ABSTRACT
Multiple research studies have demonstrated the efficacy of lactic acid bacteria in boosting both innate and adaptive immune responses. We have created a Lactococcus lactis variant that produces a modified combination protein with Fms-like tyrosine kinase 3 ligand and co-stimulator O × 40 ligand, known as HuFOLactis. The genetically modified variant was purposely created to activate T cells, NK cells, and DC cells in a laboratory setting. Furthermore, we explored the possibility of using the tumor-penetrating peptide iRGD to deliver HuFOLactis-activated immune cells to hard-to-reach tumor areas. Following brief stimulation with HuFOLactis, immune cell phenotypes and functions were assessed using flow cytometry. Confocal microscopy was employed to demonstrate the infiltrative and cytotoxic capabilities of iRGD-modified HuFOLactis-activated immune cells within tumor spheroids. The efficacy of iRGD modified HuFOLactis-activated immune cells against tumors was assessed in xenograft mouse models. HuFOLactis treatment resulted in notable immune cell activation, demonstrated by elevated levels of CD25, CD69, and CD137. Additionally, these activated immune cells showed heightened cytokine production and enhanced cytotoxicity against MKN45 cell lines. Incorporation of the iRGD modification facilitated the infiltration of HuFOLactis-activated immune cells into multicellular spheroids (MCSs). Additionally, immune cells activated by HuFOLactis and modified with iRGD, in combination with anti-PD-1 treatment, effectively halted tumor growth and prolonged survival in a mouse model of gastric cancer.
Background
In the past decade, significant progress has been made in adoptive cell transfer (ACT) therapy for cancer.Citation1 ACT can enhance immune system activity using various immune cells like T cells, natural killer cells, dendritic cells, and stem cells. The focus on ACT strategies is driven by the success of CD19 CAR-T cells in B-cell malignancies.Citation2 However, the complex and expensive process of generating these cells, along with safety concerns, hinders widespread adoption. Simplifying and reducing costs of ACT is crucial for clinical use.
Lactic acid bacteria boost immune responses by stimulating cell division, antibody production, and activating macrophages.Citation3 They also induce interferon production and enhance cytotoxicity.Citation4 Lactococcus lactis is a safe bacterium used in the food industry and is ideal for protein expression due to its safety record, lack of endotoxins, compact genome, and lower exoprotein levels compared to E. coli.Citation5 As research on the anti-tumor properties of Lactobacillus deepens, the development of novel anti-cancer drugs and the advancement of biotechnological approaches in cancer prevention hold significant importance.
T cells and natural killer (NK) cells collaborate to target cancer cells, with NK cells eliminating cells that evade T cell surveillance, thereby enhancing the efficacy of immunotherapy.Citation6 NK cells play a crucial role in recruiting dendritic cells (DCs) to tumors, which in turn amplify CD8+ T cell responses. The activation of NK cells is stimulated by IL-2 released from T cells.Citation6 The integration of these cellular components in immunotherapy holds significant potential for advancing cancer treatment, emphasizing the critical need for precise activation of T cells, NK cells, and DCs in vitro to optimize adoptive cell transfer.
The signaling of co-stimulatory molecules such as CD28, inducible T-cell co-stimulator (ICOS), CD226, OX40(CD134) and its ligand OX40L (CD252), and 4-1BB (CD137) is crucial for the effective activation, proliferation, and differentiation of T cells.Citation7 In comparison to CD28 and ICOS, OX40 exhibits certain advantages.Citation8 The OX40L-mediated co-stimulation of T cells results in the activation of the NF-κB promoter, which in turn triggers the production of Th1 cytokines, cell proliferation, and a decreased susceptibility to suppression by regulatory T cells (Treg).Citation9
DCs play a critical role in initiating immune responses through the presentation of antigens to T cells and the stimulation of their activation.Citation10 The bidirectional interactions between DCs and NK cells, known as cross-talk, involve the formation of conjugates and impact the strength and efficacy of both innate and adaptive immune responses.Citation11 The active pursuit of compounds that enhance the expansion of DCs populations is motivated by their link to a positive prognosis in cancer.Citation12 Flt3, a Fms-like tyrosine kinase, plays a major role in controlling the growth of DCs and promoting the multiplication of the initial DC progenitors.Citation13 A study demonstrated that Flt3L enhances the population of peripheral monocytes and cDCs, encompassing cDC1, cDC2, and plasmacytoid DCs, known for their cross-presentation ability. This augmentation was accompanied by significant enhancements in humoral and T-cell responses, as well as the activation of T cells, NK cells, DCs.Citation14
Drawing on the aforementioned theories, we developed a bifunctional engineered Lactococcus lactis (HuFOLactis). This system delivers a fused protein of Flt3L and co-stimulator OX40 ligand (OX40L), both of human origin, by encoding the respective genes into the bacteria for expression. The intratumoral administration of Lactis has demonstrated the potential to inhibit tumor growth by inducing cell lysis and releasing tumor-specific antigens. Furthermore, FOLactis expressing Flt3L can enhance conventional-type-1-dendritic cell (cDC1) expansion, while OX40L-expressing FOLactis can stimulate the activation of tumor-infiltrating effector T cells in mice. This dual mechanism leads to significant tumor suppression and elicits an abscopal effect through its in situ vaccination (ISV) properties. However, Due to the diversity of tumors and the variability in patient conditions, various immunotherapy approaches can be developed based on the FOLactis for the treatment of patients.
In this study, HuFOLactis was employed to stimulate PBMCs for adoptive cell therapy. The findings demonstrate that HuFOLactis can activate DCs, NK cells, and T cells. Furthermore, by modifying these activated cell subsets with iRGD, they can be precisely guided to infiltrate tumor sites, thereby augmenting anti-tumor responses synergistically. HuFOLactis provides a simple, cost-effective approach for transiently activating PBMCs ex vivo, with minimal procedural requirements and no need for extensive cell expansion. While its therapeutic efficacy is limited compared to established cell therapies like CAR-T and TCR-T, this method offers a convenient, effective, and economical option for cellular therapy, potentially serving as a supplementary treatment for specific patient cohorts.
Materials and methods
Reagents, bacterial strains, cell lines
MoBiTec (Germany) provided the Lactococcus lactis NZ9000 strain and the pNZ8148 vector. Escherichia coli strains were grown in Luria-Bertani (LB) broth at 37°C with agitation at 220 rpm. Lactis NZ9000 was grown without agitation in GM17 medium containing M17 (Difco) and 0.5% (w/v) glucose at 30°C. The MKN45 and HGC27 cell lines, which are human gastric adenocarcinoma cells, were acquired from the Cell Bank at the Shanghai Institute of Biochemistry and Cell Biology. Cell lines were grown in RPMI 1640 medium with 10% fetal bovine serum, 100 U/mL penicillin, and 100 µg/mL streptomycin at 37°C in a 5% CO2 environment. The use of Mycoplasma-free cells was ensured by phenotypic or genotypic analysis and Mycoplasma testing to confirm the validity of the cells.
Generation and characterization of HuFolactis
ICarTab Biomedical Co., Ltd. in Suzhou, China created DNA fragments of Flt3L-OX40L that were designed to be more compatible with humans. These fragments were then placed into bacterial expression vectors known as pNZ8148. 40 μl of competent cells were combined with 1 μg of the recombinant plasmid in double-distilled water for the process of electroporation. The combination of cell and plasmid was moved to an electroporation cuvette cooled on ice with a thickness of 0.2 mm, and then exposed to a single 2.5 kV pulse at 200 Ω using a Gene Pulser (Bio-Rad, USA). After electroporation, the cells were exposed to 1000 μl of a solution for recovery (GM17 broth with added 0.5 M sucrose, 20 mM MgCl2, and 20 mM CaCl2) and incubated at 30°C for 2 hours. Following that, 200 μl of the cell suspension was spread on GM17 agar with 10 μg/ml chloramphenicol and left to incubate for 2 days, leading to the successful creation of HuFOLactis. In order to activate protein production, HuFOLactis were grown in GM17 broth with an addition of 10 μg/ml chloramphenicol for one night. Subsequently, the culture was inoculated at a 1:100 ratio into fresh GM17 medium. When the OD600 reached 0.5 ~ 0.7, 10 ng/ml of nisin was introduced, and the cells were then cultured for an additional 3 hours. After centrifugation at 5000 g and 4°C for 15 minutes, the bacterial cells were collected to analyze protein expression using ELISA, aiming to identify the presence of human Flt3L and human OX40L fusion proteins in the bacterial cell lysates. SoftMax® Pro 6 software (version 6.4.2) and the SpectraMax® i3× multi-mode microplate reader (Molecular Devices) were utilized to document the findings from the ELISA test.
Preparing lysates or inactivating HuFOLactis for use
HuFOLactis were grown in GM17 liquid medium at 30°C without stirring. After reaching an optical density between 0.5 and 0.7, a concentration of 10 nanograms per milliliter of nisin was added, followed by an incubation period of 3 more hours. Afterward, bacterial cells were collected by spinning them in a centrifuge (5000 g, 4°C, 15 min), then suspended in PBS, and exposed to ultrasound using a Sonics VCX 130 ultrasound device (130 W, 20 kHz) for 30 minutes while on ice (5 son, 5 s off, 40% amplitude). After filtering the lysate (1010 CFU/ml) through a 0.2-μm filter, it was frozen and lyophilized to produce HuFOLactis lysates. Inactivated HuFOLactis was obtained by heating HuFOLactis in a 60°C water bath for 30 minutes followed by microbial culture. Upon confirmation of negative test results, in vitro cell experiments were initiated.
Dendritic cells uptake of HuFOLactis in vitro
Peripheral blood mononuclear cells (PBMCs) were isolated from donors using Ficoll density as a gradient medium and then suspended in AIM-V medium (Gibco, USA). All peripheral blood mononuclear cells were either used right away for tests or frozen in a safe liquid nitrogen storage unit. Human immature dendritic cells were produced by first subjecting PBMCs to a two-hour adherence culture. The adherent monocytes were then grown in AIM-V medium with human GM-CSF (500 U/ml, Peprotech) and IL-4 (500 U/mL, Peprotech) for 5 days. HuFOLactis cells labeled with DiO dye were mixed with dendritic cells labeled with Dil dye on the sixth day of the study. After a two-hour co-incubation, the cells were examined using a fluorescence microscope from OLYMPUS in Germany to determine their uptake by DCs. In order to study how HuFOLactis affects the maturation of DCs and their secretion of cytokines, a ratio of 1:10 of HuFOLactis was added to the DC culture on the sixth day and left to incubate for 48 hours. Afterward, dendritic cells were collected through centrifugation at 500 g for 5 minutes, then incubated with fluorescently labeled antibodies for 30 minutes at 4°C in the absence of light, followed by two washes and suspension in FACS buffer. The Human IL-12p70 Flex Set (BD Biosciences) was utilized to measure the single-cytokine IL-12p70. Samples were analyzed via flow cytometry using a Cytoflex flow cytometer, with data analysis carried out using FCAP version 3.0 array software from Soft Flow.
Immune cells activation and cytotoxicity assays
Dendritic cells (DCs) were incubated with HuFOLactis for 4–6 hours at 37°C, followed by rinsing with pre-heated PBS. Afterward, they were cultured with a mix of T cells and NK cells at a 1:10 ratio for 48 hours. After the samples were incubated, they were mixed in flow cytometry staining buffer (FACS) and then stained with the designated antibodies for 30 minutes at 4°C in the absence of light. Subsequently, they were washed twice before being resuspended in FACS buffer. Supernatant fluids were gathered to measure cytokine levels with either the BD CBA human IFN-γ kit or the BD CBA human Th1/Th2 kit.
Preparation of TM
The MKN45 cells were harvested during the logarithmic growth phase, and their membranes were isolated following established protocols. Specifically, the cells (1 × 108 cells ml–1) were lysed through repeated freeze-thaw cycles (10 min in liquid nitrogen followed by 10 min in a 37°C water bath, repeated at least six times). Subsequently, cellular debris was removed by centrifugation at 700 × g for 10 min. The MKN45 TM was then subjected to ultrasound treatment (Sonics VCX 130, USA; 130 W, 20 kHz) on ice for 2 min (with a 5 s on and off cycle at 50% amplitude), followed by centrifugation at 15,000 × g, 4°C for 30 min to collect the membranes, which were stored at −80°C for further analysis.
Oncoprotein-specific responses
Based on prior studies, lymphocytes were co-cultured with 50 μg of TM, 50 μg of TM combined with 107 Lactis lysates, or 50 μg of TM combined with 107 HuFOLactis lysates for the in vitro activation of long peptides.Citation15 Peripheral blood mononuclear cells were obtained from a donor in good health and grown in AIMV medium with the addition of GM-CSF, IL-4, and fetal bovine serum. Cells were placed in 96-well dishes with a concentration of 5 × 106 cells per well in 100 μl. Cells were exposed to 0.3 μg of resiquimod (R848, BIOFOUNT, China) on the first day, followed by treatment with 5 ng of LPS either 4 hours or 4.5 hours afterward. The cells were collected and suspended in AIMV medium supplemented with 10% FBS, 1% P/S, 50 ng/ml IL-7, and 24 IU/ml IL-2 on the second day. The medium was changed every 2–3 days up to day 10. Additionally, new cells were prepared following the same procedure on days 0–2 and added to the existing cells on day 8 for a 6-hour co-culture. Afterward, the cells were harvested through centrifugation and examined via flow cytometry utilizing antibodies against CD4-FITC, CD8-Percy5.5, IFN-γ-PE, and TNF-α-APC. We also determined the ratio of Tcm and Treg subgroups in the last cultured cells. According to our previous study, the method of intracellular cytokine detection was used.Citation16,Citation17
Flow cytometry
The phenotypic characterization of HuFOLactis activated NK and T cells involved the use of various antibody clones including anti-CD3-FITC (HIT3a, BD Bioscience), anti-CD4-Per-Cy5.5 (RPA-T4, BD Bioscience), anti-CD8-Per-Cy5.5 (RPA-T8, BD Bioscience), anti-CD8-APC (RPA-T8, BD Bioscience), anti-CD279-PE (MIH4, BD Bioscience), anti-CD45RO-APC (UCHL1, BD Bioscience), anti-CD62L-PE (DRGE-56, BD Bioscience), anti-CD25-APC (CD28.2, BD Bioscience), anti-CD69-PE (CD28.2, BD Bioscience), anti-CD56-Per-Cy5.5 (CD28.2, BD Bioscience), anti-CD137-PE (4B4-1, BD Biosciences), anti-Foxp3-PE (236A/E7, BD Bioscience), anti-CD366-PE (7D3, BD Bioscience), anti-IFN-γ-PE (B27, BD Bioscience), and anti-TNF-α-APC (MAb11, BD Bioscience). In the same way, DCs activated by HuFOLactis were collected and labeled with antibodies including anti-CD11C-FITC (B-ly6, BD Bioscience), anti-HLA-DR – Per-Cy5.5 (G46-6, BD Bioscience), anti-CD80-PE (L307.4, BD Bioscience), and anti-CD86-APC (FUN-1, BD Bioscience). Cytokine detection was performed using the BD CBA kits for human IFN-γ and IL-12p70. The BD CBA human Th1/Th2 kit from BD Biosciences was utilized for detecting multiple cytokines.
Synthesis of DSPEPEGiRGD
DSPE-PEG-Mal and C-iRGD were combined in equal proportions in a buffer solution and allowed to react for 48 hours at room temperature in a nitrogen atmosphere, with the pH maintained at 6.5. After that, the mixture was carefully dialyzed in pure water for two days to get rid of any excess iRGD. The liquid left behind was frozen, dried, and examined with some high-tech instruments to see what exactly was going on at a molecular level.
The effect of HuFOLactis actived lymphocytes modified with iRGD on multicellular spheroids
Due to the ineffectiveness of 2D cultures in cell infiltration studies, we developed three-dimensional multicellular spheres (MCS) utilizing the HGC27 gastric cancer cell line. As mentioned earlier, the HGC27 cells were placed in a 96-well dish containing RPMI1640 with 10% FBS.Citation16,Citation18 Every day, the plate was observed using a light microscope to confirm the development of consistent and dense spheroids measuring approximately 500 µm. After being cultured with HuFOLactis for 48 hours, PBMCs were labeled with CFSE and exposed to iRGD in the culture medium during the last 6 hours of cultivation. The iRGD modifed HuFOLactis activated PBMCs (HuFO-PBMC-iRGD) were then added to the spheroids at a ratio of 5:1. Following a 24-hour period of incubation at 37°C, the spheroids underwent a washing process, were then treated with 4% paraformaldehyde for fixation, and were subsequently analyzed using a ZEN 710 confocal microscope.
Xenograft mouse models
The research conducted in this study was authorized by the Ethics Committee of Drum Tower Hospital in Nanjing, China, and followed the guidelines set by the Animal Care Committee at the institution. The investigators were not blinded to the animal studies, and all possible measures were taken to minimize animal usage and alleviate their suffering. The allocation of mice was done randomly, taking into account their age and weight. Male BALB/c nude mice, aged 6–8 weeks, were injected with MKN45 cells (5 × 106 cells in 100 μl PBS) in the right axilla to create a subcutaneous tumor model.
In vivo antitumor efficacy
MKN45 cells were injected subcutaneously into the right axilla of female BALB/c nude mice aged 6–8 weeks to establish a subcutaneous tumor model, using 100 ml of PBS for suspension. PBMC-iRGD, HuFO-PBMC-iRGD, PD-1, or HuFO-PBMC-iRGD + anti-PD-1 were injected intravenously two weeks after tumor induction into the mice. Furthermore 50,000 units of synthetic human IL-2 were given through the peritoneum every second day following the transfer of cells. During adoptive cell transfer, the mice were weighed and examined for any signs of tumor growth. The tumor’s size was approximated by assuming it had an ellipsoid shape and then calculated using the formula length multiplied by the square of the width and then divided by 2. One week following the transfer of cells, flow cytometry was used to measure the levels of human T cells and DCs in the tumor tissue of mice treated with various cells. Furthermore, we identified the activation and depletion indicators of CD8+ T cells to illustrate the collaborative impacts in the immune system. Additionally, blood samples from mice were examined for serum markers including BUN, Cr, ALT, AST, IL-6, and IL-10. Finally, key organs were gathered, preserved in 4% paraformaldehyde, sliced, and stained with H&E to evaluate safety.
Statistical analysis
Statistical analysis was performed using GraphPad Prism v.9.0 statistical software (San Diego, CA, USA). All results were presented as mean ± s.e.m. The sample size for each experiment, n, is included in the results section and the associated figure legend. Statistical details for each experiment can be found in the figures and the legends. For all test, a p value of <.05 was considered significant. Flow cytometry data were acquired using CytoFLEX. Figures were created using Adobe Illustrator.
Results
Characterization of HuFolactis
In this study, we designed HuFOLactis, a similar fusion protein that expresses Flt3L and OX40L, both of human origin. Human Flt3L and human OX40L fusion proteins in bacterial cell lysates were detected by ELISA against human Flt3L and human OX40L, respectively, as shown in . Numerous studies suggest that inactivated bacteria exhibit immunomodulatory effects that closely resemble those seen in live bacteria. Inactivation leading to loss of viability and cell lysis can result in more intricate immunomodulation than anticipated.Citation19 In order to evaluate the anti-cancer properties, we carried out experiments both in live organisms and in a controlled environment using deactivated HuFOLactis.
Figure 1. HuFOLactis was characterized and it was found to be effective in boosting the activation of DCs. (a) The schematic shows intravenous injection of PBMCs modified with iRGD and activated with HuFOLactis, along with a PD-1 mAb, for improved tumor immunotherapy. Created with MedPeer. (b) The quantification of the target protein in the bacterial lysates of HuFOLactis was carried out. Bacteria (109 CFU) were collected and then sonicated to isolate the pellets. The assessment of the target protein levels in the bacterial lysates of HuFOLactis was conducted through ELISA (n = 5 biologically independent samples). (c) Analysis of the colocalization of HuFOLactis (DiO; green) in PBMC-derived DCs (DiI; red) was conducted using confocal microscopy after a two-hour incubation period. We depicted a representative out of three independent experiments yielding similar results. (d) Representative flow plots illustrating the expression levels of CD80, CD86, and HLA-DR on CD11c+ DC cells following a 48-hour in vitro co-incubation with Lactis or HuFOLactis are presented. (e) Summary of data from D showing CD80+, CD86+, and HLA-DR+ cells among CD11c+ cells (mean ± s.e.m.; n = 3 cell cultures per group). (f) IL-12p70 concentrations in DCs supernatants (mean ± s.e.m.; n = 3 cell cultures per group). (g) The influence of inactivated HuFOLactis on IL-12p70 secretion and CD80 upregulation in dendritic cells (mean ± s.e.m.; n = 3 cell cultures per group). Data were analyzed by one-way ANOVA coupled with Tukey’s multiple-comparisons test. For experiments C-G, three independent experiments were performed using PBMCs from three donors, with three cell cultures per group in each experiment. The displayed result is representative of one of these three independent experiments.
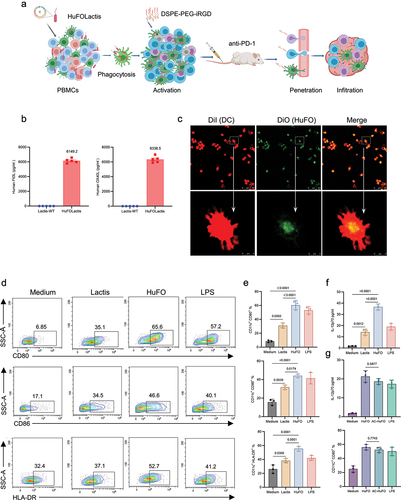
HuFolactis efficiently induced the activation of DCs in vitro
Dendritic cells (DCs) are a crucial subset of antigen-presenting cells (APCs) that specialize in priming various effector T cells, thereby influencing the immune response outcome. They play a central role in the immune system, possessing a distinctive capability to regulate both immunity and tolerance.Citation20 Hence, it is essential to trigger the proper maturation of DCs to enhance anti-tumor immunity and prevent cancer-induced immunosuppression. First, we examined the internalization of inactivated-HuFOLactis (HuFO) by DCs. HuFOLactis labeled with DiO were co-cultured with DCs labeled with Dil for a duration of two hours, followed by imaging using a fluorescence microscope to evaluate the internalization process within DCs. As shown in , after the internalization of HuFOLactis by DCs, the majority of it was confined within lysosomes. The impact of HuFOLactis on DC phenotype and cytokine secretion was then scrutinized, and the outcomes demonstrated certain variations, as depicted in .
The induction of DC mature was significantly increased with the addition of HuFOLactis. After 48 hours of HuFOLactis stimulation, flow cytometry plots revealed that DCs displayed a significant increase in CD80, CD86, and HLA-DR expression compared to DCs stimulated with Lactis (). This was supported by statistical analysis (; p = .0002, p = .0174, p = .0051, respectively). The gating strategy of dendritic cells is depicted in Supplementary Figure S2. In addition, DCs treated with HuFOLactis produced increased amounts of IL-12p70 in comparison to DCs treated with Lactis (; p = .0011). At the same time, we verified the effects of activated-HuFOLactis and inactivated- HuFOLactis on DCs phenotype and cytokine secretion. As shown in , there were no statistically significant changes in IL-12p70 secretion and CD80 expression of DCs stimulated by activated-HuFOLactis and inactivated- HuFOLactis (p = .5977, p = .7743, separately). Results from the study suggested that both activated and inactivated HuFOLactis could act as immune stimulatory agents, which could be phagocytosed by DCs, leading to an increase in DCs costimulatory molecules and cytokine secretion. In summary, the findings indicated that dendritic cells exposed to deactivated HuFOLactis resulted in activation, demonstrated by the heightened expression of costimulatory molecules and release of IL-12p70.
A short-term co-culture of PBMCs with HuFolactis
We studied the impact of HuFOLactis on NK and T cells. After 2 days of treatment with inactive Lactis or HuFOLactis in vitro, T cells and NK cells in both groups exhibited higher levels of CD25, CD69, and CD137 compared to the control group. Data from showed that T cells in the HuFOLactis group exhibited higher levels of CD25, CD69, and CD137 compared to the Lactis group (p = .0017, p = .0414, p = .0046, respectively). showed that NK cells from the HuFOLactis group had increased expression of CD25 and CD69 compared to the Lactis group (p = .0006, p = .0025, respectively).
Figure 2. After co-culturing PBMCs with HuFOLactis, both T cells and NK cells can be activated. (a, b) Representative flow plots showing the expression of CD25, CD69, and CD137 on CD3+ T cells and CD56+ NK cells after co-incubation with Lactis or HuFOLactis in vitro for 48 hours. (c) Summary of data from a showing CD25+, CD69+, and CD137+ cells among CD3+ T cells (mean ± s.e.m.; n = 3 cell cultures per group). (d) Summary of data from B showing CD25+, CD69+, and CD137+ cells among CD56+ NK cells (mean ± s.e.m.; n = 3 cell cultures per group). (e) Assessment of Th1/Th2 in coculture supernatants after PBMCs stimulated by Lactis or HuFOLactis for 48 hours in vitro (mean ± s.e.m.; n = 3 cell cultures per group). Data were analyzed by one-way ANOVA coupled with Tukey’s multiple-comparisons test. Three independent experiments were performed using PBMCs from three donors, with three cell cultures per group in each experiment. The displayed result is representative of one of these three independent experiments.
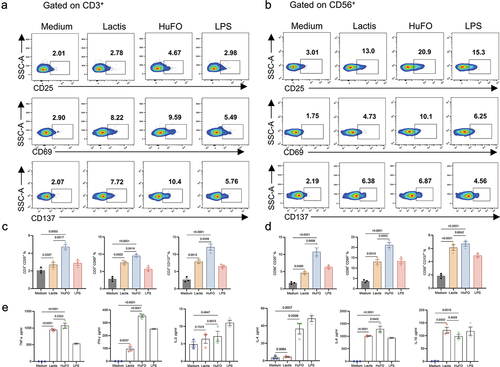
TNF-α, a cytokine produced by immune cells, can impede tumor growth and promote degenerative alterations in tumors.Citation21,Citation22 IFN-γ plays a crucial role in anti-tumor defense by inhibiting cell growth, promoting apoptosis, and enhancing the immune response, thereby aiding in the identification and elimination of cancerous cells.Citation23 The effectiveness of T and NK cells stimulated by HuFOLactis was confirmed by collecting cell supernatants and analyzing them with a BD CBA human Th1/Th2 kit after 48 hours of co-culture to quantify cytokine production. As shown in , IFN-γ and TNF-α showed higher secretion levels in the HuFOLactis group when compared to the Lactis group (p = .0352 and p = .0297, respectively). Regarding the immune suppressive function, we observed that the levels of cytokines IL-10 and IL-6 in the HuFOLactis and Lactis groups were comparable, showing no significant difference. Meanwhile, results demonstrate that HuFOLactis triggers an elevation in IL-4 production (p = .0008). However, the concentration of IL-4 is relatively low in comparison to other factors, particularly IFN-γ.
Numerous research studies have provided evidence of crosstalk and interactions between dendritic cells (DCs), T cells, and natural killer (NK) cells.Citation6,Citation11,Citation24–29 To assess interactions between different cell types, we utilized magnetic bead separation to isolate T cells, NK cells, and DC cells from PBMCs (Supplementary Figure S2a). When adding FOLactis in specific proportions to six groups of cell culture mediums (NK, T, NK+T, NK+DC, T+DC, NK+T+DC), the expression of CD69 on NK and T cells, as well as the secretion of IFN-γ in the culture supernatant, were assessed after 48 hours (Supplementary Figure S2a). The results revealed that in the three mixed cell culture groups, the expression of CD69 on T and NK cells was the highest (Supplementary Figure S2b,c), along with the highest secretion of IFN-γ in the culture supernatant (Supplementary Figure S2d). These findings suggest a mutual activation effect among the three cell types, indicating that the anti-tumor effect is expected to be optimal when mixed cell populations are reinfused in vivo.
Taken together, co-culturing HuFOLactis and PBMCs for a short period of time can activate NK cells and T cells, increase the expression of surface activation markers, and stimulate the production of anti-tumor cytokines.
HuFolactis enhance the induction of oncoprotein-specific T cells
OX40 inhibits the function of natural T regulatory (Treg) cells and counteracts the development of inducible Tregs.Citation30 The validation of the OX40L/OX40 pathway also suggests its involvement in promoting the transformation of inexperienced and experienced T cells into T follicular helper cells, while also blocking the inhibitory activity of Tregs and regulatory T follicular helper cells. Activation of OX40(CD134) through OX40L (CD134L) or OX40 agonists has been shown to boost CD4 and CD8 T-cell reactions.Citation31,Citation32
To further explore if HuFOLactis can increase the induction of oncoprotein-specific T cells in vitro, we collected PBMCs from healthy individuals and co-cultured them with crude lysates from HuFOLactis or Lactis, as well as MKN45 tumor cell membranes (TM). Following a 14-day culture period, the cells were collected and assessed for their capacity to generate IFN-γ and TNF-α when exposed to the MKN45 cells. Combining PBMCs with unrefined extracts from HuFOLactis and MKN45 cancer cell membranes (HuFO+TM) enhances the proportion of cells expressing IFN-γ and TNF-α. The HuFO+TM group had higher levels of CD4+ IFN-γ+ and CD8+ IFN-γ+ positive cells compared to the Lactis+TM group (p = .0032 and p = .0031, respectively), as shown in . Similarly, the levels of CD4+ TNF-α+ and CD8+ TNF-α+ cells were elevated in the HuFO+TM group compared to the Lactis+TM group (p = .0010 and p = .0024), as depicted in . On day 14, we also measured the proportion of Tregs, as shown in . The HuFO+TM group had a lower number of CD4+ CD25+ FoxP3+ cells compared to the Lactis+TM group (p = .0014). Central memory T cells in the HuFO+TM group had a higher percentage of CD3+ CD45RO+ CD62L+ cells compared to the Lactis+TM group (p = .0011), as illustrated in . We also conducted a reanalysis of CD25 expression in both Foxp3 positive and Foxp3 negative groups to enhance clarity regarding changes in the effector population (Supplementary Figure S3A). There is statistical significance in the expression of CD25 within the FoxP3 negative group (Supplementary Figure S3b). Meanwhile, the ratio of FoxP3−CD25+/FoxP3+CD25+ in the HuFOLactis group is greater than that in the Lactis group (Supplementary Figure S3c).
Figure 3. HuFOLactis enhance the induction of oncoprotein-specific T cells. PBMCs were co-cultured with crude lysates from HuFOLactis or lactis, as well as MKN45 tumor cell membranes (TM). After 14 days of culture, cells were harvested and tested for their ability to produce IFN-γ and TNF-α in response to the MKN45 cells. (a, e) Representative flow plots and graphs showing the expression of IFN-γ on CD4+ and CD8+ T cells (mean ± s.e.m.; n = 3 cell cultures per group). (b, f) Representative flow plots and graphs showing the expression of TNF-α on CD4+ and CD8+ T cells (mean ± s.e.m.; n = 3 cell cultures per group). (c, g) Representative flow plots and graphs showing the Tregs in the final cultured cell product (mean ± s.e.m.; n = 3 cell cultures per group). (d, h) Representative flow plots and graphs showing central memory T cells in the final cultured cell product (mean ± s.e.m.; n = 3 cell cultures per group). Data were analyzed by one-way ANOVA coupled with Tukey’s multiple-comparisons test. Three independent experiments were performed using PBMCs from three donors, with three cell cultures per group in each experiment. The displayed result is representative of one of these three independent experiments.
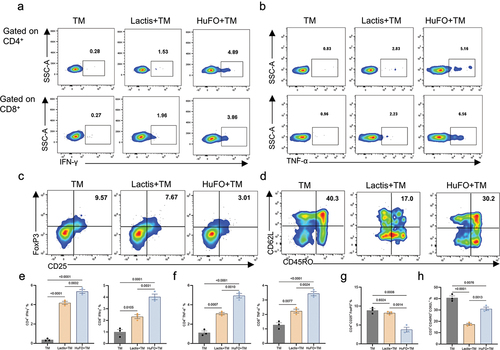
Following the above observations, our conclusion is that HuFOLactis impacts the phenotype of T cells, either through its direct actions or metabolites. This impact leads to a decrease in Treg cell proportion while preserving central memory T cell proportion, offering potential benefits for augmenting anti-tumor treatment.
HuFolactis activated PBMCs modified with iRGD possess superior penetration capacity in MCSs
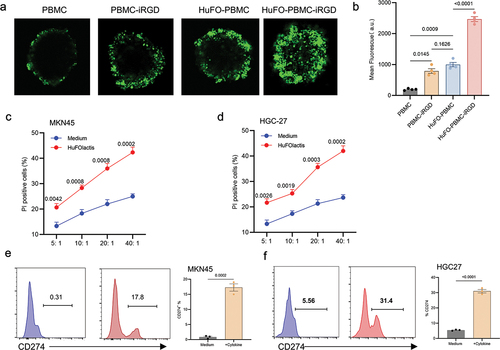
A 3D multicellular sphere (MCS) was constructed using HGC27 gastric cancer cell lines in order to determine if iRGD can improve HuFOLactis activated PBMC infiltration. The findings showed that PBMC could not infiltrate the MCSs, with only a faint signal observed on the periphery. Cells from HuFO-PBMC or PBMC-iRGD showed slightly better penetration than PBMC, but HuFO-PBMC-iRGD cells had higher fluorescence intensity than the other groups (). Using the CFSE/PI method, we validated the in vitro killing ability. HuFO-activated PBMCs were evaluated for cytotoxicity against MKN45 and HGC27 gastric cancer cell lines. The results demonstrated that PBMCs activated by HuFOLactis displayed enhanced cytotoxic effects compared to the control group in both MKN45 and HGC27 cells. (). When HuFO-activated PBMCs were co-cultured with tumor cell lines, an upregulation of PDL-1 on tumor cells was observed (). This phenomenon is hypothesized to be attributed to the secretion of high levels of IFN-γ and TNF-α by HuFO-activated T cells and NK cells, leading to the upregulation of PDL-1. When FO-activated immune cells are subjected to adoptive reinfusion, the concurrent administration of a PD-1 monoclonal antibody is expected to result in a synergistic anti-tumor response.
Figure 4. HuFOLactis activated PBMCs modified with iRGD possess superior penetration capacity in MCSs. (a) Representative morphological assessment of HGC27-MCSs was exposed to indicated CFSE stained HuFOLactis activated PBMCs at an effector to target cell ratio (E:T) of 5:1 calculated on the initial number of spheroids inoculated for 6 h before confocal microscopy. (b) Summary of data demonstrates the depth of infiltration of HGC27-MCSs by specific cells over a 6-hour period through quantitative analysis of mean fluorescence intensity (mean ± s.e.m.; n = 4 MCSs per group). (c, d) the cytotoxic reactivity of HuFOLactis activated PBMCs was measured using CFSE/PI cytotoxicity assay, the target cells were MKN45 and HGC27, respectively (mean ± s.e.m.; n = 3 cell cultures per group). (e, f) Flow cytometry was used to assess PD-L1 expression after 48 hours of culturing the MKN45 and HGC27 cell lines with HuFOLactis activated PBMCs, at an E: T ratio of 20:1 (mean ± s.e.m.; n = 3 cell cultures per group). For experiments B, data were analyzed by one-way ANOVA coupled with Tukey’s multiple-comparisons test. For experiments C and D, Data were analyzed by two-way ANOVA coupled with Tukey’s multiple-comparisons test. For experiments E and F, Data were analyzed by two-sided unpaired t-test. Three independent experiments were conducted with PBMCs from three donors, with four MCSs or three cell cultures per group in each experiment. A representative result is shown from one of the three independent experiments.
iRGD synergizes with HuFOLactis and anti-PD1 antibody inhibited tumor growth in mouse model
The study demonstrated that HuFO-PBMC-iRGD combination with anti-PD-1 exhibited the most antitumor effect (). Survival rates of tumor-bearing mice were notably improved upon treatment with HuFO-PBMC-iRGD, with further enhancement observed when combined with anti-PD-1 therapy. The most remarkable survival outcome, surpassing 80 days post-inoculation, was achieved with the combined administration of HuFO-PBMC-iRGD and anti-PD-1 (). In order to evaluate the targeted migration of HuFO-PBMC-iRGD cells the tumor model after systemic administration, we created a tumor cell suspension one week after cell infusion and examined the presence of transferred cells through flow cytometry. Remarkably, a greater percentage and number of CD3+ T cells and CD11c+ DC cells were observed in the HuFO-PBMC-iRGD group (, Supplementary Figure S4a and Supplementary Figure S4b). Immunohistochemical analysis was conducted on resected tumors 24 hours after the injection of PBMCs. Tumor sections from mice infused with HuFO-PBMC-iRGD exhibited increased infiltration of CD3+ T cells compared to the other groups (Supplementary Figure S4).
Figure 5. The combination of iRGD with HuFOLactis and anti-PD1 antibody improved mouse survival. (a) Initiating an antitumor experiment in vivo, five-week-old female Babl/c-nude mice were injected with 5 × 106 MKN45 cells subcutaneously. Two weeks later, 2 × 107 HuFO-PBMCs (activated with HuFOLactis for 24 h) either modified or not with iRGD were injected into the mice. At the same time, two groups were given a 250 µg intravenous PD-1 blockade. The observation of tumor burden and survival time in the mice was conducted. (b) Average tumor-growth curves of mice bearing MKN45 tumor with different treatments as indicated (mean ± s.e.m.; n = 10 biologically independent mice per group) (mean ± s.e.m.; n = 10 biologically independent mice per group). (c) Survival curves of mice in different groups for 80 days (n = 9 biologically independent mice per group). (d, e) Flow cytometry was employed to analyze the percentage of human CD3+ T cells and CD11c+ DCs in the tumor tissue of HuFO-PBMC with or without iRGD treatment in mice, seven days after intravenous injection of cells. (f, g) Summary of data from D and E (mean ± s.e.m.; n = 5 biologically independent mice per group). (h) The average weight of different groups for 30 days (mean ± s.e.m.; n = 6 biologically independent mice per group). (i, j) Flow cytometric analysis polyfunctional CD3+ T cells with positive staining for IFN-γ and exhausted CD3+ T cells with Tim-3 expression. (K, L) Quantifications of IFN-γ and TIM-3 expression in CD3+ T cells (mean ± s.e.m.; n = 4 biologically independent mice per group). For experiments B, p-values were determined by one-way ANOVA with Tukey’s multiple comparisons test. Differences in survival were determined by using the Kaplan–Meier method, and the p value was determined via the log-rank (Mantel–Cox) test. For experiments F, G, K and L, data were analyzed using one-way ANOVA and each data point represents one sample from an independent mouse. Animal experiments were repeated twice under similar conditions with similar results.
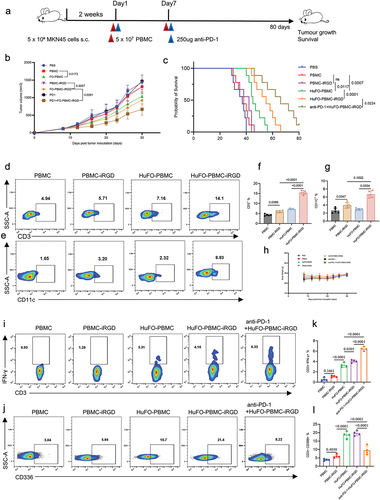
The research pinpointed activation and fatigue indicators of CD8+ T cells to demonstrate the combined impacts in the immune system. Flow cytometry analysis showed that combining HuFO-PBMC-iRGD with anti-PD-1 via intravenous administration boosted the population of multifunctional CD3+ T cells in the tumor environment. A higher percentage and greater number of CD3+IFN-γ+ T cells were detected in the anti-PD-1+ HuFO-PBMC-iRGD group ( and Supplementary Figure S5c). Conversely, the percentage and count of CD3+CD366+ T cells were found to be the lowest in the same group ( and Supplementary Figure S5d). These findings indicate that the combined therapy of HuFO-PBMC-iRGD and anti-PD1 effectively enhanced cell activation, attenuated exhaustion, and consequently prolonged overall survival.
Biosafety assessment
In mouse tumor models, no groups of mice showed abnormal weight changes when tested (). On Day 7 after treatment, there was no apparent harm observed in the heart, liver, spleen, lung, and kidney when examined histologically in all groups (Supplemental Figure S6a). Blood tests showed that there were no notable variations in BUN, Cr, ALT, and AST levels in the mice, indicating that there were no apparent harmful effects after giving HuFO-PBMC-iRGD and anti-PD1 together (Supplemental Figure S6b).
In the context of immunotherapy, the emergence of severe side effects, including cytokine storms, is a concern. In this study, we investigated systemic inflammation by examining serum levels of inflammatory cytokines seven days after treatment. The levels of IL-6 and IL-10 in the blood did not differ significantly between the all the groups, highlighting the positive safety profile of the combined treatment with HuFO-PBMC-iRGD and anti-PD1 (Supplemental Figure S4b).
Discussion
Adoptive T cell immunotherapies have shown significant anti-cancer efficacy in various clinical trials and have become a prominent area of interest in cancer treatment strategies.Citation33 However, the production of tumor-specific T cells on a large scale is often hindered by complex cell culture procedures, concerns about retrovirus-based gene transfer, and insufficient purity of T cells.
T cells, DCs, and NK cells collaborate in targeting cancer cells.Citation6,Citation11,Citation24,Citation26 NK cells can eliminate cancer cells that evade T cell recognition by downregulating MHC molecules. They induce apoptosis in cancer cells through the release of granzyme and perforin, bolstering the anti-tumor immune response. Additionally, NK cells secrete cytokines like IFN-γ, which activate other immune cells such as macrophages and T cells, further enhancing the immune response. Moreover, NK cells play a pivotal role in recruiting DCs to tumors, thereby amplifying CD8+ T cell responses. The crosstalk among DC, NK, and T cells in immune therapy is crucial. Therefore, the simultaneous activation of DC, T cells, and NK cells ex vivo through a straightforward method, coupled with adoptive transfer post short-term activation, holds significant promise for clinical application.
Our team developed an engineered strain of Lactococcus lactis capable of producing a fusion protein comprising Flt3L and OX40L. This protein functions as an in situ vaccine, influencing key elements of the antitumor immune response when administered via intratumoral injection.Citation34 However, the administration of intratumoral injections poses challenges in solid tumors with a dense consistency or high internal pressure. Additionally, the application of intratumoral injections is not viable for metastatic tumors such as abdominal and orthotopic metastases, as suitable lesions may be lacking. In this study, our aim was to utilize FOLactis to simultaneously activate DC, NK, and T cells in PBMCs in vitro, followed by adoptive transfer to exert anti-tumor effects, serving as a supplementary approach to intratumoral injection of in situ vaccine.
Our in vitro experiments demonstrate that HuFOLactis induces activation of DC, leading to increased expression of co-stimulatory molecules, including CD80, CD86, and HLA-DR. Furthermore, HuFOLactis enhances the secretion of IL-12p70 by dendritic cells, a cytokine known for its pivotal role in stimulating T and NK cells. Co-culturing PBMCs with HuFOLactis results in nonspecific activation of NK and T cells, evidenced by upregulation of activation markers CD25, CD69, and CD137, along with increased secretion of IFN-γ and TNF-α cytokines, collectively contributing to anti-tumor effects. Moreover, incubating tumor cell membrane extracts with HuFOLactis in PBMCs generates oncoprotein-specific T cells over a 14-day period. In addition, HuFOLactis can decrease Treg cells and preserve the central memory cell subset.
Efficient chemotaxis and immune cell infiltration into tumors is crucial for solid tumor therapy. However, barriers like vascular structure, extracellular matrix, and immunosuppressive microenvironment hinder immune cell aggregation. Only 2% of adoptively transferred T cells effectively infiltrate tumors, highlighting the need to increase T cell quantity for improved immunotherapy. Our team has reported that iRGD can enhance the infiltration of transferred T cells and NK cells into tumors.Citation16,Citation35 In this study, both in vitro 3D spheroid experiments and in vivo studies demonstrated that iRGD enhances the penetration of HuFOLactis-activated PBMCs into the tumor interior. Additionally, the combination of PD-1 monoclonal antibodies can lead to a more significant extension of survival.
Checkpoint inhibitors for the immune system are commonly employed to improve the immune-suppressing environment in solid tumors.Citation36 Our in vitro studies revealed a notable increase in IFN-γ secretion by HuFOLctis-stimulated PBMCs when co-cultured with tumor cells. This heightened IFN-γ production led to upregulated PD-L1 expression in the tumor cells. Combining these cells with a PD-1 monoclonal antibody for adoptive reinfusion is anticipated to yield a synergistic therapeutic effect. In vivo experiments demonstrated that the addition of an anti-PD-1 monoclonal antibody enhanced the anti-cancer efficacy. Flow cytometry analysis indicated that the HuFO-PBMC-iRGD and anti-PD1 combination resulted in elevated levels of polyfunctional CD3+ T cell infiltration in the tumor microenvironment, as evidenced by increased IFN-γ expression. Furthermore, the group treated with HuFO-PBMC-iRGD and anti-PD1 exhibited reduced Tim-3 levels on CD3+ T cells compared to the control group, suggesting mitigation of T cell exhaustion. These findings highlight the synergistic effectiveness of HuFO-PBMC-iRGD and anti-PD1 therapy in promoting T cell activation, delaying exhaustion, and enhancing anti-tumor immune responses.
Our research offers several advantages. Firstly, we utilize HuFOLactis to transiently stimulate PBMCs in vitro, leading to the concurrent activation of T cells, NK cells, and DCs. This approach is cost-effective and straightforward. Secondly, the simultaneous activation of these immune cells can collaboratively enhance anti-tumor responses. For instance, HuFOLactis-induced IL-12 secretion by DCs can amplify NK cell activity. Additionally, some DCs phagocytose HuFOLactis, migrate to tumor sites, and secrete proteins that further stimulate immune cells. The use of iRGD facilitates immune cell homing and infiltration into tumor tissues. Additionally, studies conducted in a controlled environment show that immune cells activated by HuFOLactis release high amounts of IFN-γ and TNF-α, resulting in the increase of PD-L1 expression on cancer cells. Combining HuFO-PBMC-iRGD with PD-1 monoclonal antibodies further augment the anti-tumor efficacy. However, compared to current cell therapies like CAR-T and TCR-T, the therapeutic efficacy is limited. Nonetheless, this method offers a convenient, effective, and cost-efficient cellular therapy option, serving as a supplementary treatment for certain specific patients.
In conclusion, our experimental findings indicate that HuFOLactis have the ability to activate DC, NK, and T cells in PBMC populations in vitro, and when administered post adoptive transfer in combination with iRGD and PD-1 monoclonal antibodies, it exhibits a notable anti-tumor effect and extends survival significantly. This discovery lays an important experimental groundwork for the exploration and advancement of therapeutic strategies with promising clinical applications.
Abbreviations
NK: Natural Killer;
DC: Dendritic cells;
Flt3L: Fms-like tyrosine kinase 3 ligand;
OX40L: OX40 ligand;
TM: tumor cell membranes;
ACT: Adoptive cell therapy;
MCSs: Multicellular spheroids;
DSPE-PEG: 1,2-distearoyl-sn-glycero-3-phospho-ethanolamine-polyethylene glycol;
FACS: Flow cytometry staining buffer;
CFSE: Carboxy fluorescein succinimidyl amino ester;
PI: Propidium iodide; H&E: Hematoxylin and eosin;
IFN-γ: interferon-gamma;
TNF-α: Tumor necrosis factor-alpha;
PBMCs: Peripheral blood mononuclear cells;
TME: tumor microenvironment;
Tregs: Regulatory T cells.
Authors’ contributions
JS conducted experiments, wrote the main manuscript text, and prepared figures. KX, ZQ, FL, and LL conceived and designed all experiments. JZ, MT, and QL provided protocols and tools for research. BL assisted in manuscript preparation and coordinated the research and verified the results. All authors reviewed the manuscript.
Ethics approval and consent to participate
Throughout the research, all methods were carried out in accordance with the approved guidelines. The blood collection procedure followed the approved guidelines and was approved by the Drum Tower Hospital Ethics Committee. All blood donors provided written informed consent with a statement approving scientific research for all contacts.
Supplemental Material
Download PDF (354.7 KB)Supplemental Material
Download PDF (359.9 KB)Supplemental Material
Download PDF (295.1 KB)Supplemental Material
Download PDF (241.8 KB)Supplemental Material
Download PDF (255.1 KB)Supplemental Material
Download PDF (637.1 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The original contributions presented in the study are included in the article/supplementary material.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2024.2375825
Additional information
Funding
References
- Zhao D, Zhu D, Cai F, Jiang M, Liu X, Li T, Zheng Z. Current situation and prospect of adoptive cellular immunotherapy for malignancies. Technol Cancer Res Treat. 2023 Jan;22:15330338231204198. doi:10.1177/15330338231204198.
- Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021 Apr 6;11(4):69. doi:10.1038/s41408-021-00459-7.
- Saito S, Okuno A, Kakizaki N, Maekawa T, Tsuji NM. Lactococcus lactis subsp. cremoris C60 induces macrophages activation that enhances CD4+ T cell-based adaptive immunity. Biosci Microbiota, Food Health. 2022;41(3):130–13. doi:10.12938/bmfh.2021-057.
- Zhang T, Wei X, Li Y, Huang S, Wu Y, Cai S, Aipire A, Li J. Dendritic cell-based vaccine prepared with recombinant Lactococcus lactis enhances antigen cross-presentation and antitumor efficacy through ROS production. Front Immunol. 2023;14:1208349. doi:10.3389/fimmu.2023.1208349.
- Frelet-Barrand A. Lactococcus lactis, an attractive cell factory for the expression of functional membrane proteins. Biomolecules. 2022;12(2):180. doi:10.3390/biom12020180.
- Kyrysyuk O, Wucherpfennig KW. Designing cancer immunotherapies that engage T cells and NK cells. Annu Rev Immunol. 2023;41(1):17–38. doi:10.1146/annurev-immunol-101921-044122.
- O’Neill RE, Cao X. Co-stimulatory and co-inhibitory pathways in cancer immunotherapy. Adv Cancer Res. 2019;143:145–94.
- Lu X. OX40 and OX40L interaction in cancer. Curr Med Chem. 2021;28(28):5659–73. doi:10.2174/0929867328666201229123151.
- Thapa B, Kato S, Nishizaki D, Miyashita H, Lee S, Nesline MK, Previs RA, Conroy JM, DePietro P, Pabla S, et al. OX40/OX40 ligand and its role in precision immune oncology. Cancer Metastasis Rev. 2024 Mar 25;1–3. doi:10.1007/s10555-024-10184-9.
- Moussion C, Delamarre L. Antigen cross-presentation by dendritic cells: a critical axis in cancer immunotherapy. Semin Immunol. 2024 Feb;71:101848. doi:10.1016/j.smim.2023.101848.
- Harvey AG, Graves AM, Uppalapati CK, Matthews SM, Rosenberg S, Parent EG, Fagerlie MH, Guinan J, Lopez BS, Kronstad LM, et al. Dendritic cell-natural killer cell cross-talk modulates T cell activation in response to influenza a viral infection. Front Immunol. 2022;13. doi:10.3389/fimmu.2022.1006998.
- Verneau J, Sautés-Fridman C, Sun CM. Dendritic cells in the tumor microenvironment: prognostic and theranostic impact. Semin Immunol. 2020 Apr;48:101410. doi:10.1016/j.smim.2020.101410.
- Waskow C, Liu K, Darrasse-Jèze G, Guermonprez P, Ginhoux F, Merad M, Shengelia T, Yao K, Nussenzweig M. The receptor tyrosine kinase Flt3 is required for dendritic cell development in peripheral lymphoid tissues. Nat Immunol. 2008 June;9(6):676–83. doi:10.1038/ni.1615.
- Cueto FJ, Sancho D. The Flt3L/Flt3 axis in dendritic cell biology and cancer immunotherapy. Cancers. 2021 Mar 26;13(7):1525. doi:10.3390/cancers13071525.
- Pathangey LB, McCurry DB, Gendler SJ, Dominguez AL, Gorman JE, Pathangey G, Mihalik LA, Dang Y, Disis ML, Cohen PA, et al. Surrogate in vitro activation of innate immunity synergizes with interleukin-7 to unleash rapid antigen-driven outgrowth of CD4+ and CD8+ human peripheral blood T-cells naturally recognizing MUC1, HER2/neu and other tumor-associated antigens. Oncotarget. 2017 Feb 14;8(7):10785–808. doi:10.18632/oncotarget.13911.
- Dong Y, Huang Y, Zhang Z, Chen A, Li L, Tian M, Shen J, Shao J. iRGD-modified memory-like NK cells exhibit potent responses to hepatocellular carcinoma. J Transl Med. 2023 Mar 17;21(1):205. doi:10.1186/s12967-023-04024-7.
- Liu Q, Chu Y, Shao J, Qian H, Yang J, Sha H, Cen L, Tian M, Xu Q, Chen F, et al. Benefits of an immunogenic personalized neoantigen nanovaccine in patients with high-risk gastric/gastroesophageal junction cancer. Adv Sci (Weinheim, Baden-Wurttemberg, Ger). 2022 Nov 9;10(1):e2203298. doi:10.1002/advs.202203298.
- Zhou S, Meng F, Du S, Qian H, Ding N, Sha H, Zhu M, Yu X, Wang L, Liu B, et al. Bifunctional iRGD-anti-CD3 enhances antitumor potency of T cells by facilitating tumor infiltration and T-cell activation. J Immunother Cancer. 2021 May;9(5):e001925. doi:10.1136/jitc-2020-001925.
- Taverniti V, Guglielmetti S. The immunomodulatory properties of probiotic microorganisms beyond their viability (ghost probiotics: proposal of paraprobiotic concept). Genes Nutr. 2011 Aug;6(3):261–74. doi:10.1007/s12263-011-0218-x.
- Wang Y, Xiang Y, Xin VW, Wang X-W, Peng X-C, Liu X-Q, Wang D, Li N, Cheng J-T, Lyv Y-N, et al. Dendritic cell biology and its role in tumor immunotherapy. J Hematol Oncol. 2020 Aug 3;13(1):107. doi:10.1186/s13045-020-00939-6.
- Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer. 2009 May;9(5):361–71. doi:10.1038/nrc2628.
- Zidi I, Mestiri S, Bartegi A, Amor NB. TNF-α and its inhibitors in cancer. Med Oncol (Northwood, London, England). 2010 June;27(2):185–98. doi:10.1007/s12032-009-9190-3.
- Kursunel MA, Esendagli G. The untold story of IFN-γ in cancer biology. Cytokine Growth Factor Rev. 2016 Oct;31:73–81. doi:10.1016/j.cytogfr.2016.07.005.
- MacNabb BW, Chen X, Tumuluru S, Godfrey J, Kasal DN, Yu J, Jongsma MLM, Spaapen RM, Kline DE, Kline J, et al. Dendritic cells can prime anti-tumor CD8+ T cell responses through major histocompatibility complex cross-dressing. Immunity. 2022;55(6):982–97.e8. doi:10.1016/j.immuni.2022.04.016.
- Stojanovic A, Cerwenka A. An intimate encounter: DC3s empower anti-tumor CTLs. Cancer Cell. 2021;39(9):1181–3. doi:10.1016/j.ccell.2021.08.010.
- Jacobs B, Gebel V, Heger L, Grèze V, Schild H, Dudziak D, Ullrich E. Characterization and manipulation of the crosstalk between dendritic and natural killer cells within the tumor microenvironment. Front Immunol. 2021;12:12. doi:10.3389/fimmu.2021.670540.
- Bödder J, Zahan T, van Slooten R, Schreibelt G, de Vries IJM, Flórez-Grau G. Harnessing the cDC1-NK cross-talk in the tumor microenvironment to battle cancer. Front Immunol. 2021;11:11. doi:10.3389/fimmu.2020.631713.
- Garris CS, Arlauckas SP, Kohler RH, Trefny MP, Garren S, Piot C, Engblom C, Pfirschke C, Siwicki M, Gungabeesoon J, et al. Successful anti-PD-1 cancer immunotherapy requires T cell-dendritic cell crosstalk involving the cytokines IFN-γ and IL-12. Immunity. 2018;49(6):1148–61.e7. doi:10.1016/j.immuni.2018.09.024.
- Barry KC, Hsu J, Broz ML, Cueto FJ, Binnewies M, Combes AJ, Nelson AE, Loo K, Kumar R, Rosenblum MD, et al. A natural killer–dendritic cell axis defines checkpoint therapy–responsive tumor microenvironments. Nat Med. 2018;24(8):1178–91. doi:10.1038/s41591-018-0085-8.
- Ito T, Wang YH, Duramad O, Hanabuchi S, Perng OA, Gilliet M, Qin FXF, Liu Y-J. OX40 ligand shuts down IL-10-producing regulatory T cells. Proc Natl Acad Sci USA. 2006 Aug 29;103(35):13138–43. doi:10.1073/pnas.0603107103.
- Kimura T, Fukushima S, Okada E, Kuriyama H, Kanemaru H, Kadohisa‐Tsuruta M, Kubo Y, Nakahara S, Tokuzumi A, Kajihara I, et al. Induced pluripotent stem cell-derived myeloid cells expressing OX40 ligand amplify antigen-specific T cells in advanced melanoma. Pigment Cell Melanoma Res. 2020 Sep;33(5):744–55. doi:10.1111/pcmr.12887.
- Fromm G, de Silva S, Johannes K, Patel A, Hornblower, JC, Schreiber, TH. Agonist redirected checkpoint, PD1-Fc-OX40L, for cancer immunotherapy. J Immunother Cancer. 2018 Dec 18;6(1):149. doi:10.1186/s40425-018-0454-3.
- Albarrán V, San Román M, Pozas J, Chamorro J, Rosero DI, Guerrero P, Calvo JC, González C, García de Quevedo C, Pérez de Aguado P, et al. Adoptive T cell therapy for solid tumors: current landscape and future challenges. Front Immunol. 2024;15:1352805. doi:10.3389/fimmu.2024.1352805.
- Zhu J, Ke Y, Liu Q, Yang J, Liu F, Xu R, Zhou H, Chen A, Xiao J, Meng F, et al. Engineered Lactococcus lactis secreting Flt3L and OX40 ligand for in situ vaccination-based cancer immunotherapy. Nat Commun. 2022 Dec 3;13(1):7466. doi:10.1038/s41467-022-35130-7.
- Ding N, Zou Z, Sha H, Su S, Qian H, Meng F, Chen F, Du S, Zhou S, Chen H, et al. iRGD synergizes with PD-1 knockout immunotherapy by enhancing lymphocyte infiltration in gastric cancer. Nat Commun. 2019;10(1). doi:10.1038/s41467-019-09296-6.
- Sharma P, Goswami S, Raychaudhuri D, Siddiqui BA, Singh P, Nagarajan A, Liu J, Subudhi SK, Poon C, Gant KL, et al. Immune checkpoint therapy—current perspectives and future directions. Cell. 2023 Apr 13;186(8):1652–69. doi:10.1016/j.cell.2023.03.006.
