ABSTRACT
Despite vaccines being instrumental in reducing vaccine-preventable disease, adult vaccination rates in the United States (US) are below optimal levels. To better understand factors affecting vaccination rates, we analyzed trends in adult vaccination coverage using data from the Behavioral Risk Factor Surveillance System (BRFSS) and conducted a targeted literature review (TLR) on interventions to improve adult vaccination rates in the US. Both the BRFSS analysis and the TLR focused on influenza; pneumococcal disease; tetanus and diphtheria or tetanus, diphtheria, and acellular pertussis; herpes zoster; and human papillomavirus vaccination for US adults aged 18–64 years. The TLR additionally included hepatitis A and hepatitis B vaccination. Vaccination coverage rates (VCRs) and changes in VCRs were calculated using the 2011–2019 BRFSS survey data. For the TLR, the MEDLINE and MEDLINE In-Process databases were searched for articles on vaccination interventions published between January 2015 and June 2021. The BRFSS analysis showed that changes in VCRs were generally modest and positive for most states over the study period. The TLR included 32 articles that met the eligibility criteria; intervention strategies that improved adult vaccination outcomes incorporated an educational component, vaccination reminders or reinforcement at the point of care, or authorized non-clinician members of the healthcare team to vaccinate. Furthermore, interventions combining more than one approach appeared to enhance effectiveness. The strategies identified in this TLR will be valuable for policymakers and stakeholders to inform the development and implementation of evidence-based policies and practices to improve adult vaccination coverage.
IntroductionFootnotea
Vaccines have been instrumental to public health by reducing the burden of infectious diseases globally, and widespread immunization is a major success story of modern medicine. Routine childhood vaccinations have tremendously reduced the impact and burden of communicable diseases; however, adult vaccination programs have not seen a similar level of success regarding vaccine uptake.Citation1–4 In the United States (US), the Healthy People 2020 vaccination goals were 80% for influenza among adults aged 18–64 years and 60% for pneumococcal disease among at-risk adults aged 18–64 years;Citation5 however, national vaccination coverage rate (VCR) estimates were markedly lower than these targets, with data from the 2017–2018 National Health Interview Survey in the USCitation6 indicating 46% of adults aged ≥19 years received an influenza vaccine and just 23% of at-risk adults aged 19–64 years received a pneumococcal vaccine. Vaccinating adults is critical to reduce the risk for vaccine-preventable diseases, especially for those with immunocompromising health conditions;Citation7 addressing the gap between target and achieved adult VCRs remains a priority.
Previous studies have shown that adult VCRs are variable across states,Citation2,Citation8 suggesting that state-level factors may have an important impact on vaccination coverage. In an analysis of data from the 2011–2014 Behavioral Risk Factor Surveillance System (BRFSS), interstate variability in VCRs remained high after adjusting for individual characteristics (e.g., income, education level).Citation2 Additionally, a study using 2015–2017 BRFSS data found a significant association between adult VCRs and state-level factors, including insurance coverage and participation in adult immunization information systems (IIS).Citation8 However, state-level data regarding the facilitators and initiatives for improving adult vaccination coverage remain limited.
Further research is needed to inform the development of evidence-based policies and practices that could improve VCRs and help reach target goals in adults aged 18–64 years in the US. Accordingly, we conducted a mixed methods study with the objectives of better understanding trends in national- and state-level adult vaccination coverage over time and identifying potential strategies to improve upon these trends. This study comprised a quantitative analysis of US adult vaccination coverage using BRFSS data and a targeted literature review (TLR) of interventions aimed at improving adult vaccination in the US, with a focus on adults aged 18–64 years. The objectives of this study were to examine the trends in national- and state-level VCRs, evaluate changes over time in state-level VCRs, and identify state-level factors associated with increases in adult VCRs in the US.
Materials and methods
We focused on the following routine vaccinations, which are recommended by the Advisory Committee on Immunization Practices (ACIP) for the US adult population and which vary based on factors such as an individual’s age and the presence of an immunocompromising condition or other risk factorsCitation9: seasonal influenza; pneumococcal disease; tetanus and diphtheria (Td) or tetanus, diphtheria, and acellular pertussis (Tdap); herpes zoster (HZ); and human papillomavirus (HPV).Citation10 Additionally, interventions related to hepatitis ACitation11 and hepatitis BCitation12 vaccination were included in the TLR.
BRFSS analysis
Study population
We evaluated vaccination coverage data for adults aged 18–64 years who participated in any of the 2011–2019 BRFSS surveys. To be included in the study, BRFSS survey participants must have responded to at least one vaccination question and met the age criteria and lived in the US at the time of the survey. Vaccination coverage was computed for the influenza, pneumococcal, Td/Tdap, HZ, and HPV vaccines; vaccine-specific subgroups of interest (e.g., age groups, at-risk groups) were defined according to the Centers for Disease Control and Prevention (CDC) criteria and recommendations.Citation10,Citation13
BRFSS data
The BRFSS consists of core component questions that are asked in all states without modification, optional modules that states may select for inclusion without modification, and state-added questions. Vaccine-specific BRFSS survey questions and their inclusion in the BRFSS survey from 2011–2019 are presented in Table S-1 and Table S-2, respectively (Supplementary Materials). In the present analysis, VCRs were calculated in accordance with methodology used by the CDC to analyze BRFSS data.Citation14 National- and state-level influenza and pneumococcal VCRs were calculated for each year in 2011–2019, whereas national- and state-level HZ and Td/Tdap VCRs were calculated for the years the questions pertaining to each vaccine were included as a core component. Because the HPV vaccine module was optional in all years, state-level VCRs were calculated for the states that included the optional module in 2018 or 2019 and in at least one other year, at least three years earlier.
For each vaccine with available BRFSS data, change in national- and state-level coverage was defined as the difference in vaccination coverage between the latest and earliest years divided by the number of years over which that difference was computed. Change in coverage was calculated using the years 2011 and 2019 for the influenza and pneumococcal vaccines; 2013 and 2019 for Td/Tdap; and 2014 and 2017 for HZ. In 2019, New Jersey was unable to collect enough data to meet the minimum requirements for inclusion in the aggregate BRFSS data; therefore, change in coverage was calculated using the year 2018 as the latest year, where applicable. The availability of data on the HPV vaccine varied by state; therefore, change in coverage for each state was calculated from the earliest year that the state included the optional HPV module to the latest year.
Statistical analysis
All vaccination coverage measures were summarized descriptively using point estimates and associated 95% confidence intervals. To produce coverage estimates representative of the overall US population, we used the analysis weights provided by BRFSS using the BRFSS ranking-weighting methodology.
TLR of interventions to improve vaccination coverage
Our TLR was conducted using guidelines from the Cochrane Collaboration and from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA),Citation15,Citation16 with the goal of identifying studies that describe adult vaccination interventions throughout the US. The search strategy encompassed searches of the MEDLINE and MEDLINE In-Process (via the PubMed platform) electronic medical databases. These electronic databases were searched for articles published in January 2015–June 2021; available in English; and that described adult vaccination interventions within the US. Full search terms are presented in Table S-3 (Supplementary Material). Screening was performed by a single reviewer in two phases using predefined inclusion and exclusion criteria (Table S-4, Supplementary Material): (1) screening of article titles and abstracts and (2) screening of full-text articles. Data were thematically categorized by intervention type.
Results
BRFSS analysis
Change in vaccination coverage
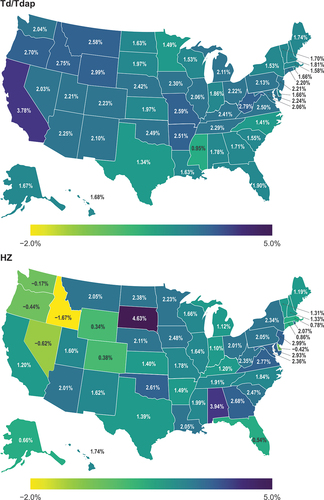
In general, most states demonstrated increases in VCRs across all five vaccine types, although decreases in vaccination coverage were observed in some states for the seasonal influenza vaccine (Louisiana, South Dakota, Tennessee, West Virginia), pneumococcal vaccine (Alabama, Arizona, Idaho), and HZ vaccine (Delaware, Idaho, Nevada, Oregon, Washington) ( and Figure S-1, Supplementary Material). The change per year in vaccination coverage across states ranged from −0.53 to 1.51 for seasonal influenza vaccination among adults aged 18–64 years, −0.27 to 1.29 for pneumococcal vaccination among adults aged 18–64 years at increased risk of pneumococcal disease, 0.95 to 3.78 for Td/Tdap vaccination among adults aged 18–64 years, and −1.67 to 4.63 for HZ vaccination among adults 60–64 years. For HPV vaccination among adults aged 18–49 years, the change per year in vaccination coverage ranged from 0.45 to 3.02 among females and 0.47 to 2.31 among males. Trends in vaccination coverage per year for influenza, pneumococcal, Td/Tdap, HZ, and HPV vaccines are presented in Supplemental Figures S-2 to S-6.
Figure 1. Change per year in influenza and pneumococcal vaccination coverage among adults in the United States.
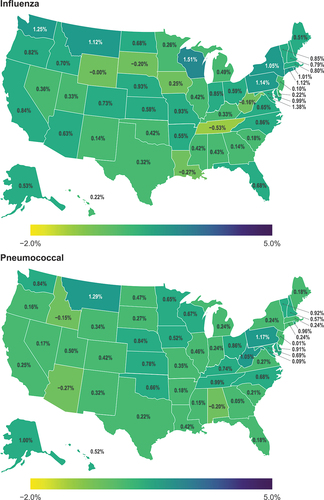
Figure 2. Change per year in Td/Tdap and HZ vaccination coverage among adults in the United States.
TLR of interventions to improve vaccination coverage
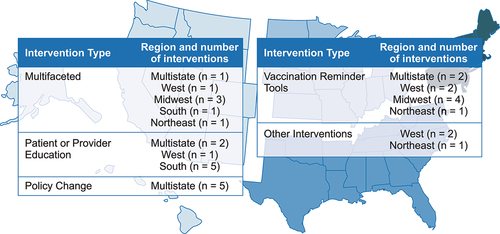
Our search identified 302 records; 188 records were excluded after title and abstract screening and 82 records were excluded after full-text review (), resulting in 32 publicationsCitation17–48 meeting the inclusion criteria. The included articles were heterogenous in terms of study design, interventions, and outcomes reported. Interventions reported in these 32 articles included multifaceted interventions (i.e., >1 intervention type utilized), patient or provider education, policy changes, vaccination reminder tools, and other interventions (i.e., free workplace vaccination, incentive and penalty-based strategies, and the adoption of Medicare Part D Tier-6). The interventions were distributed across the US (), and settings included clinics, pharmacies, community-based settings, a hospital, and a long-term care facility.
Figure 3. PRISMA flowchart.
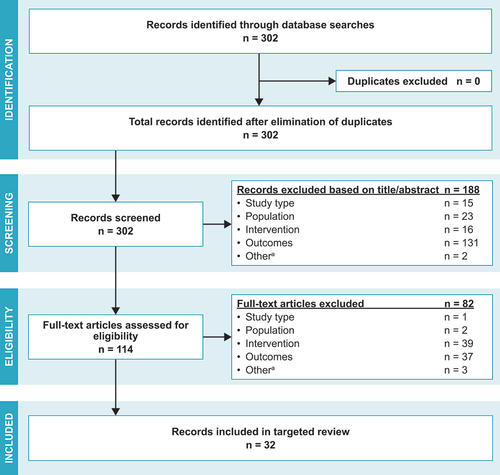
Figure 4. Regional distribution of interventions included in the TLR.
Multifaceted interventions
Seven articles reported on multifaceted interventions to improve vaccine uptake (), with approaches such as education, reminder tools, and workflow modification; all articles reported improvements in vaccination outcomes following the intervention.Citation19,Citation22,Citation24,Citation31,Citation34,Citation40,Citation46 Loskutova et al.Citation31 conducted a study with multicomponent interventions (n = 43 primary care physicians) to improve adult vaccination rates for influenza, pneumococcal, and HZ vaccines. The intervention included clinical decision support provider reminders, provider vaccination performance goals; vaccination education materials for patients, providers, and staff; monthly newsletters; and patient education visual aids, while the control group received clinical decision support provider reminders only. The authors observed significantly increased rates of influenza vaccination (p ≤ .01) and HZ vaccination (p ≤ .001) in both the reminders-only group and the multicomponent intervention group. However, pneumococcal vaccination rates did not significantly differ (p = .3) from those at baseline in the at-risk adult multicomponent intervention group.Citation36
Table 1. Key study characteristics for multifaceted interventions.
In a study by Broderick et al.Citation24 (n = 228), an intervention comprised of a provider educational session on electronic medical record (EMR)–based alerts, EMR vaccination alerts for patients, and personalized encouragement was significantly associated with less delay in time to vaccinate (p = .038). Baker et al.Citation22 (n = 1,255) implemented a multicomponent system-level intervention that consisted of electronic quality measurement, computerized point-of-care clinical decision support tools, individual performance feedback to physicians, and patient outreach in adults with rheumatoid arthritis. Over the 1-year intervention period, the authors found no improvement in influenza vaccination rates; however, the authors reported significant increases in pneumococcal (p = .002) and HZ vaccination coverage (p = .01).Citation26 Ofstead et al.Citation34 found significantly increased influenza vaccination rates (p < .01) among nursing care staff and their family members following a multicomponent intervention that consisted of educational programming and materials, vaccination tracking, and worksheets to assist with program implementation.
Wilson et al.Citation46 (n = 1,298 during a 1-year baseline data collection period; n = 17–35 during a 13-week intervention) implemented an interprofessional approach intervention that incorporated provider education, education reinforcement at the point of care (i.e., ACIP guideline infographics displayed in clinic rooms), and workflow simplification; the authors observed improvements in pneumococcal vaccination rates (3% at baseline; 23% during the final 4 weeks of the 13-week intervention), although p values were not reported. Nowalk et al.Citation40 (n = 25 primary care practices; 70,549 patients) found that a provider education and 1:1 coaching intervention significantly increased Tdap vaccination rates compared with a control group that did not receive this intervention (p < .001). Lastly, Bluml et al.Citation19 evaluated an innovative practice model intervention in community pharmacies following the principle-centered approach; briefly, when patients presented for influenza vaccinations, this approach consisted of pharmacists using IIS data to identify unmet vaccination needs for eight additional vaccine types and provide patient education. Of 1,080 people who had an influenza vaccine, unmet vaccination needs identified using the IIS included unmet needs for the pneumococcal conjugate vaccine (PCV; n = 409); pneumococcal polysaccharide vaccine (PPSV; n = 14); HPV vaccine (n = 16); and Tdap vaccine (n = 483). Following the intervention, the authors observed a significant difference in up-to-date PCV and Tdap vaccination rates (p < .001); however, there were no significant differences in PPSV or HPV vaccination rates. Overall, all the multifaceted interventions reported in this TLR included an education component, reminders, and/or reinforcement at the point of care. Collectively, these studies suggest that vaccine education and vaccination reminders, alone or in combination, may be an effective means to improve adult vaccination rates.
Patient or provider education
Eight articles reported on interventions involving patient or provider education ().Citation17,Citation20,Citation25,Citation26,Citation32,Citation35,Citation39,Citation47 A majority reported improved vaccination rates following the intervention,Citation20,Citation26,Citation32,Citation35,Citation39 and those that did not observe increased vaccination rates reported higher participant readiness to receive vaccination.Citation17,Citation25 To improve vaccination rates in a community pharmacy setting, Brackett et al.Citation17 assessed the use of motivational interviewing–based interventions (n = 19 motivational interview encounters), in which two pharmacists conducted collaborative, person-centered interviews with eligible patients to elicit intrinsic motivation to receive vaccination. Vaccination rates did not differ between the motivational interviewing intervention and control groups for the pneumococcal (n = 5), HZ (n = 6), or hepatitis B (n = 13) vaccines (p values not reported); however, based on questions assessing participant readiness to receive vaccination (5-point Likert scale), there was a significant improvement in readiness to receive both vaccinations (hepatitis B: p = .001; pneumococcal: p = .033). In a study utilizing a patient-centered, educational iBook-based app explaining the benefits of antenatal influenza and pertussis vaccination, Chamberlain et al.Citation25 (n = 300) found higher vaccination rates in the intervention group; however, these increases were not significant (influenza: p = .38; Tdap: p = .27). An integrated approach by Clark et al.Citation47 (n = 2,505) using a 2-phased intervention consisting of staff education coupled with audit, feedback, incentive, and patient education to increase pneumococcal vaccine demand significantly increased pneumococcal vaccination rates compared with the control group (p < .0001).
Table 2. Key study characteristics for patient or provider education interventions.
Healy et al.Citation39 (n = 6,577) observed an increase in antenatal Tdap vaccination from 36% to 61% after the implementation of an intervention comprised of physician education about pertussis illness in infants, Tdap vaccination recommendations, and the American College of Obstetricians and Gynecologists (ACOG) toolkit (resources to support healthcare professional communication with pregnant women about the importance of Tdap vaccination); Black women were significantly less likely (p < .001) to receive a vaccination compared to other race/ethnicity groups. Ciemins et al.Citation26 (n = 508 primary care providers in the intervention arm) found that a learning collaborative approach intervention, consisting of in-person meetings, monthly webinars, and site visits, resulted in significant improvements in influenza and pneumococcal vaccination rates among healthcare organizations participating in the intervention (p < .01) compared with matched control healthcare organizations.
In a patient education–based intervention in patients with heart failure or associated conditions, Olanipekun et al.Citation35 found that patients who received influenza vaccination information and recommendations from their physician were significantly more likely to be vaccinated against influenza than those who did not (p < .05). Furthermore, patients with heart failure who received information and recommendations from a cardiologist had the greatest odds of being vaccinated (adjusted OR, 8.2 [95% CI, 4.8–16.4]; p < .05). Lu et al.Citation32 observed that influenza VCRs were significantly higher (p < .05) in adults who received both a provider recommendation and were offered vaccination during their visit (VCR, 66.6%; n = 1,080) compared with adults who received only a recommendation without an offer (VCR, 48.4%; n = 414) or those who did not receive a recommendation or an offer (VCR, 32.0%; n = 1,450).
Lastly, a communication aids and training intervention in a modeling study by Cataldi et al.Citation20 (n = 98 clinics) led to an estimated additional 5,218 vaccinated patients and 43 cervical cancer cases prevented among the 10 clinics with the highest predicted number of cervical cancer cases prevented. However, the predicted number of cases of cervical cancer prevented by the intervention may be underestimated in this study due to time horizon differences and the analysis not accounting for people aging in (or out) of the age range of eligibility for routine HPV vaccination. In summary, education-based interventions were generally associated with improved vaccination outcomes; however, the wide variety of intervention methods and types of outcomes reported made comparisons across specific intervention types infeasible.
Policy change
Five articles reported on vaccination-related policy interventions (),Citation21,Citation27,Citation33,Citation36,Citation44 and three of these studies reported significant improvements in VCRs.Citation21,Citation27,Citation44 Tan et al.Citation21 (vaccine-eligible adult sample size not reported) implemented a standing order protocol (SOP) intervention at five health facility sites, which authorized nonphysician healthcare professionals to assess unmet vaccination needs and administer vaccines. Following SOP implementation, the authors observed increases in the number of influenza vaccinations given at all sites; however, this only resulted in a statistically significant increase in the influenza vaccination rate at two sites (p < .01). Furthermore, one site experienced an increased number of influenza vaccinations but a significant decrease in the vaccination rate (p < .01); however, the authors noted that this decrease was due to the site inheriting patients from another closed practice. Study authors also reported significant increases in pneumococcal vaccination coverage (p < .01); significantly higher HPV VCR in male participants (from 12% to 24%; p < .01); a significant increase in Tdap vaccination rates among patients aged 19–64 years (p < .01) in 4 of the 5 health facility sites; greater HZ vaccination rates, although not statistically significant; and no significant differences in hepatitis B vaccination rates in adults aged ≥19 years.Citation25
Table 3. Key study characteristics for policy change interventions.
Drozd et al.Citation27 (sample size not reported) studied the impact of policy changes allowing pharmacist-administered seasonal influenza vaccinations and found that these policy changes were associated with significant increases in influenza vaccination rates ≥6 years after the policy change (p ≤.001). Tak et al.Citation44 reported significantly higher HZ vaccination rates in states where pharmacists were authorized to give HZ vaccinations without a prescription order compared with states with prescription order requirements (p = .0022). Conversely, Patel et al.Citation36 (n = 1,002,994 adults aged 18–64 years) evaluated pharmacy-based vaccination services using data on the availability of the live attenuated influenza vaccine annually for 8,466 pharmacies during 2006–2010 as a proxy for pharmacy-based vaccination service availability. The authors found no significant association between influenza or pneumococcal vaccination rates and the availability of pharmacy-based vaccination services. McConeghy and WingCitation33 (sample size not reported) did not observe a significant change in adult influenza vaccination rates following the adoption of flexible pharmacy-based immunization statutes by states (OR, 0.9; 95% CI, −0.3 to 2.2). Overall, policy interventions that provided pharmacists, nurses, and other healthcare personnel authorization to administer vaccines, including the implementation of SOPs, where allowed by state law, generally resulted in increased vaccination rates. These findings suggest that policies that expand vaccination authorization to non-clinician care team members may positively impact adult vaccination rates.
Vaccination reminder tools
Nine articles reported on interventions using vaccination prompts and reminder tools ().Citation18,Citation23,Citation29,Citation30,Citation38,Citation41,Citation43,Citation45,Citation48 In a study by Klassing et al.Citation30 (n = 210), the intervention groups received either a phone call or a letter referencing the up-to-date CDC immunization schedule and guidelines after picking up a prescription at a pharmacy. The authors reported that the influenza vaccination rate was significantly greater (p = .02) in the letter recipient group, but found no significant differences (p = .76) in pneumococcal vaccination rates between the control and intervention groups. Benedict et al.Citation23 (n = 15,115) found that adults receiving influenza vaccination reminders were 1.15 (95% CI, 1.06–1.25) times more likely to be vaccinated than adults not receiving reminders. Szilagyi et al.Citation38 (n = 164,205) found that vaccination reminders resulted in a small but significant increase in influenza vaccination rates between the three intervention groups combined (participants received 1, 2, or 3 portal messages) compared with the control group with no message for adults aged 18–64 years (p = .001). Interestingly, the authors did not observe any dose-dependent increase in vaccination rates in the participants that received two or three messages compared with those that received one message.
Table 4. Key study characteristics for vaccination reminder tool interventions.
Two studies used a combination of patient and provider reminder tools, and both reported improved vaccination coverage. Grivas et al.Citation29 implemented an intervention during the 2011–2012 and 2012–2013 influenza seasons using an EMR notification system, laminated reminders placed in physical charts and workstations, stickers affixed to patients’ medication reconciliation list, and patient-focused reminders (e.g., educational information on the benefits of influenza vaccination) (2012–2013 season only). The intervention resulted in relative influenza vaccination rate increases of 37.6% (2011–2012 season) and 56.1% (2012–2013 season) compared with historical controls (2005–2011 seasons), although p values and sample sizes were not reported. Burns et al.Citation45 (n = 99) used an EMR-based intervention to assess patients’ pneumococcal vaccination status and notify patients and physicians of the recommended pneumococcal vaccine (i.e., PCV, PPSV). The authors found that the EMR-based intervention improved pneumococcal vaccine coverage among HIV-positive veterans at the Veterans Affairs facility that conducted the intervention (n = 22/81 eligible veterans) compared with another Veterans Affairs medical center (n = 10/84 eligible veterans; p < .05).
Several interventions focused on vaccination reminder tools for health-care providers, which were also successful in improving vaccination coverage. McAdam-Marx et al.Citation48 (n = 18,851) found significantly improved vaccination rates for at-risk adults aged 19–64 years (p < .001) following the implementation of a pneumococcal best practice alerts tool that reflects current guidelines, implemented with and without workflow redesign to identify unmet vaccination needs prior to a patient’s visit and document vaccination history during the visit. Erlandson et al.Citation41 (n = 1,478) observed an 8.3% increase in HZ vaccination rates following the implementation of weekly emails to providers with eligible patients, patient alerts during their clinic visit, and enhanced prompts for vaccination. The authors found a further increase of 17.8% for HZ vaccination following orders being placed in the patient’s chart before their clinic appointment, although p values were not reported in this study to determine statistical significance.Citation46 Sheth et al.Citation43 reported a significant increase (p < .0001) in HZ vaccination rates after implementing electronic identification of vaccine eligibility and electronic best practice alerts. Lastly, Hechter et al.Citation18 (n = 116,217) found that the vaccination coverage rate of the 3-dose series of the hepatitis B vaccine increased significantly (p < .0001) at an intervention site that utilized electronic reminders and alerts compared with the control site, where no alerts were sent. In summary, the incorporation of vaccination prompts and reminder tools generally improved vaccination outcomes, although some studies lacked information regarding statistical significance.
Other interventions
Three articles reported on other types of interventions: free workplace vaccination, incentive and penalty-based strategies, and the adoption of Medicare Part D Tier-6 ().Citation28,Citation37,Citation42 Gany et al.Citation28 (n = 53) reported 44% of unvaccinated drivers accepted an influenza vaccination (p value not reported) following the implementation of free workplace vaccination for taxi drivers, compared with a baseline of 17% of drivers being vaccinated. Podczervinski et al.Citation37 compared vaccination rates at baseline (n = 1,446) with vaccination rates following incentive- or penalty-based (n = 1,586 and n = 1,641, respectively) strategies to improve influenza vaccination coverage. Following the incentive-based strategy, a $25 gift card was awarded to all staff if 95% of employees received vaccination, whereas the penalty-based strategy required employees who declined vaccination to complete an online education module, undergo one-on-one counseling, and sign an attestation statement. The authors found that both strategies resulted in improved vaccination rates (p ≤.0001); furthermore, the penalty-based strategy resulted in greater improvement compared with the incentive-based strategy (p < .0001).Citation37 Lastly, Hechter et al.Citation42 (sample size not reported) found that the annual HZ vaccination rate was only slightly higher after the adoption of Medicare Part D Tier-6, with no patient co-pay for HZ vaccination. In addition, the Medicare Part D annual HZ vaccination rate was not statistically different (p > .05) than the annual HZ vaccination rate among patients with commercial health plans.
Table 5. Key study characteristics for other interventions.
Discussion
The findings from this study provide a deeper understanding of changes in adult vaccination coverage in the US as well as interventions aimed at improving adult VCRs. These findings also uncover the complex nature of adult vaccination and the importance of multiprong strategies to improve adult vaccine uptake. Despite the benefits of vaccination, adult VCRsCitation1–3 are considerably lower than national adult vaccination goals.Citation5
While optimal adult VCRs in the US remain unmet, our analysis of BRFSS data from 2011-2019 found generally modest and positive changes in adult VCRs for most US states, with a few states demonstrating negative changes in VCRs; however, these trends may have changed due to the COVID-19 pandemic with further negative changes in vaccine uptake.Citation50 Additionally, in agreement with the findings of previous studies,Citation2,Citation8 we observed high interstate variability in VCRs, which underscores the importance of understanding state-specific needs and barriers as well as state- and local-level interventions, policies, and programs that lead to high vaccination rates. The growing and increasingly complex CDC adult vaccination schedule in the US also highlights the importance of improving awareness of the value of adult vaccination.
Interventions to improve adult VCRs in the US mainly focused on patient or provider education, policy change, and vaccination reminder tools. The TLR findings indicate that vaccine education, reminders or reinforcement at the point of care, and authorization of non-clinician members of the healthcare team to vaccinate are all effective strategies to improve adult vaccination rates. Furthermore, policy change interventions that expand vaccination access by expanding vaccination authorization to non-clinician members of the healthcare team may also improve adult VCRs; approaches combining multiple strategies may further enhance effectiveness. Notably, these approaches may be particularly beneficial to implement in the states identified in our BRFSS analysis that did not demonstrate increases in adult VCRs over time.
While the heterogeneity across studies included in our TLR complicated the synthesis and interpretation of findings, we were able to extract key factors that impacted intervention success. In the multifaceted intervention implemented by Loskutova et al.Citation31 influenza vaccination rates significantly increased in both the multicomponent intervention group and the control group comprising clinical decision support provider reminders, which suggests that reminders were effective regardless of whether provided alone or in combination with other interventions. This underscores the importance of enhanced EMR capabilities in supporting vaccination for both provider and patient reminders. In the policy change intervention assessed by Tan et al.Citation21 vaccination rates generally increased modestly following the implementation of SOPs, but study sites that used SOPs as a foundation for additional interventions found greater success. Additionally, all the multifaceted interventions presented here reported improvements in at least one vaccination outcome, and many of these studies combined patient or provider education with reminders or reinforcement at the point of care. These patient-engaging strategies exemplify a personalized approach to address patient queries directly, which offers a personalized advantage over other strategies such as policy changes and vaccination reminder tools.
Lack of knowledge and awareness about adult vaccination among both healthcare providers and patients form barriers to improving adult vaccination rates in the US.Citation51,Citation52 These identified barriers align with our TLR findings, where the interventions that significantly improved vaccination rates often included an education or reminder component. Indeed, the simplest solution is often the most effective, and our TLR findings suggest that ensuring timely and accurate reminders, along with vaccination information that is easily understood by patients and providers, will drive improvements in adult VCRs. These findings are corroborated by an evaluation of evidence-based strategies to increase adult vaccination rates conducted by the Community Preventive Services Task Force in 2015.Citation53 Indeed, the Community Preventive Services Task Force recommends a combined approach to healthcare system–based interventions that aims to increase demand for vaccinations (e.g., patient education or reminders) and targets enhanced vaccine access, healthcare providers, or healthcare systems (e.g., provider reminders or SOPs). Similarly, the US Department of Health and Human Services aims to improve vaccine knowledge, access, and use through strategies such as increasing education regarding the benefits of vaccination and strengthening data infrastructure (e.g., IIS).Citation54
Adult vaccination is a public health priority that diverse stakeholders will need to address through interventions that include continuing education and community engagement;Citation55 however, additional research should focus on adapting these interventions to optimally improve rates for each type of vaccination across diverse patient populations and practice types. In the intervention implemented by Szilagyi et al.Citation38 patient portal reminders significantly increased influenza vaccination in patients aged 18–64 years but did not have a significant impact on vaccination rates in patients aged 0.5–17 years and ≥65 years. Furthermore, the authors observed racial and ethnic differences in the intervention outcomes, reporting that portal reminders were effective only in patients who were White and non-Hispanic. These findings suggest that tailored approaches for specific populations should be explored to address the nuanced barriers they face and optimize VCRs.
The impact of intervention setting on vaccination rates is also an important factor to consider following the COVID-19 pandemic, as complimentary settings were utilized for COVID-19 vaccination. Notably, most interventions identified in our TLR were implemented in healthcare facilities, with fewer interventions in community settings. Our TLR findings indicate an association between pharmacist authorization to vaccinate and higher vaccination rates,Citation27,Citation44 and similarly, systematic reviews have found that pharmacy-based vaccination programs may improve access and increase VCRs, which may be attributable to their convenient locations within communities.Citation27,Citation44,Citation56,Citation57 Future research may provide further insight regarding the feasibility and value of expanding convenient vaccination settings, such as pharmacies, for broader adult vaccination. Additionally, future research should also consider the role that employers may play in supporting adult vaccination.
The findings of this study are subject to several limitations. First, BRFSS data are self-reported and therefore subject to recall bias. The BRFSS does not collect information about vaccine eligibility; in the present study, we assumed that everyone who reported receiving a vaccine was eligible to receive it. Additionally, we used BRFSS data collected through 2019, which do not reflect any potential changes to adult HPV vaccination rates following the 2019 ACIP update that recommended catch-up vaccination for all persons through age 26 years and shared clinical decision-making regarding HPV vaccination for adults aged 27–45 years.Citation58 Prior to this update, HPV catch-up vaccination was recommended for females through age 26 years and males through age 21 years. Furthermore, for risk-based pneumococcal vaccination recommendations, limited data on the number of adults classified as “at-risk” makes understanding the optimal coverage rate challenging. While the Healthy People 2020 objectives include adult vaccination goals for several diseases, including influenza, pneumococcal, and HZ,Citation5 additional guidance would be beneficial for determining optimal coverage rates for adults for all recommended vaccines, by age and risk group (Table S-5, Supplemental Material).Citation59 Nevertheless, the present study provides valuable information regarding changes in adult VCRs over time, as well as lessons learned from intervention studies. Finally, in our TLR, the search strategy was restricted to studies published in 2015–2021; this restriction could have missed studies published before 2015 that may still provide useful data despite reporting on older information. Similarly, geographical restriction to the US may overlook insightful information about vaccination strategies of other countries, which may be applicable to the US, as well as limit the global generalizability of these TLR findings. Lastly, the COVID-19 pandemic may have impacted health seeking behaviors and opportunities as well as attitudes and beliefs toward vaccination, which could alter the degree to which some of these interventions were effective as well as potentially bring to light other interventions that may be effective.
Conclusion
Healthcare and public health professionals, policymakers, and other critical health system decision makers should consider the strategies identified in this literature review, especially in states that have not demonstrated increased VCRs over time, when implementing practice at the point of clinical care and when developing programs and policies to support the adult vaccination ecosystem and, ultimately, improve adult vaccination rates. This mixed methods study advocates the development of evidence-based policies and practices aimed to improve adult vaccination coverage rates.
Contributors
ALE, AB, and MP contributed to the conception and design of this study. AE, LH, DG, CS, JR, MG, and MP contributed to the acquisition, analysis, or interpretation of data for the work. All authors contributed substantially to drafting the work or revising it critically for important intellectual content. All authors provided final approval of the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Supp Material_US Adult Manuscript 17June24_clean.docx
Download MS Word (1.4 MB)Acknowledgments
The authors thank Brian Samsell, PhD, of RTI Health Solutions for medical writing assistance.
Disclosure statement
LH, DG, CS, JR, MG, and MP are full-time employees of RTI Health Solutions, an independent nonprofit research organization, which was retained by MSD to conduct the research which is the subject of this manuscript. Their compensation is unconnected to the studies on which they work. MG was a full-time employee of RTI Health Solutions at the time this study was conducted. ALE and AB are current employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, who may own stock and/or stock options in Merck & Co., Inc., Rahway, NJ, USA.
Data availability statement
The data that support the findings of the BRFSS analysis are openly available in the BRFSS Annual Survey Data at https://www.cdc.gov/brfss/annual_data/annual_data.htm. All data generated or analyzed within the TLR are included in this article and its supplementary information files.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2024.2381283
Additional information
Funding
Notes
[a] ACIP, Advisory Committee on Immunization Practices; ACOG, American College of Obstetricians and Gynecologists; BRFSS, Behavioral Risk Factor Surveillance System; CDC, Centers for Disease Control and Prevention; CI, Confidence interval; COPD, chronic obstructive pulmonary disease; EMR, electronic medical record; HPV, human papillomavirus; HZV, herpes zoster virus; IIS, immunization information systems; NR, Not reported; OR, Odds ratio; PCV, pneumococcal conjugate vaccine; PPSV, pneumococcal polysaccharide vaccine; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; SOP, standing order protocol; Td, tetanus and diphtheria; Tdap, tetanus, diphtheria, and acellular pertussis; TLR, targeted literature review; UCLA, University of California, Los Angeles; US, United States; VCR, vaccination coverage rate.
References
- Centers for Disease Control and Prevention (CDC). Vaccination coverage among adults. 2020 [accessed 2022 Dec 19]. https://www.cdc.gov/vaccines/imz-managers/coverage/adultvaxview/pubs-resources/vaccination-coverage-adults-2019-2020.html.
- La EM, Trantham L, Kurosky SK, Odom D, Aris E, Hogea C. An analysis of factors associated with influenza, pneumoccocal, Tdap, and herpes zoster vaccine uptake in the US adult population and corresponding inter-state variability. Hum Vaccin Immunother. [2018 Feb 1];14(2):430–16. doi: 10.1080/21645515.2017.1403697 .
- Williams WW, Lu PJ, O’Halloran A, Kim DK, Grohskopf LA, Pilishvili T, Skoff TH, Nelson NP, Harpaz R, Markowitz LE, et al. Surveillance of vaccination coverage among adult populations — United States, 2015. MMWR Surveill Summ. [2017 May 5];66(11):1–28. doi:10.15585/mmwr.ss6611a1.
- Rodrigues CMC, Plotkin SA. Impact of vaccines; health, economic and social perspectives. Front Microbiol. 2020;11:1526. doi: 10.3389/fmicb.2020.01526.
- Office of Disease Prevention and Health Promotion. Healthy people 2020 objectives for immunizations and infectious diseases. 2019 [accessed 2021 June 24]. https://www.healthypeople.gov/2020/topics-objectives/topic/immunization-and-infectious-diseases/objectives.
- Lu PJ, Hung MC, Srivastav A, Grohskopf LA, Kobayashi M, Harris AM, Dooling KL, Markowitz LE, Rodriguez-Lainz A, Williams WW. Surveillance of vaccination coverage among adult populations —United States, 2018. MMWR Surveill Summ. [2021 May 14];70(3):1–26. doi:10.15585/mmwr.ss7003a1.
- Corbett S. Impact of the COVID-19 pandemic on administration of adult vaccinations. N C Med J. 2021 Mar;82(2):126–129. doi: 10.18043/ncm.82.2.126.
- Garbinsky D, Hunter S, La EM, Poston S, Hogea C. State-level variations and factors associated with adult vaccination coverage: a multilevel modeling approach. Pharmacoecon Open. 2021 Sep;5(3):411–423. doi: 10.1007/s41669-021-00262-x.
- Murthy N, Wodi AP, Bernstein H, Ault KA. Advisory committee on immunization P, advisory committee on immunization P. Recommended adult immunization schedule, United States, 2022. Ann Intern Med. 2022 Mar;175(3):432–443. doi:10.7326/M22-0036.
- Centers for Disease Control and Prevention (CDC). Adult immunization schedule. 2023 Apr 27. [accessed 2023 Aug 30]. https://www.cdc.gov/vaccines/schedules/hcp/imz/adult.html.
- Nelson NP, Weng MK, Hofmeister MG, Moore KL, Doshani M, Kamili S, Koneru A, Haber P, Hagan L, Romero JR, et al. Prevention of hepatitis a virus infection in the United States: recommendations of the advisory committee on immunization practices, 2020. MMWR Recomm Rep. [2020 Jul 3];69(5):1–38. doi:10.15585/mmwr.rr6905a1.
- Weng MK, Doshani M, Khan MA, Frey S, Ault K, Moore KL, Hall EW, Morgan RL, Campos-Outcalt D, Wester C, et al. Universal hepatitis B vaccination in adults aged 19–59 years: updated recommendations of the advisory committee on immunization practices — United States, 2022. MMWR Morb Mortal Wkly Rep. [2022 Apr 1];71(13):477–483. doi:10.15585/mmwr.mm7113a1 .
- Hr HC, Ortega-Sanchez I, Bialek SR, Bialek SR, Centers for Disease Control and Prevention. Update on recommendations for use of herpes zoster vaccine. MMWR Morb Mortal Wkly Rep. [2014 Aug 22];63(33):729–731.
- Centers for Disease Control and Prevention (CDC). Complex sampling weights and preparing 2019 BRFSS module data for analysis 2020. [accessed 2023 Sep 28]. https://www.cdc.gov/brfss/annual_data/2019/pdf/Complex-Smple-Weights-Prep-Module-Data-Analysis-2019-508.pdf.
- Cochrane. Cochrane handbook for systematic reviews of interventions version 6.3. 2022. www.training.cochrane.org/handbook.
- Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, Moher D, Peters MDJ, Horsley T, Weeks L, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. [2018 Oct 2];169(7):467–473. doi:10.7326/M18-0850.
- Brackett A, Butler M, Chapman L. Using motivational interviewing in the community pharmacy to increase adult immunization readiness: a pilot evaluation. J Am Pharm Assoc (2003). 2015 Mar;55(2):182–186. doi: 10.1331/JAPhA.2015.14120.
- Hechter RC, Qian L, Luo Y, Ling Grant DS, Baxter R, Klein NP, Nunley KV, Aukes L., Hogea C, Krishnarajah G, Patterson BJ. Impact of an electronic medical record reminder on hepatitis B vaccine initiation and completion rates among insured adults with diabetes mellitus. Vaccine. [2019 Jan 3];37(1):195–201. doi: 10.1016/j.vaccine.2018.06.035.
- Bluml BM, Brock KA, Hamstra S, Tonrey L. Evaluation of the impact of an innovative immunization practice model designed to improve population health: results of the project IMPACT immunizations pilot. Popul Health Manag. 2018 Feb;21(1):55–62. doi: 10.1089/pop.2017.0049.
- Cataldi JR, Håbesland M, Anderson-Mellies A, Dempsey AF, Cockburn M. The potential population-based impact of an HPV vaccination intervention in Colorado. Cancer Med. 2020 Feb;9(4):1553–1561. doi: 10.1002/cam4.2803.
- Tan LJ, VanOss R, Ofstead CL, Wetzler HP. Maximizing the impact of, and sustaining standing orders protocols for adult immunization in outpatient clinics. Am J Infect Control. 2020 Mar;48(3):290–296. doi: 10.1016/j.ajic.2019.07.023.
- Baker DW, Brown T, Lee JY, Ozanich A, Liss DT, Sandler DS, Ruderman EM. A multifaceted intervention to improve influenza, pneumococcal, and herpes zoster vaccination among patients with rheumatoid arthritis. J Rheumatol. 2016 June;43(6):1030–7. doi:10.3899/jrheum.150984.
- Benedict KM, Santibanez TA, Kahn KE, Pabst LJ, Bridges CB, Kennedy ED. Receipt and effectiveness of influenza vaccination reminders for adults, 2011-2012 season, United States. Influenza Other Respir Viruses. 2018 Sep;12(5):605–612. doi: 10.1111/irv.12568.
- Broderick R, Ventura I, Soroosh S, Franco L, Giles JT. Reducing missed opportunities for influenza vaccination in patients with rheumatoid arthritis: evaluation of a multisystem intervention. J Rheumatol. 2018 Aug;45(9):1220–1228. doi:10.3899/jrheum.170763.
- Chamberlain AT, Seib K, Ault KA, Rosenberg ES, Frew PM, Cortés M, Whitney EAS, Berkelman RL, Orenstein WA, Omer SB. Improving influenza and Tdap vaccination during pregnancy: a cluster-randomized trial of a multi-component antenatal vaccine promotion package in late influenza season. Vaccine. [2015 Jul 9];33(30):3571–3579. doi:10.1016/j.vaccine.2015.05.048.
- Ciemins EL, Jerry M, Powelson J, Leaver-Schmidt E, Joshi V, Casanova D, Kennedy JW, Penso J. Impact of a learning collaborative approach on influenza and pneumococcal immunization rates in US adults: a mixed methods approach. Popul Health Manag. 2020 Feb;23(1):29–37. doi:10.1089/pop.2019.0019.
- Drozd EM, Miller L, Johnsrud M. Impact of pharmacist immunization authority on seasonal influenza immunization rates across states. Clin Ther. 2017 Aug;39(8):1563–80.e17. doi: 10.1016/j.clinthera.2017.07.004.
- Gany F, Rau-Murthy R, Mujawar I, Prasad L, Roberts N. Increasing influenza vaccination in New York City taxi drivers: a community driven approach. Vaccine. [2015 May 21];33(22):2521–2523. doi:10.1016/j.vaccine.2015.03.027.
- Grivas PD, Devata S, Khoriaty R, Boonstra PS, Ruch J, McDonnell K, Hernandez-Aya L, Wilfong J, Smerage J, Ison MG, et al. Low-cost intervention to increase influenza vaccination rate at a comprehensive cancer center. J Cancer Educ. 2017 Dec;32(4):871–877. doi:10.1007/s13187-016-1017-2.
- Klassing HM, Ruisinger JF, Prohaska ES, Melton BL. Evaluation of pharmacist-initiated interventions on vaccination rates in patients with asthma or COPD. J Community Health. 2018 Apr;43(2):297–303. doi:10.1007/s10900-017-0421-9.
- Loskutova NY, Smail C, Callen E, Staton EW, Nazir N, Webster B, Pace WD. Effects of multicomponent primary care-based intervention on immunization rates and missed opportunities to vaccinate adults. BMC Fam Pract. [2020 Feb 29];21(1):46. doi:10.1186/s12875-020-01115-y.
- Lu PJ, Srivastav A, Amaya A, Dever JA, Roycroft J, Kurtz MS, O’Halloran A, Williams WW. Association of provider recommendation and offer and influenza vaccination among adults aged ≥18 years – United States. Vaccine. [2018 Feb 1];36(6):890–898. doi:10.1016/j.vaccine.2017.12.016.
- McConeghy KW, Wing C. A national examination of pharmacy-based immunization statutes and their association with influenza vaccinations and preventive health. Vaccine. [2016 June 24];34(30):3463–8. doi:10.1016/j.vaccine.2016.04.076.
- Ofstead CL, Amelang MR, Wetzler HP, Tan L. Moving the needle on nursing staff influenza vaccination in long-term care: results of an evidence-based intervention. Vaccine. [2017 Apr 25];35(18):2390–2395. doi:10.1016/j.vaccine.2017.03.041.
- Olanipekun T, Effoe VS, Olanipekun O, Igbinomwanhia E, Kola-Kehinde O, Fotzeu C, Bakinde N, Harris R. Factors influencing the uptake of influenza vaccination in African American patients with heart failure: findings from a large urban public hospital. Heart Lung. 2020 May;49(3):233–237. doi:10.1016/j.hrtlng.2019.12.003.
- Patel AR, Breck AB, Law MR. The impact of pharmacy-based immunization services on the likelihood of immunization in the United States. J Am Pharm Assoc (2003). 2018 Sep;58(5):505–514.e2. doi: 10.1016/j.japh.2018.05.011.
- Podczervinski S, Stednick Z, Helbert L, Davies J, Jagels B, Gooley T, Casper C, Pergam SA. Employee influenza vaccination in a large cancer center with high baseline compliance rates: comparison of carrot versus stick approaches. Am J Infect Control. [2015 Mar 1];43(3):228–233. doi:10.1016/j.ajic.2014.11.025.
- Szilagyi PG, Albertin C, Casillas A, Valderrama R, Duru OK, Ong MK, Vangala S, Tseng C-H, Rand CM, Humiston SG, et al. Effect of patient portal reminders sent by a health care system on influenza vaccination rates: a randomized clinical trial. JAMA Intern Med. [2020 Jul 1];180(7):962–970. doi:10.1001/jamainternmed.2020.1602.
- Healy CM, Ng N, Taylor RS, Rench MA, Swaim LS. Tetanus and diphtheria toxoids and acellular pertussis vaccine uptake during pregnancy in a metropolitan tertiary care center. Vaccine. [2015 Sep 11];33(38):4983–4987. doi:10.1016/j.vaccine.2015.07.018.
- Nowalk MP, Lin CJ, Pavlik VN, Brown AE, Zhang S, Moehling KK, Raviotta JM, South-Paul JE, Hawk M, Ricci EM, et al. Using the 4 Pillars™ practice transformation program to increase adult Tdap immunization in a randomized controlled cluster trial. Vaccine. [2016 Sep 22];34(41):5026–5033. doi:10.1016/j.vaccine.2016.07.053.
- Erlandson KM, Streifel A, Novin AR, Hawkins KL, Foster C, Langness J, Bessesen M, Falutz J, Moanna A, Looney D, et al. Low rates of vaccination for herpes zoster in older people living with HIV. AIDS Res Hum Retroviruses. 2018 Jul;34(7):603–606. doi:10.1089/aid.2017.0315.
- Hechter RC, Qian L, Yan S, Luo Y, Krishnarajah G, Tseng HF. Impact of the change of copay policy in Medicare part D on zoster vaccine uptake among Medicare beneficiaries in a managed care organization. BMC Health Serv Res. [2017 Jul 21];17(1):503. doi:10.1186/s12913-017-2441-7.
- Sheth H, Moreland L, Peterson H, Aggarwal R. Improvement in herpes zoster vaccination in patients with rheumatoid arthritis: a quality improvement project. J Rheumatol. 2017 Jan;44(1):11–17. doi:10.3899/jrheum.160179.
- Tak CR, Gunning K, Kim J, Sherwin CM, Ruble JH, Nickman NA, Biskupiak JE. The effect of a prescription order requirement for pharmacist-administered vaccination on herpes zoster vaccination rates. Vaccine. [2019 Jan 21];37(4):631–636. doi:10.1016/j.vaccine.2018.12.003.
- Burns CM, Banks RE, Wilson BM, Carter RR, Jump RLP, Perez F. A virtual clinic improves pneumococcal vaccination coverage among patients living with HIV at a veterans affairs medical center. AIDS Care. 2018 Feb;30(2):146–149. doi:10.1080/09540121.2017.1390542.
- Wilson J, Swee M, Mosher H, Scott-Cawiezell J, Levins L, Fort K, Kumar B. Using lean six sigma to improve pneumococcal vaccination rates in a veterans affairs rheumatology clinic. J Healthc Qual. 2020 May;42(3):166–1674. doi:10.1097/jhq.0000000000000218.
- Clark RC, Jackson J, Hodges D, Gilliam B, Lane J. Improving pneumococcal immunization rates in an ambulatory setting. J Nurs Care Qual. 2015 Jul;30(3):205–11. doi: 10.1097/ncq.0000000000000110.
- McAdam-Marx C, Tak C, Petigara T, Jones NW, Yoo M, Briley MS, Gunning K, Gren L. Impact of a guideline-based best practice alert on pneumococcal vaccination rates in adults in a primary care setting. BMC Health Serv Res. [2019 Jul 10];19(1):474. doi:10.1186/s12913-019-4263-2.
- Basurto-Dávila R, Meltzer MI, Mills DA, Beeler Asay GR, Cho BH, Graitcer SB, Dube NL, Thompson MG, Patel SA, Peasah SK. School-based influenza vaccination: health and economic impact of Maine’s 2009 influenza vaccination program. Health Serv Res. 2017 Dec;52 (Suppl 2):2307–2330. doi: 10.1111/1475-6773.12786.
- Eiden AL, DiFranzo A, Bhatti A, Echo Wang H, Bencina G, Yao L, Saxena K, Chen Y-T, Kujawski SA. Changes in vaccine administration trends across the life-course during the COVID-19 pandemic in the United States: a claims database study. Expert Rev Vaccines. 2023 Jan;22(1):481–494. doi:10.1080/14760584.2023.2217257.
- Ventola CL. Immunization in the United States: recommendations, barriers, and measures to improve compliance: part 2: adult vaccinations. P T. 2016;41(8):492–506. https://www.ncbi.nlm.nih.gov/pubmed/27504066.
- Tan L. Adult vaccination: now is the time to realize an unfulfilled potential. Hum Vaccin Immunother. 2015;11(9):2158–2166. doi: 10.4161/21645515.2014.982998.
- Community Preventive Services Task Force (CPSTF). Increasing appropriate vaccination: health care system-based interventions implemented in combination. 2015 Feb 13 [Accessed 2022 Nov 4]. https://www.thecommunityguide.org/sites/default/files/assets/Vaccination-Health-Care-System-Based.pdf.
- US Department of Health and Human Services. Vaccines national strategic plan 2021-2025. 2021. Accessed 2023 Dec 8. https://www.hhs.gov/sites/default/files/HHS-Vaccines-Report.pdf.
- Pollard AJ, Bijker EM. A guide to vaccinology: from basic principles to new developments. Nat Rev Immunol. 2021 Feb;21(2):83–100. doi:10.1038/s41577-020-00479-7.
- Murray E, Bieniek K, Del Aguila M, Egodage S, Litzinger S, Mazouz A, Mills H, Liska J. Impact of pharmacy intervention on influenza vaccination acceptance: a systematic literature review and meta-analysis. Int J Clin Pharm. 2021 Oct 01;43(5):1163–1172. doi:10.1007/s11096-021-01250-1.
- Burson RC, Buttenheim AM, Armstrong A, Feemster KA. Community pharmacies as sites of adult vaccination: a systematic review. Hum Vaccin Immunother. 2016;12(12):3146–59. doi: 10.1080/21645515.2016.1215393.
- Sp ME, Chesson HW, Unger ER, Romero JR, Markowitz LE, Markowitz LE. Human papillomavirus vaccination for adults: updated recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2019;68(32):698–702. doi:http://dx.doi.org/10.15585/mmwr.mm6832a3.
- Centers for Disease Control and Prevention (CDC). Recommended vaccines by disease. 2022 [accessed 2024 June 7]. https://www.cdc.gov/vaccines/vpd/vaccines-diseases.html.
