ABSTRACT
In order to proactively identify emerging issues that may impact the risk assessment and risk management functions of the Indian biosafety regulatory system, the Ministry of Environment, Forests and Climate Change sought to understand the nature and diversity of genetically engineered crops that may move to product commercialization within the next 10 y. This paper describes the findings from a questionnaire designed to solicit information about public and private sector research and development (R&D) activities in plant biotechnology. It is the first comprehensive overview of the R&D pipeline for GE crops in India.
INTRODUCTION
In India, the regulation of genetically engineered (GE) organisms is prescribed in rules notified by Ministry of Environment and Forests (now the Ministry of Environment, Forests and Climate Change; MoEF&CC), Government of India on December 5, 1989 under the Environment (Protection) Act 1986. These rules, commonly referred to as Rules 1989, cover the manufacture, import, use, research and release of GE organisms and derived products in India. (Rules for the Manufacture, Citation1989) The key regulatory bodies responsible for implementation of the Rules 1989 are the Genetic Engineering Appraisal Committee (GEAC) in MoEF&CC, and the Review Committee on Genetic Manipulation (RCGM) in the Department of Biotechnology (DBT).
Regulatory systems cannot remain static; technological innovation is a dynamic driver of how biotechnology is being applied to develop new products and processes internationally. Since these are regulated activities in India, it is important for GEAC and RCGM to remain apprised of these changes so that risk assessment guidance, supporting resources, and outreach and training of stakeholders can be appropriately modified. In order to proactively identify emerging issues that may impact the risk assessment and risk management functions of the Indian biosafety regulatory system, MoEF&CC conducted a survey to understand the nature and diversity of genetically engineered crops (also referred to as transgenic crops herein) that may move to product commercialization within the next 10 y. This is the first comprehensive overview of the R&D pipeline for GE crops in India.
MATERIALS AND METHODS
An electronic questionnaire was prepared to identify what GE crops and traits are currently under development or are anticipated to be developed within the next 10 y in India (2014–2024). It was distributed to 1050 individual scientists from 320 public and private sector organizations that are involved in plant breeding R&D in India based on known research activities e.g., through engagement with Institutional Biosafety Committees. The questionnaire was comprised of 2 categories of questions: (1) general questions related to information about the respondent's institution and R&D activities; and (2) specific questions to provide information about specific transgenic plants currently under development (see Box 1). A total of 243 responses to the questionnaire were received by the closing date of May 1, 2014 which was a response rate of 23%.
RESULTS AND DISCUSSION
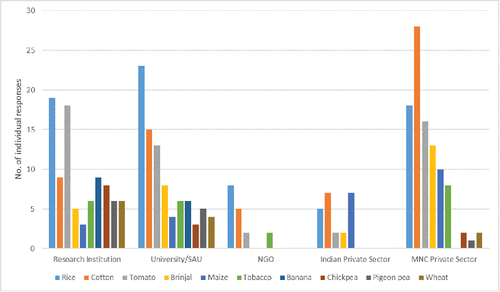
India has a very rich and innovative R&D pipeline. Respondents to the product pipeline questionnaire identified over 85 different plant species currently being used in experimental work, including plants used for food, livestock feed, fiber, fuel, and dietary or medicinal purposes. The ten most prevalent crops are presented in , and a comprehensive list of all of the crop species identified by respondents is in . Trait variation was also found to be extensive, ranging from resistance traits for biotic stressors, to abiotic stress tolerances (e.g., drought, salt, heavy metals, etc.) to truly novel nutritional, medicinal or metabolic phenotypes as seen in .
FIGURE 2. Aggregated responses for traits that are being studied in R&D programs (total number of responses received for each trait). MS/FR = Male-sterility, fertility restoration pollination control system.
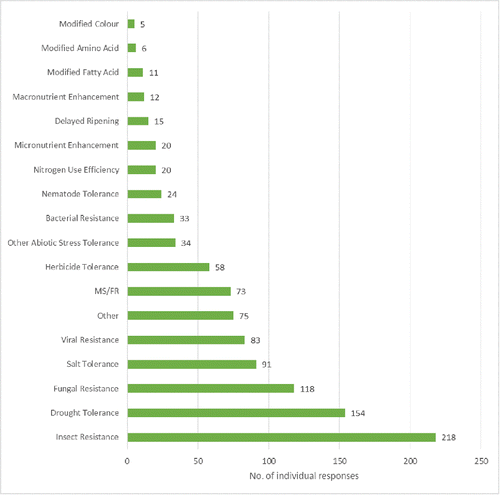
TABLE 1. A list of all crops/plants in the R&D pipeline as identified by respondents to the questionnaire (the number in parentheses indicates the number of respondents who listed the crop/plant)
and demonstrate that Indian plant biotechnology R&D is being used to develop plant products that are relevant to Indian agriculture today, but it is also forward looking. For example, there is significant research on traits that are relevant to mitigating the impacts of climate change on agriculture, which will be important to ensuring that agricultural productivity is maintained and ultimately improved. (Swaminathan and Kesavan, Citation2012) Productivity constraints in crops that are particularly relevant to smallholder farmers (e.g., pulses, millets) are also receiving significant attention, with important implications for improved food and nutrition security. (Erskine et al., Citation2011, National Academy of Agricultural Sciences, Citation2013)
The majority of R&D projects reported in the questionnaire are early phase. For example, 80% of the respondents indicated that their projects were limited to basic research, transformation and regeneration, or early phase event selection in contained (i.e., laboratory, growth chamber or greenhouse) conditions, and only 20% had progressed to event selection in confined field trials. Plant transformation is a commonly used and very important research tool, and it is likely that many of the R&D projects identified by respondents are experimental system or proof-of-concept projects. This is important information as it emphasizes that the biosafety regulatory system must be responsive to all types of agricultural biotechnology research, be it for knowledge generation or product development. Differentiating basic R&D projects from those that are intended for advancement through to commercial product release will help in informing prospective biosafety risk assessment and regulation needs, and so should be determined through a separate, systematic study.
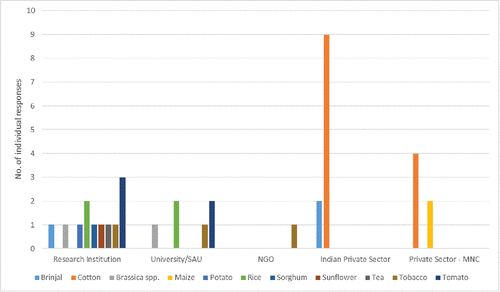
Public sector plant biotechnology R&D in India is very strong, with 73% of all respondents self-identifying from public sector research institutions, state agricultural or other universities. Of the 57 respondents who identified as private sector, 78% were from Indian companies indicating that the domestic private sector is strongly represented in plant biotechnology R&D. The majority of all 127 respondents who anticipate eventual commercialization expect India to be the first adopter of the GE crops they are developing. This is a strong indication that the GE plants under development by Indian scientists are intended for the Indian market, and that the R&D investments made in India should result in benefits for Indian farmers and consumers. Eighty-eight respondents to Q21 indicated that they anticipate submitting applications for commercial release (i.e., cultivation, and use in food and/or feed) of their GE crops, with 8% and 6% of these identifying 2015 and 2016 as target years for approval. Anticipated products for commercialization from the private sector are limited to brinjal, cotton and maize, whereas applications from the public sector encompass a much broader range of crops ().
FIGURE 3. Applications for commercial approvals that respondents anticipate will be submitted to Indian regulatory authorities by the end of 2016.
The results of the questionnaire also demonstrated a significant gap between respondents' expectations for product delivery to farmers (i.e., commercial release of GE crops) and the reality of what is actually required to achieve this, including the time it takes to address biosafety regulatory requirements. This means that many respondents are either unaware of what is required by regulators to demonstrate that a novel GE plant is as safe as its conventional counterpart, or have unrealistic expectations about how promptly products can advance from contained research, through Biosafety Research Level I (BRLI) and Biosafety Research Level II (BRLII) field trials (DBT and MoEF&CC, Citation2008), to approvals for cultivation and consumption/use. This means that, at a minimum, additional education and outreach is needed at the institutional level to improve understanding about the staged pathway to product deployment. Product developers must have a realistic understanding of both the time and financial implications of meeting regulatory requirements in order to make informed decisions about whether project objectives can be achieved.
Internationally there is considerable experience as regards assessing the environmental and food safety of GE plants expressing insect resistance and/or herbicide tolerance traits, including published risk assessments conducted by competent authorities in many countries. While makes clear that these traits are already common in Indian R&D programs, the Indian regulatory system must also prepare for a potential scenario of new plants expressing new traits with which there is relatively less experience in India and elsewhere. This will be greatly facilitated if the development of tools and guidance needed to address these future challenges is initiated in the near term. Some suggestions are provided below.
Environmental risk and food safety assessment of GE crops are comparative exercises requiring specific kinds of information about the non-transformed host plant and its derived food and feed products. Biology and crop composition documents such as those published by the Organization for Economic Cooperation and Development (OECD, Citation2006) are very useful resources for this purpose. The Government of India has published biology documents that complement the OECD documents by providing information that is specific to the Indian context for cotton, (MoEF&CC and DBT, Citation2011) maize, (MoEF&CC and DBT, Citation2011) okra, (MoEF&CC and DBT, Citation2011) and rice, (MoEF&CC and DBT, Citation2011) and MoEF&CC is currently developing the same for chickpea, pigeon pea, sorghum, papaya, mustard, tomato, rubber and potato. The intent of these crop-specific biology documents is to describe information that is directly relevant to environmental risk assessment in a format that is accessible to risk assessors and regulators. The document is an overview of pertinent biological information on the untransformed (i.e., conventional or non-transgenic) species that can be used as a reference to help define the baseline and scope for the conventional comparator against which transformed organisms will be compared in the risk assessment. As seen in , there are many other crop species that could potentially be submitted for regulatory permits and approvals in India, and so additional biology documents will need to be prepared after a careful assessment that determines which of these new plant species are intended for eventual BRLI and BRLII confined field trials and commercialization (as some are being studied for basic research under contained conditions only). For less familiar crop species, it is likely that gaps in information needed for risk assessment (and hence the preparation of biology documents) may have to be addressed through field research. For example, understanding the reproductive biology of the non-transgenic plant species is essential to determine appropriate isolation practices so that confined BRLI and BRLII field trials of the experimental, transgenic plants can be effectively managed. Similarly, it will be necessary to identify important food and feed nutrients, as well as anti-nutrients, toxins or allergens that naturally occur in those plant species that will be used for food and/or feed so that any changes in these that lie outside of the normal range of variation can be identified and evaluated as part of the safety assessment process.
Risk assessors may wish to consider if there are any new, plausible risk hypotheses (Garcia-Alonso and Raybould, Citation2014, Wolt et al., Citation2010) to be addressed when assessing the potential environmental impacts of transgenic plants expressing abiotic stress tolerance traits (see ). If there are, then how these should be addressed must be clearly described in an appropriate guidance document. As seen in , drought tolerance is the second most popular trait in the R&D pipeline, and so product developers need to know what additional kinds of studies (if any) will be necessary to meet their regulatory obligations.
In regards to the safety assessment of foods derived from plants with altered nutritional or metabolic profiles (see ), the Government of India's Guidelines for the Safety Assessment of Foods Derived from Genetically Engineered Plants was updated in 2012 to include a framework to address the safety assessment of foods derived from transgenic plants modified for nutritional or health benefits. (ICMR, Citation2008) This update made sure that the Indian guidance was consistent with the Codex Alimentarius Commission's Guideline for the Conduct of Food Safety Assessment of Foods Derived from Recombinant-DNA Plants CAC/GL 45–2003. (CAC, Citation2008) Ensuring that risk assessors have the technical capacity to apply this framework is important as a significant number of R&D projects were identified that relate to nutritional or metabolic changes (see ). also lists a number of medicinal plant species which raises interesting questions relative to existing regulations for traditional medicines, and how GE versions of these plants should be managed from risk assessment and regulatory oversight perspectives.
This survey, while being the first comprehensive overview of the R&D pipeline for GE crops in India, is preliminary only and the results indicate that more detailed follow-up is required. For example, it would be very instructive to follow-up with public sector institutions to get a much clearer idea as to how many of the research projects being undertaken are aspirational versus realistic in terms of future commercial release. This is needed to ensure institutional preparedness for compliance with the regulatory system, as well as for post-approval considerations such as stewardship, market access and trade implications.
ABBREVIATIONS
| GE | = | Genetically engineered |
| GEAC | = | Genetic Engineering Appraisal Committee |
| MoEF&CC | = | The Ministry of Environment, Forests and Climate Change, Government of India |
| R&D | = | Research and development |
| RCGM | = | Review Committee on Genetic Manipulation |
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Morven McLean, ILSI Research Foundation, for her assistance with this study.
Funding
This work was conducted under the auspices of the Phase II Capacity Building Project for Implementation of the Cartagena Protocol on Biosafety with funding from the United Nations Environment Program.
REFERENCES
- CAC. Guideline for the Conduct of Food Safety Assessment of Foods Derived from Recombinant-DNA Plants, CAC/GL 45–2003. Geneva: Codex Alimentarius Commission, 2008.
- DBT and MoEF&CC. Guidelines and Standard Operating Procedures (SOPs) for Confined Field Trials of Regulated, Genetically Engineered (GE) Plants. New Delhi: Department of Biotechnology, Ministry of Science and Technology and Ministry of Environment, Forests and Climate Change, 2008.
- Erskine W, Sarker A, Kumar S. Investing in lentil improvement toward a food secure world. Food Security 2011; 3(2):127-139; http://dx.doi.org/10.1007/s12571-011-0124-5
- Garcia-Alonso M, Raybould A. Protection goals in environmental risk assessment: a practical approach. Transgenic Res 2014; 23(6):945-56; PMID:24154954; http://dx.doi.org/10.1007/s11248-013-9760-1
- ICMR. Guidelines for the Safety Assessment of Foods Derived from Genetically Engineered Plants. New Delhi: Indian Council of Medial Research, 2008.
- MoEF&CC and DBT. Biology of Oryza sativa L. (rice). Series of Crop Specific Biology Documents New Delhi: Ministry of Environment, Forests and Climate Change and the Department of Biotechnology, Ministry of Science and Technology, 2011.
- MoEF&CC and DBT. Biology of Abelmoschus esculentus L. (okra). Series of Crop Specific Biology Documents 2011. New Delhi: Ministry of Environment, Forests and Climate Change and the Department of Biotechnology, Ministry of Science and Technology.
- MoEF&CC and DBT. Biology of Zea mays (maize). Series of Crop Specific Biology Documents. New Delhi: Ministry of Environment, Forests and Climate Change and the Department of Biotechnology, Ministry of Science and Technology, 2011.
- MoEF&CC and DBT. Biology of Gossypium spp. (cotton). Series of Crop Specific Biology Documents. New Delhi: Ministry of Environment, Forests and Climate Change and the Department of Biotechnology, Ministry of Science and Technology, 2011.
- National Academy of Agricultural Sciences. Role of millets in nutritional security of India. New Delhi: National Academy of Agricultural Sciences 2013; Policy Paper 66.
- OECD. Safety Assessment of Transgenic Organisms: OECD Consensus Documents, Volumes 1–4 2006, 2010. Organization of Economic Cooperation and Development, Paris.
- Rules for the Manufacture, Use/Import/Export and Storage of Hazardous Microorganisms/Genetically Engineered Organisms or Cells notified under Environment Protection Act of 1986. Government of India: Ministry of Environment and Forests, 1989.
- Swaminathan MS, Kesavan PC. Agricultural research in an era of climate change. Agric Res 2012; 1(1):3-11; http://dx.doi.org/10.1007/s40003-011-0009-z
- Wolt JD, Keese P, Raybould A, Fitzpatrick JW, Burachik M, Gray A, Olin SS, Schiemann J, Sears M, Wu F. Problem formulation in the environmental risk assessment for genetically modified plants. Transgenic Res 2010; 19(3):425-436; PMID:19757133; http://dx.doi.org/10.1007/s11248-009-9321-9