exhaustion, namely the conversion of CD8 +T cells to NK-like T cells, and identified the transcription factors ID3 and SOX4 as key contributors to cell dysfunction. They further showed that CRISPR/Cas9 technology-mediated knockouts of ID3 and SOX4 can delay CAR-T cell exhaustion. Moreover, CRISPR/Cas9 technology can also indirectly reduce the treatment cost of CAR-T cells by shortening the manufacturing time of CAR-T cells (reducing the culture cost of CAR-T cells) and improving the persistence/proliferation ability (reducing the dosage of subsequent CAR-T cells).Citation65,Citation77 Consequently, it is of great practical value to construct CAR-T cells based on CRISPR/Cas9 technology. Furthermore, CAR-M cellsCitation80,Citation81 and CAR-NK cells,Citation82,Citation83 as extensions of CAR-T cell technology, have also demonstrated efficacy in the treatment of hematological malignancies and solid tumors. For example, researchers from The University of Texas MD Anderson Cancer Center conducted a phase I/II clinical trial.Citation84 In this latest clinical trial, 37 patients with recurrent or refractory B-cell malignant tumors received cord blood-derived CAR-NK cell therapy targeting CD19. The results showed that the overall response rate was 48.6% after 100 days of treatment, and the one-year progression-free survival rate and overall survival rate were 32% and 68% respectively. The clinical trial reported excellent safety without severe cytokine release syndrome, neurotoxicity, or graft-versus-host disease.Citation84 In recent years, the tandem chimeric antigen receptor (CAR) structure has gained significant popularity in the design of CAR-T cells due to its distinct advantages. This structure expands the recognition range of tumor antigens of CAR-T cells, and it will produce synergistic stimulation when recognizing and combining two tumor antigens at the same time, further enhancing the anti-tumor effect of CAR-T cells.
Furthermore, two articles about solid tumors were identified through citation burst analysis ().Citation44,Citation47 This phenomenon is due to the fact that these two documents have been cited many times by researchers in the field of “CAR-T cell therapy for hematological malignancies” in a short time. This indicates that these articles may hold significant research and reference value within this specific field, resulting in their frequent citation. These two studies involve the field of CAR-M cells and the application of CRISPR/Cas9 technology in the construction of CAR-T cells respectively. Klichinsky et al.Citation39 showed that CAR-M cells can specifically phagocytize tumor cells and exhibit good infiltration ability in solid tumors. Moreover, CAR-M cells can stimulate the anti-tumor immune activity of T cells by shaping the inflammatory tumor microenvironment. Tang et al.Citation42 knocked out endogenous TGF-β receptor II (TGFBR2) in CAR-T cells by CRISPR/Cas9 technology, which not only reduced the exhaustion of CAR-T cells but also improved the anti-tumor activity of CAR-T cells in tumor microenvironment rich in TGF-β. After analysis, we found that although these two articles involved solid tumor research, their core content was still close to the emerging topics 4 and 5 summarized above.
CAR-T cell therapy exhibits significant promise and potential, as evidenced by its notable achievements in the management of hematological malignancies. In the future, CAR-T cell therapy is expected to be extended to other types of cancer and play a greater role in individualized treatment. Furthermore, the technical improvement of CAR-T cell therapy and the accumulation of clinical experience will further improve the therapeutic effect and safety. Nevertheless, several challenges persist in the realm of CAR-T cell therapy, including drug resistance, elevated recurrence rates, toxic reactions, and limited in vivo persistence. Thus, further research and development are needed to solve these problems to further improve CAR-T therapy.
Conclusion
Miao et al.Citation51 have done bibliometrics research on the whole field of CAR-T cells. They included related articles and reviews from 2009 to 2021. Different from them, this study focuses on the related research of CAR-T in the treatment of hematological malignancies (excluding the literature related to solid tumors) and only includes relevant articles. Consequently, the literature retrieval strategy of this study is more targeted. Additionally, this study also included the studies in 2022 and 2023. Due to different research directions and emphases, the information obtained in this study, especially the research hotspots and emerging topics, is different from the research of Miao et al.Citation51 (readers can read and compare themselves).
CAR-T cell therapy has emerged as a prominent approach for the treatment of hematological malignancies, leading to a substantial increase in the number of scholarly publications dedicated to this area of research. Consequently, our study aimed to comprehensively analyze this research domain through bibliometric analysis. Our study indicates a steady growth in related publications within this research field over the years. The countries, research institutions, journals, and scientists that contributed the most to publications are the United States, the University of Pennsylvania, Frontiers in Immunology, and Carl H. June. The most frequently cited journal is the New England Journal of Medicine. The scientists with more cited frequency and co-cited frequency are Carl H. June and Shannon L. Maude respectively. We not only shed light on its overall landscape but also investigated its developmental trajectory and the evolution of prominent research areas. Furthermore, we identified the prevailing research hotspots and delineated six emerging themes, which may provide more targeted guidance and more valuable reference for researchers in the field of CAR-T cell therapy for hematological malignancies.
Author contributions
Q.H.: Writing-Original draft preparation, manuscript, investigation, and figure preparation. H.L.: Investigation, figure preparation, manuscript. Y.Z.: Conceptualization, Methodology, Supervision, manuscript. All authors approved the final version of the manuscript and agreed to be accountable for all specs of the work.
Annexes 2.pdf
Download PDF (16 MB)Figure S1.pdf
Download PDF (131.7 KB)Annexes 1.pdf
Download PDF (96.1 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2024.2371664
Additional information
Funding
References
- Cheng Q, Tan J, Liu R, Kang L, Zhang Y, Wang E, Li Y, Zhang J, Xiao H, Xu N, et al. CD20-specific chimeric antigen receptor-expressing T cells as salvage therapy in rituximab-refractory/relapsed B-cell non-Hodgkin lymphoma. Cytotherapy. 2022;24(10):1026–18. Epub 2022/06/13. doi: 10.1016/j.jcyt.2022.05.001. PubMed PMID: 35691818.
- Pan J, Tang K, Luo Y, Seery S, Tan Y, Deng B, Liu F, Xu X, Ling Z, Song W, et al. Sequential CD19 and CD22 chimeric antigen receptor T-cell therapy for childhood refractory or relapsed B-cell acute lymphocytic leukaemia: a single-arm, phase 2 study. Lancet Oncol. 2023;24(11):1229–41. Epub 2023/10/21. doi: 10.1016/s1470-2045(23)00436-9. PubMed PMID: 37863088.
- Wang T, Tang Y, Cai J, Wan X, Hu S, Lu X, Xie Z, Qiao X, Jiang H, Shao J, et al. Coadministration of CD19- and CD22-Directed Chimeric Antigen Receptor T-Cell Therapy in Childhood B-Cell Acute Lymphoblastic Leukemia: A Single-Arm, Multicenter, Phase II Trial. J Clin Oncol. 2023;41(9):1670–83. Epub 2022/11/09. doi: 10.1200/jco.22.01214. PubMed PMID: 36346962; PubMed Central PMCID: PMCPMC10419349.
- Tschernia NP, Heiling H, Deal AM, Cheng C, Babinec C, Gonzalez M, Morrison JK, Dittus C, Dotti G, Beaven AW, et al. Patient-reported outcomes in CD30-directed CAR-T cells against relapsed/refractory CD30+ lymphomas. J Immunother Cancer. 2023;11(8). Epub 2023/08/02. e006959. doi: 10.1136/jitc-2023-006959. PubMed PMID: 37527906; PubMed Central PMCID: PMCPMC10394544.
- Tambaro FP, Singh H, Jones E, Rytting M, Mahadeo KM, Thompson P, Daver N, DiNardo C, Kadia T, Garcia-Manero G, et al. Autologous CD33-CAR-T cells for treatment of relapsed/refractory acute myelogenous leukemia. Leukemia. 2021;35(11):3282–6. Epub 2021/04/10. doi: 10.1038/s41375-021-01232-2. PubMed PMID: 33833386; PubMed Central PMCID: PMCPMC8550958.
- Li H, Li J, Wu J, Shi Z, Gao Y, Song W, Li J, Li Z, Zhang M. A second-generation CD38-CAR-T cell for the treatment of multiple myeloma. Cancer Med. 2023;12(9):10804–15. Epub 2023/04/12. doi: 10.1002/cam4.5818. PubMed PMID: 37039305; PubMed Central PMCID: PMCPMC10225187.
- Liu M, Zhang L, Zhong M, Long Y, Yang W, Liu T, Huang X, Ma X. CRISPR/Cas9-mediated knockout of intracellular molecule SHP-1 enhances tumor-killing ability of CD133-targeted CAR T cells in vitro. Exp Hematol Oncol. 2023;12(1):88. Epub 2023/10/07. doi: 10.1186/s40164-023-00450-x. PubMed PMID: 37803455; PubMed Central PMCID: PMCPMC10559533.
- Jetani H, Navarro-Bailón A, Maucher M, Frenz S, Verbruggen C, Yeguas A, Vidriales MB, González M, Rial Saborido J, Kraus S, et al. Siglec-6 is a novel target for CAR T-cell therapy in acute myeloid leukemia. Blood. 2021;138(19):1830–42. Epub 2021/07/22. doi: 10.1182/blood.2020009192. PubMed PMID: 34289026; PubMed Central PMCID: PMCPMC9642786.
- Miao L, Zhang J, Huang B, Zhang Z, Wang S, Tang F, Teng M, Li Y. Special chimeric antigen receptor (CAR) modifications of T cells: a review. Frontiers in oncology. Front Oncol. 2022;12:832765. Epub 2022/04/09. doi: 10.3389/fonc.2022.832765. PubMed PMID: 35392217; PubMed Central PMCID: PMCPMC8981721.
- Wang C, Yu M, Zhang W. Neoantigen discovery and applications in glioblastoma: an immunotherapy perspective. Cancer Lett. 2022;550:215945. Epub 2022/10/11. doi: 10.1016/j.canlet.2022.215945. PubMed PMID: 36216148.
- Chong EA, Alanio C, Svoboda J, Nasta SD, Landsburg DJ, Lacey SF, Ruella M, Bhattacharyya S, Wherry EJ, Schuster SJ, et al. Pembrolizumab for B-cell lymphomas relapsing after or refractory to CD19-directed CAR T-cell therapy. Blood. 2022;139(7):1026–38. Epub 2021/09/09. doi: 10.1182/blood.2021012634. PubMed PMID: 34496014; PubMed Central PMCID: PMCPMC9211527.
- Zhou M, Chen M, Shi B, Di S, Sun R, Jiang H, Li Z. Radiation enhances the efficacy of EGFR-targeted CAR-T cells against triple-negative breast cancer by activating NF-κB/Icam1 signaling. Mol Ther. 2022;30(11):3379–93. Epub 2022/08/06. doi: 10.1016/j.ymthe.2022.07.021. PubMed PMID: 35927951; PubMed Central PMCID: PMCPMC9637637.
- Wang AX, Ong XJ, D’Souza C, Neeson PJ, Zhu JJ. Combining chemotherapy with CAR-T cell therapy in treating solid tumors. Front Immunol. 2023;14:1140541. Epub 2023/03/24. doi: 10.3389/fimmu.2023.1140541. PubMed PMID: 36949946; PubMed Central PMCID: PMCPMC10026332.
- Chen C, Hu Z, Liu S, Tseng H. Emerging trends in regenerative medicine: a scientometric analysis in CiteSpace. Expert opinion on biological therapy. 2012;12(5):593–608. Epub 2012/03/27. doi: 10.1517/14712598.2012.674507. PubMed PMID: 22443895.
- Chen C. Searching for intellectual turning points: progressive knowledge domain visualization. Proc Natl Acad Sci USA. 2004;101(Suppl 1):5303–10. Epub 2004/01/16. doi: 10.1073/pnas.0307513100. PubMed PMID: 14724295; PubMed Central PMCID: PMCPMC387312.
- van Eck NJ, Waltman L, van Eck NJ. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. 2010;84(2):523–38. Epub 2010/06/30. doi: 10.1007/s11192-009-0146-3. PubMed PMID: 20585380; PubMed Central PMCID: PMCPMC2883932.
- Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, Bader P, Verneris MR, Stefanski HE, Myers GD, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439–48. Epub 2018/02/01. doi: 10.1056/NEJMoa1709866. PubMed PMID: 29385370; PubMed Central PMCID: PMCPMC5996391.
- Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, Braunschweig I, Oluwole OO, Siddiqi T, Lin Y, et al. Axicabtagene ciloleucel CAR T-Cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531–44. Epub 2017/12/12. doi: 10.1056/NEJMoa1707447. PubMed PMID: 29226797; PubMed Central PMCID: PMCPMC5882485.
- Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, Jäger U, Jaglowski S, Andreadis C, Westin JR, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45–56. Epub 2018/12/07. doi: 10.1056/NEJMoa1804980. PubMed PMID: 30501490.
- Park JH, Rivière I, Gonen M, Wang X, Sénéchal B, Curran KJ, Sauter C, Wang Y, Santomasso B, Mead E, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med. 2018;378(5):449–59. Epub 2018/02/01. doi: 10.1056/NEJMoa1709919. PubMed PMID: 29385376; PubMed Central PMCID: PMCPMC6637939.
- Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, Oluwole OO, Lin Y, Braunschweig I, Hill BT, Timmerman JM, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol. 2019;20(1):31–42. Epub 2018/12/07. doi: 10.1016/s1470-2045(18)30864-7. PubMed PMID: 30518502; PubMed Central PMCID: PMCPMC6733402.
- Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–17. Epub 2014/10/16. doi: 10.1056/NEJMoa1407222. PubMed PMID: 25317870; PubMed Central PMCID: PMCPMC4267531.
- Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, Fry TJ, Orentas R, Sabatino M, Shah NN, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet (London, England). Lancet. 2015;385(9967):517–28. Epub 2014/10/17. doi: 10.1016/s0140-6736(14)61403-3. PubMed PMID: 25319501; PubMed Central PMCID: PMCPMC7065359.
- Turtle CJ, Hanafi LA, Berger C, Gooley TA, Cherian S, Hudecek M, Sommermeyer D, Melville K, Pender B, Budiarto TM, et al. CD19 CAR–T cells of defined CD4+: CD8+ composition in adult B cell ALL patients. J Clin Invest. 2016;126(6):2123–38. Epub 2016/04/26. doi: 10.1172/jci85309. PubMed PMID: 27111235; PubMed Central PMCID: PMCPMC4887159.
- Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, Mehta A, Purev E, Maloney DG, Andreadis C, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet (Lond, Engl). 2020;396(10254):839–52. Epub 2020/09/06. doi: 10.1016/s0140-6736(20)31366-0. PubMed PMID: 32888407.
- Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, Chung SS, Stefanski J, Borquez-Ojeda O, Olszewska M, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Science translational medicine. Sci Transl Med. 2014;6(224):224ra25. Epub 2014/02/21. doi: 10.1126/scitranslmed.3008226. PubMed PMID: 24553386; PubMed Central PMCID: PMCPMC4684949.
- Nastoupil LJ, Jain MD, Feng L, Spiegel JY, Ghobadi A, Lin Y, Dahiya S, Lunning M, Lekakis L, Reagan P, et al. Standard-of-Care axicabtagene ciloleucel for relapsed or refractory large B-cell lymphoma: results from the US lymphoma CAR T consortium. J Clin Oncol. 2020;38(27):3119–28. Epub 2020/05/14. doi: 10.1200/jco.19.02104. PubMed PMID: 32401634; PubMed Central PMCID: PMCPMC7499611.
- Jacobson CA, Hunter BD, Redd R, Rodig SJ, Chen PH, Wright K, Lipschitz M, Ritz J, Kamihara Y, Armand P, et al. Axicabtagene ciloleucel in the non-trial setting: outcomes and correlates of response, resistance, and toxicity. J Clin Oncol. 2020;38(27):3095–106. Epub 2020/07/16. doi: 10.1200/jco.19.02103. PubMed PMID: 32667831; PubMed Central PMCID: PMCPMC7499617.
- Shah NN, Johnson BD, Schneider D, Zhu F, Szabo A, Keever-Taylor CA, Krueger W, Worden AA, Kadan MJ, Yim S, et al. Bispecific anti-CD20, anti-CD19 CAR T cells for relapsed B cell malignancies: a phase 1 dose escalation and expansion trial. Nat Med. 2020;26(10):1569–75. Epub 2020/10/07. doi: 10.1038/s41591-020-1081-3. PubMed PMID: 33020647.
- Dean EA, Mhaskar RS, Lu H, Mousa MS, Krivenko GS, Lazaryan A, Bachmeier CA, Chavez JC, Nishihori T, Davila ML, et al. High metabolic tumor volume is associated with decreased efficacy of axicabtagene ciloleucel in large B-cell lymphoma. Blood Adv. 2020;4(14):3268–76. Epub 2020/07/24. doi: 10.1182/bloodadvances.2020001900. PubMed PMID: 32702097; PubMed Central PMCID: PMCPMC7391155.
- Shah NN, Highfill SL, Shalabi H, Yates B, Jin J, Wolters PL, Ombrello A, Steinberg SM, Martin S, Delbrook C, et al. CD4/CD8 T-cell selection affects chimeric antigen receptor (CAR) T-cell potency and toxicity: updated results from a phase I Anti-CD22 CAR T-cell trial. J Clin Oncol. 2020;38(17):1938–50. Epub 2020/04/15. doi: 10.1200/jco.19.03279. PubMed PMID: 32286905; PubMed Central PMCID: PMCPMC7280047.
- Brudno JN, Lam N, Vanasse D, Shen YW, Rose JJ, Rossi J, Xue A, Bot A, Scholler N, Mikkilineni L, et al. Safety and feasibility of anti-CD19 CAR T cells with fully human binding domains in patients with B-cell lymphoma. Nat Med. 2020;26(2):270–80. Epub 2020/01/22. doi: 10.1038/s41591-019-0737-3. PubMed PMID: 31959992; PubMed Central PMCID: PMCPMC7781235.
- Parker KR, Migliorini D, Perkey E, Yost KE, Bhaduri A, Bagga P, Haris M, Wilson NE, Liu F, Gabunia K, et al. Single-cell analyses identify brain mural cells expressing CD19 as potential off-tumor targets for CAR-T immunotherapies. Cell. 2020;183(1):126–42.e17. Epub 2020/09/23. doi: 10.1016/j.cell.2020.08.022. PubMed PMID: 32961131; PubMed Central PMCID: PMCPMC7640763.
- Logue JM, Zucchetti E, Bachmeier CA, Krivenko GS, Larson V, Ninh D, Grillo G, Cao B, Kim JC, Chavez J, et al. Immune reconstitution and associated infections following axicabtagene ciloleucel in relapsed or refractory large B-cell lymphoma. Haematologica. 2021;106(4):978–86. Epub 2020/04/25. doi: 10.3324/haematol.2019.238634. PubMed PMID: 32327504; PubMed Central PMCID: PMCPMC8017820.
- Kadauke S, Myers RM, Li Y, Aplenc R, Baniewicz D, Barrett DM, Barz Leahy A, Callahan C, Dolan JG, Fitzgerald JC, et al. Risk-adapted preemptive tocilizumab to prevent severe cytokine release syndrome after CTL019 for pediatric B-cell acute lymphoblastic leukemia: a prospective clinical trial. J Clin Oncol. 2021;39(8):920–30. Epub 2021/01/09. doi: 10.1200/jco.20.02477. PubMed PMID: 33417474; PubMed Central PMCID: PMCPMC8462622.
- Stadtmauer EA, Fraietta JA, Davis MM, Cohen AD, Weber KL, Lancaster E, Mangan PA, Kulikovskaya I, Gupta M, Chen F, et al. CRISPR-engineered T cells in patients with refractory cancer. Sci (New Y, NY). 2020;367(6481):eaba7365. Epub 2020/02/08. doi: 10.1126/science.aba7365. PubMed PMID: 32029687.
- Tong C, Zhang Y, Liu Y, Ji X, Zhang W, Guo Y, Han X, Ti D, Dai H, Wang C, et al. Optimized tandem CD19/CD20 CAR-engineered T cells in refractory/relapsed B-cell lymphoma. Blood. 2020;136(14):1632–44. Epub 2020/06/20. doi: 10.1182/blood.2020005278. PubMed PMID: 32556247; PubMed Central PMCID: PMCPMC7596761 interests.
- de Larrea C F, Staehr M, Lopez AV, Ng KY, Chen Y, Godfrey WD, Purdon TJ, Ponomarev V, Wendel H-G, Brentjens RJ, et al. Defining an optimal dual-targeted CAR T-cell therapy approach simultaneously targeting BCMA and GPRC5D to prevent BCMA escape–driven relapse in multiple Myeloma. Blood Cancer Discov. 2020;1(2):146–54. Epub 2020/10/23. doi: 10.1158/2643-3230.Bcd-20-0020. PubMed PMID: 33089218; PubMed Central PMCID: PMCPMC7575057.
- Awasthi R, Pacaud L, Waldron E, Tam CS, Jäger U, Borchmann P, Jaglowski S, Foley SR, van Besien K, Wagner-Johnston ND, et al. Tisagenlecleucel cellular kinetics, dose, and immunogenicity in relation to clinical factors in relapsed/refractory DLBCL. Blood advances. Blood Adv. 2020;4(3):560–72. Epub 2020/02/12. doi: 10.1182/bloodadvances.2019000525. PubMed PMID: 32045475; PubMed Central PMCID: PMCPMC7013261.
- Dai H, Wu Z, Jia H, Tong C, Guo Y, Ti D, Han X, Liu Y, Zhang W, Wang C, et al. Bispecific CAR-T cells targeting both CD19 and CD22 for therapy of adults with relapsed or refractory B cell acute lymphoblastic leukemia. J Hematol Oncol. 2020;13(1):30. Epub 2020/04/05. doi: 10.1186/s13045-020-00856-8. PubMed PMID: 32245502; PubMed Central PMCID: PMCPMC7126394.
- Lonial S, Lee HC, Badros A, Trudel S, Nooka AK, Chari A, Abdallah A-O, Callander N, Lendvai N, Sborov D, et al. Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): a two-arm, randomised, open-label, phase 2 study. Lancet Oncol. 2020;21(2):207–21. Epub 2019/12/21. doi: 10.1016/s1470-2045(19)30788-0. PubMed PMID: 31859245.
- Gauthier J, Hirayama AV, Purushe J, Hay KA, Lymp J, Li DH, Yeung CCS, Sheih A, Pender BS, Hawkins RM, et al. Feasibility and efficacy of CD19-targeted CAR T cells with concurrent ibrutinib for CLL after ibrutinib failure. Blood. 2020;135(19):1650–60. Epub 2020/02/23. doi: 10.1182/blood.2019002936. PubMed PMID: 32076701; PubMed Central PMCID: PMCPMC7205814.
- Sheih A, Voillet V, Hanafi LA, DeBerg HA, Yajima M, Hawkins R, Gersuk V, Riddell SR, Maloney DG, Wohlfahrt ME, et al. Clonal kinetics and single-cell transcriptional profiling of CAR-T cells in patients undergoing CD19 CAR-T immunotherapy. Nat Commun. 2020;11(1):219. Epub 2020/01/12. doi: 10.1038/s41467-019-13880-1. PubMed PMID: 31924795; PubMed Central PMCID: PMCPMC6954177.
- Klichinsky M, Ruella M, Shestova O, Lu XM, Best A, Zeeman M, Schmierer M, Gabrusiewicz K, Anderson NR, Petty NE, et al. Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat Biotechnol. 2020;38(8):947–53. Epub 2020/05/04. doi: 10.1038/s41587-020-0462-y. PubMed PMID: 32361713; PubMed Central PMCID: PMCPMC7883632.
- Asnani M, Hayer KE, Naqvi AS, Zheng S, Yang SY, Oldridge D, Ibrahim F, Maragkakis M, Gazzara MR, Black KL, et al. Retention of CD19 intron 2 contributes to CART-19 resistance in leukemias with subclonal frameshift mutations in CD19. Leukemia. 2020;34(4):1202–7. Epub 2019/10/09. doi: 10.1038/s41375-019-0580-z. PubMed PMID: 31591467; PubMed Central PMCID: PMCPMC7214268.
- Jackson Z, Roe A, Sharma AA, Lopes F, Talla A, Kleinsorge-Block S, Zamborsky K, Schiavone J, Manjappa S, Schauner R, et al. Automated manufacture of autologous CD19 CAR-T cells for treatment of non-Hodgkin lymphoma. Front Immunol. 2020;11:1941. Epub 2020/08/28. doi: 10.3389/fimmu.2020.01941. PubMed PMID: 32849651; PubMed Central PMCID: PMCPMC7427107.
- Tang N, Cheng C, Zhang X, Qiao M, Li N, Mu W, Wei X-F, Han W, Wang H. TGF-β inhibition via CRISPR promotes the long-term efficacy of CAR T cells against solid tumors. JCI Insight. 2020;5(4):e133977. Epub 2020/01/31. doi: 10.1172/jci.insight.133977. PubMed PMID: 31999649; PubMed Central PMCID: PMCPMC7101140.
- Liu E, Marin D, Banerjee P, Macapinlac HA, Thompson P, Basar R, Nassif Kerbauy L, Overman B, Thall P, Kaplan M, et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N Engl J Med. 2020;382(6):545–53. Epub 2020/02/06. doi: 10.1056/NEJMoa1910607. PubMed PMID: 32023374; PubMed Central PMCID: PMCPMC7101242.
- Wang N, Hu X, Cao W, Li C, Xiao Y, Cao Y, Gu C, Zhang S, Chen L, Cheng J, et al. Efficacy and safety of CAR19/22 T-cell cocktail therapy in patients with refractory/relapsed B-cell malignancies. Blood. 2020;135(1):17–27. Epub 2019/11/08. doi: 10.1182/blood.2019000017. PubMed PMID: 31697824.
- Yang Z, Wang Y. Clinical development of chimeric antigen receptor-T cell therapy for hematological malignancies. Chin Med J. 2023;136(19):2285–96. Epub 2023/06/26. doi: 10.1097/cm9.0000000000002549. PubMed PMID: 37358555; PubMed Central PMCID: PMCPMC10538902.
- Miao L, Zhang J, Zhang Z, Wang S, Tang F, Teng M, Li Y. A bibliometric and knowledge-map analysis of CAR-T cells from 2009 to 2021. Front Immunol. 2022;13:840956. Epub 2022/04/05. doi: 10.3389/fimmu.2022.840956. PubMed PMID: 35371087; PubMed Central PMCID: PMCPMC8971369.
- Elsallab M, Ellithi M, Hempel S, Abdel-Azim H, Abou-El-Enein M. Long-term response to autologous anti-CD19 chimeric antigen receptor T cells in relapsed or refractory B cell acute lymphoblastic leukemia: a systematic review and meta-analysis. Cancer gene therapy. 2023;30(6):845–54. Epub 2023/02/08. doi: 10.1038/s41417-023-00593-3. PubMed PMID: 36750666; PubMed Central PMCID: PMCPMC10281866.
- Zheng XH, Zhang XY, Dong QQ, Chen F, Yang SB, Li WB. Efficacy and safety of chimeric antigen receptor-T cells in the treatment of B cell lymphoma: a systematic review and meta-analysis. Chin Med J. 2020;133(1):74–85. Epub 2020/01/11. doi: 10.1097/cm9.0000000000000568. PubMed PMID: 31923107; PubMed Central PMCID: PMCPMC7028209.
- Shahzad M, Nguyen A, Hussain A, Ammad-Ud-Din M, Faisal MS, Tariq E, Ali F, Butt A, Anwar I, Chaudhary SG, et al. Outcomes with chimeric antigen receptor t-cell therapy in relapsed or refractory acute myeloid leukemia: a systematic review and meta-analysis. Front Immunol. 2023;14:1152457. Epub 2023/05/12. doi: 10.3389/fimmu.2023.1152457. PubMed PMID: 37168849; PubMed Central PMCID: PMCPMC10164930.
- Roex G, Timmers M, Wouters K, Campillo-Davo D, Flumens D, Schroyens W, Chu Y, Berneman ZN, Lion E, Luo F, et al. Safety and clinical efficacy of BCMA CAR-T-cell therapy in multiple myeloma. J Hematol Oncol. 2020;13(1):164. Epub 2020/12/05. doi: 10.1186/s13045-020-01001-1. PubMed PMID: 33272302; PubMed Central PMCID: PMCPMC7713173.
- Zinzi A, Gaio M, Liguori V, Cagnotta C, Paolino D, Paolisso G, Castaldo G, Nicoletti GF, Rossi F, Capuano A, et al. Late relapse after CAR-T cell therapy for adult patients with hematologic malignancies: a definite evidence from systematic review and meta-analysis on individual data. Pharmacol Res. 2023;190:106742. Epub 2023/03/25. doi: 10.1016/j.phrs.2023.106742. PubMed PMID: 36963592.
- Zeng W, Zhang P. Resistance and recurrence of malignancies after CAR-T cell therapy. Experimental cell research. 2022;410(2):112971. Epub 2021/12/16. doi: 10.1016/j.yexcr.2021.112971. PubMed PMID: 34906583.
- Zhang ZZ, Wang T, Wang XF, Zhang YQ, Song SX, Ma CQ. Improving the ability of CAR-T cells to hit solid tumors: challenges and strategies. Pharmacol Res. 2022;175:106036. Epub 2021/12/18. doi: 10.1016/j.phrs.2021.106036. PubMed PMID: 34920118.
- Kouro T, Himuro H, Sasada T. Exhaustion of CAR T cells: potential causes and solutions. J Transl Med. 2022;20(1):239. Epub 2022/05/24. doi: 10.1186/s12967-022-03442-3. PubMed PMID: 35606821; PubMed Central PMCID: PMCPMC9125881.
- Hong R, Hu Y, Huang H. Biomarkers for chimeric antigen receptor T cell therapy in acute lymphoblastic leukemia: prospects for personalized management and prognostic prediction. Front Immunol. 2021;12:627764. Epub 2021/03/16. doi: 10.3389/fimmu.2021.627764. PubMed PMID: 33717147; PubMed Central PMCID: PMCPMC7947199.
- Li Y, Ming Y, Fu R, Li C, Wu Y, Jiang T, Li Z, Ni R, Li L, Su H, et al. The pathogenesis, diagnosis, prevention, and treatment of CAR-T cell therapy-related adverse reactions. Front Pharmacol. 2022;13:950923. Epub 2022/11/01. doi: 10.3389/fphar.2022.950923. PubMed PMID: 36313336; PubMed Central PMCID: PMCPMC9616161.
- Miao L, Zhang Z, Ren Z, Li Y. Reactions related to CAR-T cell therapy. Front Immunol. 2021;12:663201. Epub 2021/05/18. doi: 10.3389/fimmu.2021.663201. PubMed PMID: 33995389; PubMed Central PMCID: PMCPMC8113953.
- Wei W, Chen ZN, Wang K. CRISPR/Cas9: a powerful strategy to improve CAR-T cell persistence. Int J Mol Sci. 2023;24(15):12317. Epub 2023/08/12. doi: 10.3390/ijms241512317. PubMed PMID: 37569693; PubMed Central PMCID: PMCPMC10418799.
- Dimitri A, Herbst F, Fraietta JA. Engineering the next-generation of CAR T-cells with CRISPR-Cas9 gene editing. Molecular cancer. 2022;21(1):78. Epub 2022/03/20. doi: 10.1186/s12943-022-01559-z. PubMed PMID: 35303871; PubMed Central PMCID: PMCPMC8932053.
- Naeem M, Hazafa A, Bano N, Ali R, Farooq M, Razak SIA, Lee TY, Devaraj S. Explorations of CRISPR/Cas9 for improving the long-term efficacy of universal CAR-T cells in tumor immunotherapy. Life Sci. 2023;316:121409. Epub 2023/01/22. doi: 10.1016/j.lfs.2023.121409. PubMed PMID: 36681183.
- Zhang X, Zhu L, Zhang H, Chen S, Xiao Y. CAR-T cell therapy in hematological malignancies: current opportunities and challenges. Front Immunol. 2022;13:927153. Epub 2022/06/28. doi: 10.3389/fimmu.2022.927153. PubMed PMID: 35757715; PubMed Central PMCID: PMCPMC9226391.
- Hovhannisyan L, Riether C, Aebersold DM, Medová M, Zimmer Y. CAR T cell-based immunotherapy and radiation therapy: potential, promises and risks. Mol Cancer. 2023;22(1):82. Epub 2023/05/13. doi: 10.1186/s12943-023-01775-1. PubMed PMID: 37173782; PubMed Central PMCID: PMCPMC10176707.
- Sugita M, Yamazaki T, Alhomoud M, Martinet J, Latouche JB, Golden E, Boyer O, Van Besien K, Formenti SC, Galluzzi L, et al. Radiation therapy improves CAR T cell activity in acute lymphoblastic leukemia. Cell Death Disease. 2023;14(5):305. Epub 2023/05/05. doi: 10.1038/s41419-023-05829-6. PubMed PMID: 37142568; PubMed Central PMCID: PMCPMC10160073.
- Luke JJ, Patel MR, Blumenschein GR, Hamilton E, Chmielowski B, Ulahannan SV, Connolly RM, Santa-Maria CA, Wang J, Bahadur SW, et al. The PD-1- and LAG-3-targeting bispecific molecule tebotelimab in solid tumors and hematologic cancers: a phase 1 trial. Nat Med. 2023;29(11):2814–24. Epub 2023/10/20. doi: 10.1038/s41591-023-02593-0. PubMed PMID: 37857711; PubMed Central PMCID: PMCPMC10667103.
- Xiao X, Ma Z, Li Z, Deng Y, Zhang Y, Xiang R, Zhu L, He Y, Li H, Jiang Y, et al. Anti-BCMA surface engineered biomimetic photothermal nanomissile enhances multiple myeloma cell apoptosis and overcomes the disturbance of NF-κB signaling in vivo. Biomaterials. 2023;297:122096. Epub 2023/04/20. doi: 10.1016/j.biomaterials.2023.122096. PubMed PMID: 37075614.
- Ruella M, Korell F, Porazzi P, Maus MV. Mechanisms of resistance to chimeric antigen receptor-T cells in haematological malignancies. Nature reviews drug discovery. 2023;22(12):976–95. Epub 2023/11/01. doi: 10.1038/s41573-023-00807-1. PubMed PMID: 37907724.
- Wudhikarn K, Perales MA. Infectious complications, immune reconstitution, and infection prophylaxis after CD19 chimeric antigen receptor T-cell therapy. Bone marrow transplantation. 2022;57(10):1477–88. Epub 2022/07/16. doi: 10.1038/s41409-022-01756-w. PubMed PMID: 35840746; PubMed Central PMCID: PMCPMC9285870.
- Telli Dizman G, Aguado JM, Fernández-Ruiz M. Risk of infection in patients with hematological malignancies receiving CAR T-cell therapy: systematic review and meta-analysis. Expert review of anti-infective therapy. 2022;20(11):1455–76. Epub 2022/09/24. doi: 10.1080/14787210.2022.2128762. PubMed PMID: 36148506.
- Siddiqi T, Soumerai JD, Dorritie KA, Stephens DM, Riedell PA, Arnason J, Kipps TJ, Gillenwater HH, Gong L, Yang L, et al. Phase 1 TRANSCEND CLL 004 study of lisocabtagene maraleucel in patients with relapsed/refractory CLL or SLL. Blood. 2022;139(12):1794–806. Epub 2021/10/27. doi: 10.1182/blood.2021011895. PubMed PMID: 34699592; PubMed Central PMCID: PMCPMC10652916.
- Gill S, Vides V, Frey NV, Hexner EO, Metzger S, O’Brien M, O’Brien, M., Hwang, WT, Brogdon, J.L., Davis, MM, Fraietta, JA, Gaymon, AL. Anti-CD19 CAR T cells in combination with ibrutinib for the treatment of chronic lymphocytic leukemia. Blood Adv. 2022;6(21):5774–85. Epub 2022/03/30. doi: 10.1182/bloodadvances.2022007317. PubMed PMID: 35349631; PubMed Central PMCID: PMCPMC9647791.
- Minson AG, Hamad N, Cheah CY, Tam CS, Blombery P, Westerman DA, Ritchie DS, Morgan H, Holzwart N, Lade S, et al. CAR T-cells and time-limited Ibrutinib as treatment for relapsed/refractory mantle cell lymphoma: phase II TARMAC study. Blood J. 2023;143(8):673–84. Epub 2023/10/26. doi: 10.1182/blood.2023021306. PubMed PMID: 37883795.
- Tao R, Han X, Bai X, Yu J, Ma Y, Chen W, Zhang D, Li Z. Revolutionizing cancer treatment: enhancing CAR-T cell therapy with CRISPR/Cas9 gene editing technology. Front Immunol. 2024;15:1354825. Epub 2024/03/07. doi: 10.3389/fimmu.2024.1354825. PubMed PMID: 38449862; PubMed Central PMCID: PMCPMC10914996.
- Dötsch S, Svec M, Schober K, Hammel M, Wanisch A, Gökmen F, Jarosch S, Warmuth L, Barton J, Cicin-Sain L, et al. Long-term persistence and functionality of adoptively transferred antigen-specific T cells with genetically ablated PD-1 expression. Proc Natl Acad Sci USA. 2023;120(10):e2200626120. Epub 2023/03/01. doi: 10.1073/pnas.2200626120. PubMed PMID: 36853939; PubMed Central PMCID: PMCPMC10013756.
- Good CR, Aznar MA, Kuramitsu S, Samareh P, Agarwal S, Donahue G, Ishiyama K, Wellhausen N, Rennels AK, Ma Y, et al. An NK-like CAR T cell transition in CAR T cell dysfunction. Cell. 2021;184(25):6081–100.e26. Epub 2021/12/04. doi: 10.1016/j.cell.2021.11.016. PubMed PMID: 34861191; PubMed Central PMCID: PMCPMC8827167.
- Li W, Wang F, Guo R, Bian Z, Song Y. Targeting macrophages in hematological malignancies: recent advances and future directions. J Hematol Oncol. 2022;15(1):110. Epub 2022/08/18. doi: 10.1186/s13045-022-01328-x. PubMed PMID: 35978372; PubMed Central PMCID: PMCPMC9387027.
- Liu M, Liu L, Song Y, Li W, Xu L. Targeting macrophages: a novel treatment strategy in solid tumors. J Transl Med. 2022;20(1):586. Epub 2022/12/13. doi: 10.1186/s12967-022-03813-w. PubMed PMID: 36510315; PubMed Central PMCID: PMCPMC9743606.
- Li Y, Basar R, Wang G, Liu E, Moyes JS, Li L, Kerbauy LN, Uprety N, Fathi M, Rezvan A, et al. KIR-based inhibitory CARs overcome CAR-NK cell trogocytosis-mediated fratricide and tumor escape. Nat Med. 2022;28(10):2133–44. Epub 2022/09/30. doi: 10.1038/s41591-022-02003-x. PubMed PMID: 36175679; PubMed Central PMCID: PMCPMC9942695.
- Huang R, Wen Q, Zhang X. CAR-NK cell therapy for hematological malignancies: recent updates from ASH 2022. J Hematol Oncol. 2023;16(1):35. Epub 2023/04/08. doi: 10.1186/s13045-023-01435-3. PubMed PMID: 37029381; PubMed Central PMCID: PMCPMC10082521.
- Marin D, Li Y, Basar R, Rafei H, Daher M, Dou J, Mohanty V, Dede M, Nieto Y, Uprety N, et al. Safety, efficacy and determinants of response of allogeneic CD19-specific CAR-NK cells in CD19(+) B cell tumors: a phase 1/2 trial. Nat Med. 2024;30(3):772–84. Epub 2024/01/19. doi: 10.1038/s41591-023-02785-8. PubMed PMID: 38238616; PubMed Central PMCID: PMCPMC10957466.