ABSTRACT
An international meeting of genetically modified (GM) food safety assessors from the main importing and exporting countries from Asia and the Americas was held in Buenos Aires, Argentina, between June 26th and 28th, 2013. Participants shared their evaluation approaches, identified similarities and challenges, and used their experience to propose areas for future work. Recommendations for improving risk assessment procedures and avenues for future collaboration were also discussed. The deliberations of the meeting were also supported by a survey of participants which canvassed risk assessment approaches across the regions from which participants came. This project was initiated by Argentine Agri-Food Health and Quality National Service (SENASA, Ministry of Agriculture, Argentina), with the support of the International Life Sciences Institute (ILSI) and other partner institutions. The importance of making all possible efforts toward more integrated and harmonized regulatory oversight for GM organisms (GMOs) was strongly emphasized. This exercise showed that such harmonization is a feasible goal that would contribute to sustain a fluid trade of commodities and ultimately enhance food security. Before this can be achieved, key issues identified in this meeting will have to be addressed in the near future to enable regulatory collaboration or joint work. The authors propose that the recommendations coming out of the meeting should be used as a basis for continuing work, follow up discussions and concrete actions.
Abbreviations
| GLP | = | good laboratory practices |
| GM | = | genetically modified |
| GMOs | = | genetically modified organisms |
| HESI | = | ILSI's Health and Environmental Sciences Institute |
| IFBiC | = | ILSI's International Food Biotechnology Committee |
| ILSI | = | International Life Sciences Institute |
| OECD | = | Organisation for Economic Co-operation and Development |
| SENASA | = | Agri-Food Health and Quality National Service (Servicio Nacional de Sanidad y Calidad Agroalimentaria of Argentina) |
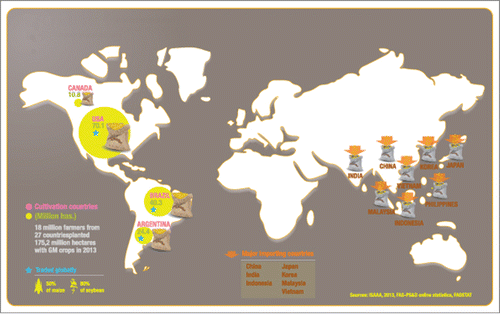
Since their introduction in 1994, transgenic crops have been widely adopted by a growing number of countries. According to the most recent ISAAA report, over 18 million farmers from some 30 countries planted 175 million ha of GM crops in 2013 (James, Citation2013). The main GM crops producers are in the Americas: the US, Brazil and Argentina currently produce 80% of the soybean and over 50% of the maize grain traded globally. In Asia, Japan and Korea are the largest maize importers, whereas China, India and the Philippines cultivate GM crops but are also net importers of large amounts of GM commodities. In fact, China (the world's largest soybean importer) is expected to break a record for soybean imports in 2014, with imports estimated to be over 60 million tons (James, Citation2013; USDA, Citation2014; AMIS, Citation2014; Fig. 1).
Despite the increase in global trade of GM commodities, different country profiles, regulatory systems, history of adoption and public perception issues make it difficult to harmonize safety assessment criteria and synchronize approval decisions. Therefore, cultivation and import approvals for GM crops, result in trade disruptions (Burachik, Citation2013) and potential problems in the food and feed supply flow (FAO, Citation2014) these differences create a growing need for dialog among countries to understand each others approaches to the safety assessment of GM crops, share experiences and build trust.
In recognition of these needs, Argentine Agri-Food Health and Quality National Service (SENASA, Ministry of Agriculture), proposed an international meetingFootnote1 to foster dialog, build confidence, and discuss differences in the perception of the hazards involved and in the subsequent interpretations of the safety assessment process for GM plant materials based on the Codex Alimentarius Plant Guidelines (Codex Alimentarius, Citation2003a, Citation2003b).
SENASA and ILSI recognized that to address the identified needs, it would be critical to gather experts from both producing and importing countries of GM crops and their derived products, focusing on the Americas and Asia, which are important trade partners. Also identified was the need to provide attendees with information on the approaches, concerns and regulatory requirements of participant nations to support their deliberations, along with information sharing during and subsequent to the meeting. To achieve this, a survey was conducted prior to the meeting. The anonymous returns were provided to and discussed by participants during the meeting. This paper describes the pre-meeting survey and the conduct, deliberations and recommendations of the meeting itself.
Pre-Meeting Survey
A survey form was sent out to all participating regulators prior to the meeting, canvassing aspects of GMO safety assessment in each country. To ensure that respondents would not be constrained or inhibited in their responses, survey returns were tallied generically, without attribution to specific countries or individuals, and were taken as indicative of the different evaluation models represented at the meeting. The survey included questions on 6 topics:
Regulatory frameworks
Implementation of Codex guidelines
Decision making process and evaluation models
Interactions between reviewers, petitioners and stakeholders
Inter-government interactions
Resources used in the evaluation process
All but one of the represented countries reported having a specific regulatory system in place to perform the safety assessment of GMOs. The respondent of the only exception indicated that their process was under development. Of the existing regulatory systems, most had already gone through one or more revisions or updates. In general, the focus is on GMOs (that is, process-based regulations), with the exception of 2 countries that have a broader scope, regulating all breeding techniques or including GMOs within a general framework for all plant-derived foods.
All represented countries indicated that they conform with Codex Guidelines, although some (4/12) have additional requirements. Examples of such requirements include whole-food sub-chronic toxicity studies, protein heat stability data, and evaluation of herbicide metabolites in herbicide-tolerant crops. In one case, public perception was identified as a factor driving specific safety assessment validations by local designated entities. All respondents clarified that additional studies or information may be eventually required on a case by case basis.
Figure 3. Additional messages and advice to specific sectors involved in GMO development and regulation.

Most countries follow a similar model for safety reviews, utilizing government regulatory staff and multidisciplinary advisory committees that make recommendations to decision-makers. Different governmental entities, such as Ministries of Agriculture, Environment, Health, Food Safety Agencies, or inter-Ministerial commissions are involved. Others establish review committees or work with technical biosafety commissions also charged with approval decisions.
With one exception, all countries have implemented permanent or ad hoc Technical Committees with experts in different disciplines performing the assessment, which can be permanent or renew their membership periodically. In a few cases, where there is a lack of a permanent regulatory staff to manage and conduct assessments, the process is fulfilled by the Technical Committee itself.
The same scientific disciplines (basically, Biology, Molecular Biology and Genetics, Plant and Food Sciences and Agronomy) are represented in all assessments committees, whose members come from public organizations (academia, government, professional associations), although seldom including plant breeders or plant geneticists. Experts on particular areas (such as nutritionists, toxicologists, entomologists and specific crop specialists) are called for focused advice when needed.
The information and resources to support safety reviews are similar for all countries (scientific literature, OECD consensus documents, databases and specific reports from developers) and 10/12 attendants use the ILSI crop composition database as a reference.
The familiarity with the Problem Formulation approach for Environmental Risk Assessment (Codex Alimentarius, Citation2003b) was also explored. While most respondents acknowledged being familiar with it, it was not clear if it is systematically applied or used by any of the respondents.
Ten out of 12 respondents countries publish decision documents or summarized reviews on their websites. All respondents indicated that their countries interact in some way with agencies in other countries or consider their assessments as a reference. Finally, most countries agreed on their willingness to collaborate with other agencies or governments. Interestingly, this exercise revealed no particular patterns in terms of requirements, regulatory focus, or evaluation models that consistently differentiated the safety evaluation in GMO-producing countries from those that only import GMOs, within the scope of the questions asked.
Conduct of the Meeting
To achieve a broad representation of producer and importing countries and of the key disciplines involved in GMO safety assessments, 23 members of regulatory agencies and 6 staff members from international organizations and academia from 13 countries (Argentina, Australia, Brazil, Canada, China, India, Indonesia, Japan, Korea, Malaysia, Philippines, US and Vietnam), together with 17 industry representatives from 5 countries (Argentina, Brazil, China. Japan and US) met in the city of Buenos Aires for 2 and half days. In addition, 5 experts from the different IFBiC and HESI Task Forces gave overviews of the state of the science on key safety assessment-related topics. In line with its mission and tripartite approach (academia, government and industry), ILSI provided a neutral forum for participants to share different approaches and experiences in the safety evaluation of GM crops, and to identify opportunities to work in more integrated ways.
Participants were invited based on their professional capacities and experience, and were explicitly not expected to represent the view or position of their countries or agencies at this meeting, but rather, focus on their experience and insights. Therefore, all comments and opinions were reported and included as group conclusions or recommendations, without being attributed to any individual in particular. This way, participants were free to share their experiences, rather than official positions of the parties. The complete agenda and report can be accessed at: http://www.ilsi.org/FoodBioTech/Documents/2013%20International% 20Meeting%20on%20Comparative%20Approaches/Meeting%20Materials/Summary_Report_Meeting_Safety_Assessment_of_GM.pdf
A series of presentations by experts in their fields was provided to stimulate discussions and to provide all participants with a baseline understanding of key issues for later consideration. These presentations covered key topics relevant to food safety assessment and included: the Codex Alimentarius (Codex Alimentarius, Citation2003a, Citation2003b) Plant Guidelines (history; scientific basis and implementation); an introduction to different safety assessment approaches (Wolt et al., Citation2010); current knowledge on natural variability and composition during plant breeding (Zhou et al., Citation2011; Herman and Price, Citation2013; Herman et al., Citation2009; Harrigan et al., Citation2010; Brune et al., Citation2013; Privalle et al., 2013; CitationHoekenga et al., 2013; Yang et al., Citation2013), domestication (Tanksley, Citation2004; Sang, Citation2009; Lenser and Theissen, Citation2013; Doebley et al., Citation2006, Flint-Garcia, Citation2013), and modification; approaches to the safety assessment of proteins and GM crops-derived food and feed (Delaney et al., Citation2008; Bushey et al., Citation2014); the role of whole-food animal feeding studies in the food safety assessment (Bartholomaeus et al., Citation2013; Ricroch, Citation2013; Snell et al., Citation2012), from which their lack of power to detect toxins became apparent, which then called the ethics of their use into question, and an overview of plant genome plasticity in relation to the likelihood of trait interactions in stacked GM crops (Weber et al., Citation2012) which made it evident that naturally occurring genetic changes are far greater than anything produced with modern biotechnology. Examples of safety assessment approaches from the perspective of exporter and importer countries were also presented by experts from Brazil and China, respectively.
Breakout sessions were held after the presentation of each general topic to share country-specific approaches to safety assessment. The scientific basis for the interpretation and application of the Codex Guidelines, their common and particular challenges were also discussed, aimed at identifying needs and areas for future work.
Meeting Conclusions
Compositional analysis
The conduct and purpose of compositional analysis should be revisited given the current state of knowledge on natural variability (Harrigan et al., Citation2010; Yang et al., Citation2013), genome plasticity and the experience with GM technology (Herman et al., Citation2009; Brune et al., Citation2013; Privalle et al., Citation2013; CitationHoekenga et al., 2013). Specific questions that need to be addressed include: Can compositional analysis be justified in its current form? If so, is its purpose still to identify unintended effects (Herman and Price, Citation2013)? Or should it be to monitor nutritional equivalence (Zhou et al., Citation2011; Privalle et al., Citation2013; CitationHoekenga et al., 2013) or toxins?
Revisiting the purpose of compositional analysis could provide scope to reframe it to focus only on critical nutrients and anti-nutrients for some trait/crop combinations, instead of a full compositional dataset, most of which has no bearing on safety.
Even against the background of the above critiques, some participants expressed the need to extend the composition databases to include data from additional countries, GM crops, new crops, and data obtained by alternative analytical methods.
Best Practice documents, less stringent, but still apt to be validated as equivalent to GLPs, should be developed on Quality Assurance Guidelines for Regulatory Sciences for compositional and other studies. This would greatly help local and public sector developers in meeting regulatory requirements.
Protein safety
The evaluation criteria for protein allergenicity and toxicity are fairly well accepted (Ladics et al., Citation2011) in general, although the weight of evidence approach could be re-defined and streamlined.
Open access platforms should be generated to share protein data and whole food safety information, regulatory decisions (in several languages), relevant documents, agencies reports on controversial studies and news related to new developments.
Whole Food Testing
There is a need to train and inform toxicologists and regulators on the key differences between food risk assessment and chemical risk assessment.
In most cases, there is no safety-based justification for whole-food subchronic toxicity tests with rodents or other animals (Bartholomaeus et al., Citation2013; Ricroch, Citation2013; Snell et al., Citation2012; Kuiper et al., Citation2001). More evidence based, specific, guidance relative to studies using whole food is needed, so as to have a better insight than is provided by currently available guidance documents (Kuiper et al., Citation2001; EFSA, 2008; EFSA, Citation2011) on when, or if, these could be scientifically warranted.
Participants recognized that all too often animal studies are conducted for the benefit of public perception rather than for scientific merit. However, it was also acknowledged that there are substantial ethical (e.g., animal welfare), reputational and risk communication issues involved with such studies.
Stacked Traits
Stacking of traits from diverse germplasm sources has been safely done by conventional breeding for many decades, and is the basis of today's modern crop varieties.
The experience with transgenic and conventional stacks has been “safe apart = safe together." The potential for harm is both theoretically remote and without supporting evidence.
For most cases, it would be possible to assess the potential for interactions leading to a hazard using available information on the individual events and the crop, using a Problem Formulation approach, or by following IFBiC´s or similar flow charts (Wolt et al., Citation2010; Steiner et al., Citation2013). Depending on jurisdiction, all stacks may merit a theoretical assessment to determine if any possible hazards have an associated plausible risk; in a few cases, laboratory or field studies may be required to obtain more data. Should a laboratory study be advisable, a first-tier evaluation would be recommended (Delaney et al., Citation2008), to determine the need for additional safety assessments.
Interagency Collaboration
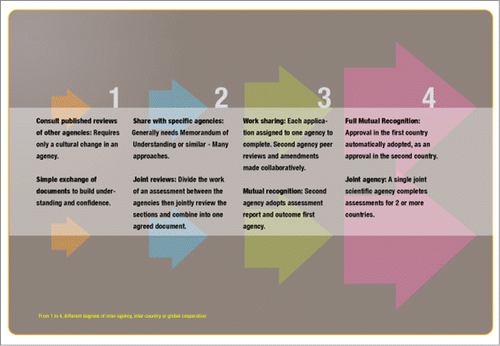
Attendees from Latin America, North America and Australia-New Zealand discussed their experiences of interagency collaboration and outlined the various levels of possible cooperation. These ranged from information-sharing in specific situations (like Low Level Presence of unapproved products in shipments of approved materials, LLP (FAO, Citation2014) and formal discussions at the regional level to explore harmonization possibilities and challenges (among MERCOSUR countries) to the creation of joint regulatory agencies like Australia and New Zealand (FSANZ). A model of the potential stages and levels of interagency engagement and collaboration was developed (Fig. 2), which highlights opportunities requiring little formal interagency agreement through to broader, highly formal collaborative agreements.
Recommendations
The objective of this meeting was to share experiences, learn from each other, build trust and hopefully, serve as a model to promote continued discussions and cooperation between different regions toward improved dialog and coordination on GM crop and food safety assessment.
It was readily evident that all countries represented by the participants approach the safety assessment of GM plants in a similar manner and conform with Codex as the general reference.
There was a clear consensus on the need to advance and update criteria for safety assessment, particularly in the light of the current knowledge on natural variability and on genome plasticity, the effects of domestication and breeding on the plant genome as compared with genetic engineering, and the experience with GM crop cultivation and consumption.
The following recommendations were made on opportunities to improve the risk assessment processes:
Risk assessors should be professional, highly trained and capable of clearly communicate their decisions to the authorities and the public.
Given that conventional plant breeding is intended to be the baseline for the evaluation of transgenic crops as described in the original Codex documents, more plant breeding and plant genetics experts should be participating in the expert committees and regulatory structures responsible for assessing GMO safety.
A process whereby countries collaboratively reach an agreement on the safety of transgenic proteins/crops should be developed. Regional or sub-regional approaches should be taken.
Criteria are needed to determine when a GM event becomes “conventional” after several years of use, so that it can be acceptable as a comparator for further events.
Criteria should be developed to identify solid, relevant evidence, used to support decision making, and rejecting irrelevant/poor quality or misrepresented studies.
Online databases need be developed/improved to provide better access to information, data, and decisions from other regulatory agencies and related bodies. As a minimum, these should share links with each other.
Resources should be developed to help local developers work with high quality standards for the generation of data of regulatory studies. While such data need not meet GLP criteria, it should meet sound Quality Assurance validation.
Expand compositional databases to include information from additional GM crops and regions.
Harmonization of evaluation criteria for breeding stacks; this is an area where there is the greatest potential for agreement.
The importance of making all possible efforts toward more integrated and harmonized regulatory oversight for GMOs cannot be over emphasized. This exercise showed that such harmonization is a feasible goal that would contribute to a fluid trade of commodities and ultimately, food security. But before this can be achieved, the key issues that have been identified will have to be addressed in the near future to enable regulatory collaboration and joint work. The authors propose that the recommendations coming out of the meeting will be used as a basis for continuing work, follow up discussions and concrete actions (Fig. 3).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
1011886_Supplementary_Materials.zip
Download Zip (374.6 KB)Acknowledgments
We want to express our gratitude to attendees, for their commitment and active participation; to speakers,Footnote2 who gave their time and expertise, to IFBIC and its membership, board staff who made this participation possible. To sponsoring organizations: Croplife International, ArgenBio, PBS (Program for Biosafety Systems of the Biodiversity Convention), ILSI Brazil, ILSI SEA and ILSI´s Research Foundation´s Center for Environmental Risk Assessment (CERA). We also want to recognize ILSI India, ILSI Korea and ILSI Focal Point in China for their valuable assistance. A special recognition to Facilitators and Rapporteurs: Drs. Taiwo Koyejo, Gabriela Levitus, Andres Maggi, Don McKenzie, Morven McLean, Clara Rubinstein and Eugenio Ulian and also to Dr Kevin Glenn and Anna Bickel for helpful discussion and assistance with the manuscript. And finally and especially, to SENASA (Agri-Food Health and Quality National Service) and to ILSI Argentina´s members and staffs for organizing and hosting this meeting in Buenos Aires.
Notes
1 This proposal was supported by ILSI Argentina, the International Food Biotechnology Committee (IFBIC) as well as by other ILSI entities such as the Center for Environmental Risk Assessment (CERA) of the ILSI Research Foundation, HESI (the Health and Environmental Sciences Institute of ILSI) and ILSI branches from other participating countries.
2 Link to List of Speakers: http://www.ilsi.org/FoodBioTech/Documents/2013%20International%20Meeting%20on%20Comparative%20Approaches/Meeting%20Materials/c_Speakers_Meeting_Safety_Assessment_of_GM.pdf
REFERENCES
- AMIS. Market Monitor. Agricultural market information system. N 19, June 2014. Available at: www.amis-outlook.org
- Bartholomaeus A, Parrott W, Bondy G. The use of whole food animal studies in the safety assessment of genetically modified crops: limitations and recommendations. Crit Rev Toxicol 2013; 43 S2:1-24; PMID:24164514; http://dx.doi.org/10.3109/10408444.2013.842955
- Brune PD, Culler AH, Ridley WP, Walker K. Safety of GM crops: compositional analysis. J Agric Food Chem 2013; 61:8243-7; PMID:24266762; http://dx.doi.org/10.1021/jf401097q
- Burachik M. The trade dispute about genetically engineered products: argentina against the european communities. AgBioForum 2013; 16, 170-6
- Bushey DF. Bannon GA, Delaney BF, Graser G, Hefford M, Jiang X, Lee TC, Madduri KM, Pariza M, Privalle LS. et al. Characteristics and safety assessment of intractable proteins in genetically modified crops. Reg Toxicol Pharmacol 2014; 69:154-70; PMID:24662477; http://dx.doi.org/10.1016/j.yrtph.2014.03.003
- Codex Alimentarius. Guideline for the conduct of food safety assessment of foods derived from recombinant-DNA plants (CAC/GL 45-2003). Codex Alimentarius Commission. Rome, Italy: Joint FAO/WHO Food Standards Programme; 2003a
- Codex Alimentarius. Principles for the risk analysis of foods derived from modern biotechnology. CAC/GL 44-20032003. Codex Alimentarius Commission. Rome, Italy: Joint FAO/WHO Food Standards Programme; 2003b
- Delaney B. Astwood JD, Cunny H, Conn RE, Herouet-Guicheney C, Macintosh S, Meyer LS, Privalle L, Gao Y, Mattsson J., et al. Evaluation of protein safety in the context of agricultural biotechnology. Food Chem Toxicol 2008; 46 2:S71-97; PMID:18348900; http://dx.doi.org/10.1016/j.fct.2008.01.045
- Doebley JF, Gaut BS, Smith BD The molecular genetics of crop domestication. Cell 2006; 127:1309-21; PMID:17190597; http://dx.doi.org/10.1016/j.cell.2006.12.006
- EFSA., Safety and nutritional assessment of GM plants and derived food and feed: The role of animal feeding trials. Report of the EFSA GMO panel working group on animal feeding trials. Food Chem Toxicol 2008; 46, S2-S70; PMID:18328408
- EFSA. Scientific opinion on guidance for risk assessment of food and feed from genetically modified plants. EFSA J 2011; 9:2150
- FAO. Technical Consultation on Low Levels of Genetically Modified (GM) Crops in International Food and Feed Trade. Rome, Italy, 2014, Narrative Report. 20-1. 2014. Available from: http://www.fao.org/fileadmin/user_upload/agns/topics/LLP/AGD803_6_Report_En.pdf
- Flint-Garcia SA. Genetics and consequences of crop domestication. J Agric Food Chem 2013; 61:8267−76; PMID:23718780; http://dx.doi.org/10.1021/jf305511d
- Harrigan GG. Lundry D, Drury S, Berman K, Riordan SG, Nemeth MA, Ridley WP, Glenn KC. Natural variation in crop composition and the impact of transgenesis. Nat Biotechnol 2010; 28:402-4; PMID:20458297; http://dx.doi.org/10.1038/nbt0510-402
- Herman RA., Chassy BM, Parrott W. Compositional assessment of transgenic crops: an idea whose time has passed. Trend Biotechnol 2009; 27:555-7; PMID:19699543; http://dx.doi.org/10.1016/j.tibtech.2009.07.003
- Herman RA, Price WD. Unintended compositional changes in genetically modified (GM) crops 20 years of reasearch. J Agric Food Chem 2013; 61:11695-701; PMID:23414177; http://dx.doi.org/10.1021/jf400135r
- Hoekenga OA, Srinivasan J, Barry G, Bartholomaeus A. Compositional analysis of genetically modified (GM) crops: key issues and future needs. J Agric Food Chem 61, 8248−53; PMID:23746303; http://dx.doi.org/10.1021/jf401141r
- James C. Global status of commercialized biotech/GM crops: 2013. ISAAA Brief No. 46. ISAAA: Ithaca, NY; 2013
- Kuiper HA, Kleter GA, Noteborn PJM, Kok EJ. Assessment of the food safety issues related to genetically modified foods. Plant J 2001; 27, 503-28; PMID:11576435; http://dx.doi.org/10.1046/j.1365-313X.2001.01119.x
- Ladics GS. Cressman RF, Herouet-Guicheney C, Herman RA, Privalle L, Song P, Ward JM, McClain S. Bioinformatics and the allergy assessment of agricultural biotechnology products: industry practices and recommendations. Regul Toxicol Pharmacol 2011; 60:46-53; PMID:21320564; http://dx.doi.org/10.1016/j.yrtph.2011.02.004
- Lenser T, Theissen G. Molecular mechanisms involved in convergent crop domestication. Trend Plant Sci 2013; 18, 704-14; PMID:24035234; http://dx.doi.org/10.1016/j.tplants.2013.08.007
- Privalle LS, Gillikin N, Wandelt C, Bringing a transgenic crop to market: where compositional analysis fits. J Agric Food Chem 2013; 61:8260−6; PMID:23534903; http://dx.doi.org/10.1021/jf400185q
- Ricroch AE. Assessment of GE food safety using ‘-omics’ techniques and long-term animal feeding studies. New Biotechnol 2013; 30, 349-54; PMID:23253614; http://dx.doi.org/10.1016/j.nbt.2012.12.001
- Sang T Genes and mutations underlying domestication transitions in grasses. Plant Physiol 2009; 149, 63-70; PMID:19126696; http://dx.doi.org/10.1104/pp.108.128827
- Snell C. Bernheim A, Bergé JB, Kuntz M, Pascal G, Paris A, Ricroch AE. Assessment of the health impact of GM plant diets in long-term and multigenerational animal feeding trials: a literature review. Food Chem Toxicol 2012; 50:1134-48; PMID:22155268; http://dx.doi.org/10.1016/j.fct.2011.11.048
- Steiner HY. Halpin C, Jez JM, Kough J, Parrott W, Underhill L, Weber N, Hannah LC. Evaluating the potential for adverse interactions within genetically engineered breeding stacks. Plant Physiol 2013; 161 1587-94; PMID:23460691; http://dx.doi.org/10.1104/pp.112.209817
- Tanksley SD, The genetic developmental and molecular bases of fruit size and shape variation in tomato. Plant Cell 2004; 16 2004:S181-S189; PMID:15131251; http://dx.doi.org/10.1105/tpc.018119
- USDA. World Agricultural Supply and Demand Estimates. Agricultural marketing service. economic research service. foreign agricultural service. WASDE 530. June 11, 2014. Available at: http://usda.mannlib.cornell.edu/
- Weber N, Halpin C, Hannah LC, Jez JM, Kough J, Parrott W. Editor's choice: Crop genome plasticity and its relevance to food and feed safety of genetically engineered breeding stacks. Plant Physiol 2012; 160:1842-1853; PMID:23060369; ; 10.1104/pp.112.204271
- Wolt JD. Keese P, Raybould A, Fitzpatrick JW, Burachik M, Gray A, Olin SS, Schiemann J, Sears M, Wu F. Problem formulation in the environmental risk assessment for genetically modified plants. Transgen Res 2010; 19, 425-36; PMID:19757133; http://dx.doi.org/10.1007/s11248-009-9321-9
- Yang XS, Staub JM, Pandravada A, Riordan SG, Yan Y, Bannon GA, Martino-Catt SJ. Omics technologies reveal abundant natural variation in metabolites and transcripts among conventional maize hybrids. Food Nutr Sci 2013; 4:335-41; http://dx.doi.org/10.4236/fns.2013.43044
- Zhou J. Harrigan GG, Berman KH, Webb EG, Klusmeyer TH, Nemeth MA. Stability in the composition equivalence for grain from insect protected maize and seed from glyphosate-tolerant soybean to conventional counterparts over multiple season, locations, and breeding germplasms. J Agric Food Chem 2011; 59:8822-8; PMID:21797257; http://dx.doi.org/10.1021/jf2019038