ABSTRACT
The global agricultural landscape regarding the commercial cultivation of genetically modified (GM) crops is mosaic. Meanwhile, a new plant breeding technique, genome editing is expected to make genetic engineering-mediated crop breeding more socially acceptable because it can be used to develop crop varieties without introducing transgenes, which have hampered the regulatory review and public acceptance of GM crops. The present study revealed that product- and process-based concepts have been implemented to regulate GM crops in 30 countries. Moreover, this study analyzed the regulatory responses to genome-edited crops in the USA, Argentina, Sweden and New Zealand. The findings suggested that countries will likely be divided in their policies on genome-edited crops: Some will deregulate transgene-free crops, while others will regulate all types of crops that have been modified by genome editing. These implications are discussed from the viewpoint of public acceptance.
INTRODUCTION
Two decades have passed since the advent of the Flavr Savr tomato (Solanum lycopersicum), which was the first genetically modified (GM) food crop approved for consumption in the United States. However, the business failed. (Bruening and Lyons, Citation2000) Subsequently, the commercial cultivation of GM crops, including soybeans (Glycine max), maize (Zea mays), cotton (Gossypium arboreum) and canola (Brassica napus), has expanded in at least 28 countries (ISAAA, Citation2015). The leading countries are the United States, Brazil, Argentina, India, Canada, China and Paraguay. In contrast, no GM crops have been commercially cultivated in the Russian Federation, (The_US_Library_of_Congress, Citation2014) most of the EU countries (except in Spain, Portugal, Czech Republic, Slovakia and Romania), (Lucht, Citation2015) New Zealand, (The_US_Library_of_Congress, Citation2014) the Republic of Korea, (The_US_Library_of_Congress, Citation2014) Japan (The_US_Library_of_Congres, Citation2014) and a number of countries. One of major concerns surrounding GM crops is the possibility that their transgenes, which are derived from an unrelated cross-incompatible species, could have adverse effects on the environment and/or human health. Some countries, represented by the United States, regulate GM crops on a product-basis; others, including EU member states, adopt process-based GM crop regulations that demand a more rigorous risk assessment throughout the process of GM crop development (Araki and Ishii, Citation2015; Davidson, Citation2010; Hartung and Schiemann, Citation2014).
Zinc-finger nucleases (ZFNs), (Klug, Citation2010) transcription activator-like effector nucleases (TALENs) (Joung and Sander, Citation2013) and the clustered regularly interspaced short palindromic repeat (CRISPR)/Cas9 system (Hsu et al., Citation2014) (so-called genome editing), can create DNA double-strand breaks (DSBs) at target sites, and subsequently add an exogenous gene or copy a variant via homology-directed repair (HDR) together with a DNA template. Genome editing can also create insertions or deletions (indels) of various lengths via non-homologous end-joining (NHEJ) without a DNA template, mostly resulting in gene disruption. In recent plant research, NHEJ has frequently been used to generate new varieties of major crops, including rice (Oryza sativa), wheat (Triticum), barley (Hordeum vulgare), potato (Solanum tuberosum L.), sweet orange (Citrus sinensis), tomato, soybean and maize (Araki and Ishii, Citation2015; Hartung and Schiemann, Citation2014; Ishii and Araki, Citation2016; Nagamangala Kanchiswamy et al., Citation2015; Nagamangala Kanchiswamy et al., Citation2015; Voytas and Gao, Citation2014). Such reports suggest that NHEJ will be a preferred approach in genome editing-mediated crop breeding because the resultant transgene-free plants may be considered to fall outside of GM crop regulations. This might also increase the possibility that the crops will be socially accepted. Indeed, the U.S. Department of Agriculture (USDA) officially responded to an inquiry by a developer in 2012, concluding for the first time that a variety of transgene-free maize generated via NHEJ using ZFNs was not subject to their regulations because the maize variety did not contain a ‘plant pest’ (Araki et al., Citation2014). This raises the question as to whether, regulators in other countries view transgene-free crops from a product-based or process-based stand point.
We selected 34 countries and investigated whether they implement their regulations regarding GM crops on a product- or process-basis. We then investigated the current status of regulatory responses to genome-edited crops without transgenes. Based on these findings, we discuss possible scenarios for future regulatory landscape and the possible social acceptance of genome-edited crops.
RESULTS
The Regulatory Concepts of GM Crops in Different Countries
In contrast to conventional breeding techniques, such as random mutagenesis, GM crops have – in many countries - remained the subject of GMO regulations that review the potential impact of exogenous DNA and/or relevant genetic modification on the environment and food safety prior to field trials and food consumption (Davidson, Citation2010; Chen and Lin, Citation2013; Li et al., Citation2015). Before investigating the regulatory responses to genome edited crops, it is worth understanding the different regulatory concepts of GMO regulations that have been enacted worldwide because the existing regulatory concepts may impact the regulation of genome edited crops.
In the EU, GM crops are subject to regulatory review via detailed procedures under EU Directive 2001/18/EC (2001) and EC Regulation 258/97 (1997); these are considered to be process-based GMO regulations (Araki and Ishii, Citation2015; Davidson, Citation2010; Araki et al., Citation2014; Sprink et al., Citation2016). Currently, maize is the only GM crop to be commercially cultivated in the EU. It is cultivated in limited areas (0.1 million hectares or less) in some of the 28 member states (Spain, Portugal, Czech Republic, Slovakia and Romania). (ISAAA, Citation2015) In contrast, the USA is considered to regulate GM crops primarily under 7 CFR Part 340 and Part 360, which imply that the risks associated with GM crops should be assessed based on the final product rather than on the processes involved in producing the product (Araki and Ishii, Citation2015; Araki et al., Citation2014; Policy, Citation2016). With a total of 70.9 million hectares of GM maize, soybean, cotton canola, sugar beet, alfalfa, papaya, squash and potato, the USA is the world's biggest producer of GM crops (ISAAA, Citation2015). The great differences between the EU and the USA suggest that process-based regulations discourage GM crop cultivation, whereas, product-based regulations encourage it (Davidson, Citation2010; Policy, Citation2016). We therefore decided to investigate whether different countries implement product-based or process-based GMO regulations.
We judged the regulatory concepts according to the definition of GMO in the relevant regulations (Table S1). If the definition of GMO was based on a final product or the GM organism that possesses a specific genetic status (such as ‘a novel combination of genetic material obtained through the use of modern biotechnology’), the concept was deemed to be a product-based regulation. If the definition simply referred to genetically modified organisms or focused on the process of genetic modification (such as ‘the genetic material has been altered in a way that does not occur naturally by mating and/or natural recombination’), the concept was regarded as a process-based regulation. We selected 28 countries in which GM crops are currently cultivated for commercial purposes. Moreover, we added the EU, and the UK which may change its regulations after it secedes from the EU. We also included Japan and Republic Korea because the 2 countries are in close proximity to China. New Zealand was added to the analysis due to its close proximity to Australia where GM crops are commercially cultivated. Furthermore, the Russian Federation was added because it has the world's largest acreage.
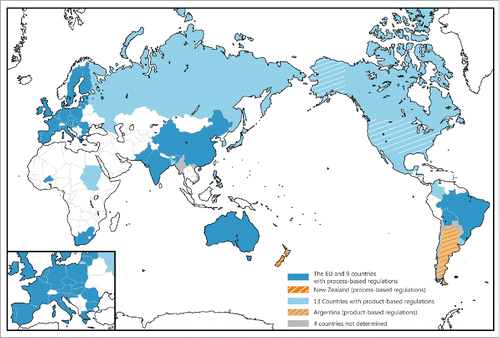
As a result, the concepts of GMO regulations in a total of 34 countries were analyzed (). The regulatory concepts of 5 countries in which GM crops are commercially cultivated (Paraguay, Myanmar, Chile and Vietnam) were unclear (N.D.) due to the lack of a clear definition in the relevant regulation or no accessibility of legal documents. Regarding the remaining 30 countries (24 countries with commercial cultivation and 6 countries with no commercial cultivation), we judged that South Africa, Mexico, and Japan have different regulatory concepts to those described by Ramessar et al. (Ramessar et al., Citation2008). Such differences in regulatory concepts may be attributed to the different interpretations of legal documents. However, we contend that Japan adopts product-based regulations, not process-based regulations, as suggested by a report by the Science Council of Japan (Science_Council_of_Japan Citation2014). Nevertheless, the results of our analysis were largely in accord with those by Ramessar et al. (Ramessar et al., Citation2008) in that the USA, Argentina, Canada, the Philippines and Bangladesh were deemed to have product-based regulations, while Brazil, India, China, Bolivia, Australia, Burkina Faso, the EU, New Zealand and the UK were considered to have process-based regulations, and in that the regulatory concept of Chile was considered to be unclear (N.D.) ().
TABLE 1. The regulatory concept of genetically modified (GM) crops in 34 countries.
Regarding the 24 countries in which GM crops are commercially cultivated, 11 countries were deemed to adopt product-based regulations, whereas 13 countries were considered to have process-based regulations (). Our analysis suggests that are no significant differences in the number of regulatory concepts that are adopted among the 24 countries. This tendency is largely similar, even in the top 10 GM crop cultivating countries: 4 countries with product-based regulations (the USA, Argentina, Canada and Uruguay); 5 countries with process-based regulations (Brazil, India, China, Pakistan and South Africa); and one country in which the regulatory concept was considered to be unclear (Paraguay). In the countries in which no GM crops are commercially cultivated, 3 countries have employed product-based regulations, whereas EU, New Zealand and UK adopt process-based regulation. Importantly, the Russian Federation, which has adopted product-based regulations, currently prohibits the commercial cultivation of GM crops.
The Cartagena Protocol on Biosafety (CPB) is an international agreement that aims to ensure the safe handling, transport and use of living modified organisms (LMOs; a technical legal term that is closely related to GMOs) (The_Convention_on_Biological_Diversity, Citation2016). As of August 2016, it had been ratified by 170 countries; however, the USA, Argentina, Canada, Australia, Chile and the Russian Federation, have not ratified the protocol. It is noteworthy that GM crops are cultivated for commercial purposes in the 5 above-mentioned countries. Among these countries, the USA, Argentina and Canada, which are included in the top 10 countries for the cultivation of GM crops, have adopted product-based regulations (). More importantly, there is division in the regulatory concepts that are adopted in the countries that ratified the CPB, regardless of their experience with the commercial cultivation of GM crops ().
The Regulatory Responses to Genome-Edited Crops
It has been suggested that NHEJ will be a preferred approach in crop breeding that employs genome editing because the resultant plants are considered to harbor no transgenes, which is one of the major concerns surrounding GM crops. The transgene-free plants developed via genome editing might bypass product-based GMO regulation. Moreover, some experts and interest groups have advocated that such transgene-free crops should not be regulated, even under process-based GMO regulations (Hartung and Schiemann, Citation2014; Camacho et al., Citation2014; European_Academies'_Science_Advisory_Council, Citation2015; European_Plant_Science_Organisation , Citation2015; European_Seed_Association, Citation2015). Others disagree (GM_Freeze, Citation2016; GMWATCH, Citation2014; Green_Peace, Citation2015; IFOAM_EU, Citation2015).
As noted in the Introduction, an official statement made by the USDA Animal and Plant Health Inspection Service (APHIS) 2012 noted that if a maize variety resulting from NHEJ using ZFNs has no transgenes derived from a ‘plant pest’, then it would be considered to be a non-regulated article. However, this regulatory decision was made through consideration on a case-by-case basis. More importantly, in 2015, the world's first regulation for NPBTs, including genome editing, was issued in Argentina (Resolution no.173/2015). It explicitly indicates that some products without a transgene may not fall under their product-based GMO regulations (Whelan and Lema, Citation2015). Most plant genome editing experiments use Agrobacterium-mediated transformation to deliver genome editing nucleases into plant cells (Araki and Ishii, Citation2015; Ishii and Araki, Citation2016). In such cases, the Ti plasmid that is used can be incorporated in the plant genome, which may be interpreted as a transgene even under product-based GMO regulations. Indeed, recent plant research demonstrated the genomic insertion of plasmid-derived DNA sequences by transfecting Arabidopsis protoplasts with Cas9 and gRNA plasmids (Kim and Kim, Citation2016). However, the deregulation shown in the Argentine regulations would be likely if it is shown that the plasmid-derived DNA is not present in the final product. Moreover, the deregulation would be more likely if researchers use CRISPR/Cas9 in the form of a ribonucleoprotein which can be delivered into plant cells without the use of a DNA vector (Woo et al., Citation2015).
We comprehensively surveyed the regulatory movements in relation to genome-edited crops or crop breeding by genome editing (). In 2016, New Zealand, which adopts process-based GMO regulation, amended a relevant regulation under the Hazardous Substances and New Organisms (HSNO) Act of 1996 (EPA, 2015). This amendment was performed after the High Court overruled the Environmental Protection Authority (EPA) decision in 2014 that the deregulation of transgene-free plans produced via NHEJ of genome editing (Ishii and Araki, Citation2016; Kershen, Citation2015). The amendment in the regulations showed a very different regulatory direction to Argentina, which clarified the definitions of what is and is not a GMO following consultation by the EPA. It was decided that plants bred using conventional chemical and radiation mutagenesis, which were in use in New Zealand on or before July 29, 1998, are not considered to be GMOs under the HSNO Act of 1996 (EPA, Citation2015). That is, the amended regulation can be interpreted as including plants modified via NHEJ within its regulatory scope.
TABLE 2. The regulatory status of plant breeding by genome editing.
An interesting movement has occurred regarding the regulatory view on CRISPR/Cas9-mediated gene disrupted Arabidopsis in Sweden (). Researchers in universities consulted the Swedish Board of Agriculture (the Swedish government's expert authority on the agricultural and food policies) about whether 2 different Arabidopsis varieties in which PsbS was disrupted and a point mutation was introduced in A120 respectively fall under the EU regulations. The Board concluded that such transgene-free plants are not subject to regulations based on the EU's definition of GMOs; however, it should be noted that this conclusion does not reflect decisions from either the Ministry of Agriculture or the EU. This movement, in which a governmental authority in an EU member state explicitly expressed their regulatory view in relation to genome-edited plants, is, except regulatory decisions regarding a herbicide-resistant canola variety developed using another NPBT (oligonucleotide-directed mutagenesis) (Fladung, Citation2016), unique in the EU, which has remained silent about whether plant breeding by genome editing or genome-edited plants fall under their regulations (Sprink et al., Citation2016).
We found that the USDA APHIS has responded to 6 regulatory inquiries by private and public developers since the first regulatory conclusion stating that ZFN-modified maize varieties do not include ‘plant pest’ in 2012 (). Remarkably, the APHIS concluded in the 6 inquiries under 7 CFR Part 340 and Part 360 that soybean, rice, wheat, white button mushroom and maize, which were all modified via NHEJ using TALENs or CRISPR/Cas9, are ‘non-regulated’ subjects as they do not constitute a non-plant pest and/or non-noxious weed. The purposes of such crop developments include the alternation of nutrient composition, disease resistance and the prevention of color deterioration in plants or mushroom. Therefore, such crops can be tested in open field, without the federal oversight in the USA. To date, the APHIS has deregulated the crop varieties in the 6 inquiries on a case-by-case-basis. The repeated deregulations suggest that similar crops that lack transgenes will undergo virtually no risk assessment in the USA.
In sum, Argentina established a new product-based regulation that paves the way for the rapid development of transgene-free crops generated by NPBTs such as genome editing. The USA also assumes a favorable attitude toward regulations pertaining to crop breeding by genome editing. It has not considered amending or establishing a new regulation. Likewise, a Swedish authority made their stance toward the deregulation of transgene-free plants. Conversely, New Zealand amended its process-based GMO regulations in order to regulate any type of crop breeding by genome editing. It should be noted that no countries have enacted a relevant law or amended a GMO law in the era of genome editing. The regulatory responses in New Zealand and Argentina remain at the level of an authoritative decree or enforcement ordinance under relevant law(s) ().
DISCUSSIONS
In the present report, we analyzed the GMO regulations in as many as 34 countries and confirmed the regulatory concepts in 30 countries (). The results show no significant differences in the number of countries with product- and process-based regulations in countries where GM crops are commercially cultivated. Remarkably, in the major GM crop producing countries, 3 countries with product-based regulations did not ratify the CPB (the USA, Argentina and Canada), while it was ratified by Brazil and India- 2 countries devoted to the cultivation of GM crops. It may not be useful to excessively argue about which of these regulatory concepts is favorable to developers (Kuzma, Citation2016). Indeed, there is no commercial cultivation of GM crops in Japan or the Republic of Korea, which employ product-based regulations. Furthermore, despite the fact that it employs product-based regulations, the Russian Federation has prohibited the commercial cultivation of GM crops.
We presented the current status of the regulatory responses to genome-edited crops in the USA, Argentina, Sweden and New Zealand (). The regulatory responses in the USA and Argentina suggest that transgene-free crops will be tested in the open field soon and might be approved for food consumption by health regulators in the near future. Conversely, it is unlikely that such crops will soon be cultivated in New Zealand. Although the regulatory responses in New Zealand and Argentina remain at the level of an authoritative decree or enforcement ordinance under relevant law(s), such amendments in relevant regulations will likely impact other countries because several years or longer are often required to amend an existing law, or enact a new law (). Countries with a product-based GMO policy that have experienced the commercial cultivation of GM crops are likely follow Argentina. In contrast, the EU is likely to follow New Zealand because the EU has employed a cautious, process-based policy regarding GMO (Davidson, Citation2010; Hartung and Schiemann, Citation2014). The UK is noteworthy because the country can change to a product-based GMO policy after it secedes from the EU. Regarding Japan and the Republic of Korea, which have adopted product-based regulations and which have not experienced the commercial cultivation of GM crops, we predict that the 2 countries will follow the USA, and not Argentina. Indeed, a GMO committee in the Japan Ministry of the Environment expressed their preliminary views regarding NPBTs including genome-edited crops, indicating that they will review genome edited crops on a case-by-case-basis according to the existing GMO law (Ministry_of_the_Environment, Citation2016). With regard to the Russian Federation which has adopted a product-based GMO policy, the President signed Federal Law 358-FZ on July 3, 2016, which prohibits the cultivation of GM plants and strengthens state control and the monitoring of processing and imports of GMOs (USDA, Citation2016). The Government attitude suggests that it is unlikely that crop breeding by genome editing will be encouraged because its encouragement may induce the imports of genome-edited products. These considerations suggest that countries throughout the world will be divided regarding their policies toward genome-edited crops (). Some countries will deregulate transgene-free crops, whereas others will regulate all types of crops modified by genome editing.
Figure 1. The international regulatory landscape regarding genetically modified organisms (GMOs). The 29 countries are colored according to the survey on the regulatory concepts that they employ regarding GMOs. The blue countries have adopted process-based GMO regulations. The light blue countries have employed process-based GMO regulations. The USA (product-based GMO regulations), Argentina (product-based GMO regulations) and New Zealand (process-based GMO regulations) are highlighted with stripes because they have made the regulatory responded to genome-edited crops.
Although the USA is the world's largest commercial cultivator of GM crops, not all people accept GM food products (Lucht, Citation2015; Li et al., Citation2015; Wunderlich and Gatto, Citation2015). The regulatory stance of the USDA APHIS seems unfavorable because they have applied their GMO regulations to crops generated using genome editing techniques that substantially differ from conventional GMO techniques. It would be vital to interrogate developers about how off-target mutations, which can potentially occur during genome editing (Klug, Citation2010; Joung and Sander, Citation2013; Hsu et al., Citation2014), were investigated in the resultant plants (Araki and Ishii, Citation2015; Ishii and Araki, Citation2016; Huang et al., Citation2016). Although off-target mutations may result in a silent mutation or a loss of function, some could lead to a gain of function through such mechanisms as a frameshift mutation, potentially affecting food safety or the environment (Araki et al., Citation2014) (Araki and Ishii, Citation2015; Ishii and Araki, Citation2016). Notably, the negative attitude of people toward GMOs is in part associated with a lack of trust in relevant regulations and/or developers (Lucht, Citation2015; Wunderlich and Gatto, Citation2015; Siegrist, Citation1999; Tanaka, Citation2004; Zilberman et al., Citation2013). To enhance public trust in genome edited crops, developers should investigate the occurrence of off-target mutations in the resultant plants.
The CPB aims to oversee LMOs resulting from modern biotechnology that may have adverse effects on biological diversity and to also take risks to human health into account (The_Convention_on_Biological_Diversity, Citation2016). It is noteworthy that the cultivation of an herbicide-resistant rice variety with an ALS variant, which was developed using a non-GM technique, has led to the emergence of herbicide-resistant weeds through hybridization with wild species in Italy and the USA (Busconi et al., Citation2012; Burgos et al., Citation2014). This accident underscores the significance of carefully considering the potential environmental impacts of a phenotype or multiple phenotypes which can be readily introduced in a crop by robust genome editing tools, in addition to the ecological characteristics of the field surrounding the cultivated land. Although it is important to confirm the absence of any residual transgenes, more attention should be paid to the phenotype of the transgene-free product (Ishii and Araki, Citation2016). With regard to New Zealand, the amended regulations under the HSNO Act of 1996 are ambiguous regarding the details of the regulatory review of a genome-edited crop (EPA, Citation2015), which may hamper communications among regulators, developers and the public.
CONCLUSION
The findings suggest that countries will likely be divided regarding their policies on genome-edited crops, potentially leading to a mosaic global agriculture landscape with regard to the commercial cultivation of genome-edited crops.
To avoid this scenario, clearer and more appropriate policies should be established through the discussion of various issues surrounding crops that are developed using genetic engineering tools. The countries that have ratified the CPB have different regulatory concepts, regardless of their experience of GM crop cultivation for commercial purposes (). Although the global inconsistence in GMO regulations has led to a social argument about which regulatory concept is better, what is more important is people's trust in food crop regulations. The CPB which has been ratified by 170 countries can take the initiative to harmonize the regulations pertaining to GM and genome-edited crops. In each country, regulators should make every effort to shape appropriate policy regarding genome-edited crops, inviting representatives of citizen groups in addition to scholars and developers.
ABBREVIATIONS
| CPB | = | Cartagena Protocol on Biosafety |
| CRISPR/Cas9 | = | Clustered Regularly Interspaced Short Palindromic Repeat (CRISPR)/Cas 9 |
| DNA | = | deoxyribonucleic acid |
| DSB | = | double-strand break |
| GMO | = | genetically modified organisms |
| HDR | = | homology-directed repair |
| LMO | = | Living modified organism |
| NHEJ | = | non-homologous end-joining |
| NPBT | = | New Plant Breeding Technique |
| TALENs | = | TAL Effector Nucleases |
| ZFNs | = | Zinc Finger Nucleases |
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
The authors declare that they have no conflict of interest in association with the present study.
KGMC_1261787_Supplemental_Material.zip
Download Zip (13.3 KB)FUNDING
This work was supported by a Hokkaido University faculty grant to T.I.
REFERENCES
- Araki M, Ishii T. Towards social acceptance of plant breeding by genome editing. Trends Plant Sci 2015; 20:145-9; PMID:25726138; http://dx.doi.org/10.1016/j.tplants.2015.01.010
- Araki M, Nojima K, Ishii T. Caution required for handling genome editing technology. Trends Biotechnol 2014; 32:234-7; PMID:24767735; http://dx.doi.org/10.1016/j.tibtech.2014.03.005
- Bruening G, Lyons JM. The case of the FLAVR SAVR tomato. California Agriculture 2000; 54:6-7; http://dx.doi.org/10.3733/ca.v054n04p6
- Burgos NR, Singh V, Tseng TM, Black H, Young ND, Huang Z, Hyma KE, Gealy DR, Caicedo AL. The impact of herbicide-resistant rice technology on phenotypic diversity and population structure of United States weedy rice. Plant Physiol 2014; 166:1208-20; PMID:25122473; http://dx.doi.org/10.1104/pp.114.242719
- Busconi M, Rossi D, Lorenzoni C, Baldi G, Fogher C. Spread of herbicide-resistant weedy rice (red rice, Oryza sativa L.) after 5 years of Clearfield rice cultivation in Italy. Plant Biol (Stuttg) 2012; 14:751-9; PMID:22443148; http://dx.doi.org/10.1111/j.1438-8677.2012.00570.x
- Camacho A, Van Deynze A, Chi-Ham C, Bennett AB. Genetically engineered crops that fly under the US regulatory radar. Nature biotechnology 2014; 32:1087-91; PMID:25380439; http://dx.doi.org/10.1038/nbt.3057
- Chen H, Lin Y. Promise and issues of genetically modified crops. Curr Opin Plant Biol 2013; 16:255-60; PMID:23571013; http://dx.doi.org/10.1016/j.pbi.2013.03.007
- Davidson J. GM plants: Science, politics and EC regulations. Plant Science 2010; 178:94-8; http://dx.doi.org/10.1016/j.plantsci.2009.12.005
- EPA. The Environmental Protection Authority (EPA): GMO regulations clarified. http://wwwepagovtnz/publications-resources/bulletin/May-2016/Pages/GMO-regulations-clarified.aspx. Accessed 26 August, 2016, 2015.
- European_Academies'_Science_Advisory_Council. Statement: New breeding techniques. http://wwweasaceu/fileadmin/PDF_s/reports_statements/Easac_14_NBT.pdf. Accessed March 7, 2016, 2015.
- European_Plant_Science_Organisation. Statement: Crop Genetic Improvement Technologies. 2015 Feb 26 [accessed 2016 Aug 26] http://www.epsoweb.org/file/2147.
- European_Seed_Association. Regulatory approaches to modern plant breeding - the case of mutagenesis and new gene editing technologies. 2015 Jul 20 [accessed 2016 Aug 26] https://www.euroseeds.eu/system/files/publications/files/esa_15.0543_0.pdf
- Fladung M. Cibus' herbicide-resistant canola in European limbo. Nat Biotechnol 2016; 34:473-4; PMID:27153271; http://dx.doi.org/10.1038/nbt.3558
- GM_Freeze. The case for regulating Gene Edited crops. 2016 Feb 7 [accessed 2016 Aug 26] http://www.gmfreeze.org/news-releases/266/.
- GMWATCH. “Genome editing”: GM by another name. http://wwwgmwatchorg/news/archive/2014/15546 genome-editing-gm-by-another-name. Accessed February 19, 2016, 2014.
- Green_Peace. Policy briefing Gene-editing of plants – GM through the back door? 2015 Nov 30 [accessed 2016 Aug 26] http://www.greenpeace.org/eu-unit/Global/eu-unit/reports-briefings/2015/Greenpeace_Gene-editing_30112015%20-%202.pdf
- Hartung F, Schiemann J. Precise plant breeding using new genome editing techniques: opportunities, safety and regulation in the EU. Plant J 2014; 78:742-52; PMID:24330272; http://dx.doi.org/10.1111/tpj.12413
- Hsu PD, Lander ES, Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell 2014; 157:1262-78; PMID:24906146; http://dx.doi.org/10.1016/j.cell.2014.05.010
- Huang S, Weigel D, Beachy RN, Li J. A proposed regulatory framework for genome-edited crops. 2016; 48:109-11.
- IFOAM_EU. New plant breeding techniques position paper. 2015 Dec 10 [accessed 2016 Aug 26] http://www.ifoam-eu.org/sites/default/files/ifoameu_policy_npbts_position_final_20151210.pdf
- International Service for the Acquisition of Agri-biotech Applications (ISAAA) Brief 51–2015. http://isaaaorg/resources/publications/briefs/51/executivesummary/default.asp. Accessed 26 August, 2016, 2015.
- Ishii T, Araki M. Consumer acceptance of food crops developed by genome editing. Plant cell reports 2016; 35:1507-18; PMID:27038939; http://dx.doi.org/10.1007/s00299-016-1974-2
- Joung JK, Sander JD. TALENs: a widely applicable technology for targeted genome editing. Nat Rev Mol Cell Biol 2013; 14:49-55; PMID:23169466; http://dx.doi.org/10.1038/nrm3486
- Kershen DL. Sustainability council of New Zealand trust v the environmental protection authority: gene editing technologies and the law. GM Crops Food 2015; PMID:26618752; http://dx.doi.org/10.1080/21645698.2015.1122859
- Kim J, Kim JS. Bypassing GMO regulations with CRISPR gene editing. Nat Biotechnol 2016; 34:1014-5; PMID:27727209; http://dx.doi.org/10.1038/nbt.3680
- Klug A. The discovery of zinc fingers and their applications in gene regulation and genome manipulation. Annu Rev Biochem 2010; 79:213-31; PMID:20192761; http://dx.doi.org/10.1146/annurev-biochem-010909-095056
- Li Y, Hallerman EM, Liu Q, Wu K, Peng Y. The development and status of Bt rice in China. Plant Biotechnol J 2016;14(3):839-48.
- Lucht JM. Public Acceptance of Plant Biotechnology and GM Crops. Viruses 2015; 7:4254-81; PMID:26264020; http://dx.doi.org/10.3390/v7082819
- Ministry_of_the_Environment. Summary of minutes: the expert committee regarding such as living modified organisms (Second in FY2015). 2016 Jan 22 [accessed 2016 Aug 26] http://www.env.go.jp/council/12nature/y127-02.html.
- Nagamangala Kanchiswamy C, Malnoy M, Velasco R, Kim JS, Viola R. Non-GMO genetically edited crop plants. Trends Biotechnol 2015; 33:489-91; PMID:25978870; http://dx.doi.org/10.1016/j.tibtech.2015.04.002
- Nagamangala Kanchiswamy C, Sargent DJ, Velasco R, Maffei ME, Malnoy M. Looking forward to genetically edited fruit crops. Trends Biotechnol 2015; 33:62-4; PMID:25129425; http://dx.doi.org/10.1016/j.tibtech.2014.07.003
- Kuzma J. Policy : Reboot the debate on genetic engineering. Nature 2016; 531:165-7; PMID:26961641; http://dx.doi.org/10.1038/531165a
- Ramessar K, Capell T, Twyman RM, Quemada H, Christou P. Trace and traceability–a call for regulatory harmony. Nat Biotechnol 2008; 26:975-8; PMID:18779799; http://dx.doi.org/10.1038/nbt0908-975
- Science_Council_of_Japan. New Plant Breeding Techniques: Current status and issues (in Japanese). http://wwwscjgojp/ja/info/kohyo/division-16.html. Accessed 26 August, 2016, 2014.
- Siegrist M. A Causal model explaining the perception and acceptance of gene technology. J Applied Social Psychol 1999; 29:2093-106; http://dx.doi.org/10.1111/j.1559-1816.1999.tb02297.x
- Sprink T, Eriksson D, Schiemann J, Hartung F. Regulatory hurdles for genome editing: process- vs. product-based approaches in different regulatory contexts. Plant Cell Rep 2016; 35:1493-506; PMID:27142995; http://dx.doi.org/10.1007/s00299-016-1990-2
- Tanaka Y. Major psycological facotors affecting acceptance of gene-recombination technology. Risk Analysis 2004; 24:1575-83; PMID:15660613; http://dx.doi.org/10.1111/j.0272-4332.2004.00551.x
- The_Convention_on_Biological_Diversity. The cartagena protocol on biosafety. https://bchcbdint/protocol. Accessed 19 January 2016, 2016.
- The_US_Library_of_Congress. Restrictions on genetically modified organisms: Japan. http://wwwlocgov/law/help/restrictions-on-gmos/japan.php. Accessed 19 January 2016, 2014.
- The_US_Library_of_Congress. Restrictions on genetically modified organisms: New Zealand. http://wwwlocgov/law/help/restrictions-on-gmos/new-zealand.php. Accessed 19 January 2016, 2014.
- The_US_Library_of_Congress. Restrictions on genetically modified organisms: Russian federation. https://wwwlocgov/law/help/restrictions-on-gmos/russia.php. Accessed 26 August 2016, 2014
- The_US_Library_of_Congress. Restrictions on genetically modified organisms: Republic of Korea. https://wwwlocgov/law/help/restrictions-on-gmos/south-korea.php. Accessed 26 August, 2016, 2014
- USDA. GAIN report: Russia bans cultivation and breeding of GE crops and animals. http://gainfasusdagov/Recent%20GAIN%20Publications/Russia%20Bans%20Cultivation%20and%20Breeding%20of%20GE%20Crops%20and%20Animal_Moscow_Russian%20Federation_7-12-2016.pdf. Accessed 26 August, 2016, 2016.
- Voytas DF, Gao C. Precision genome engineering and agriculture: opportunities and regulatory challenges. PLoS Biol 2014; 12:e1001877; PMID:24915127; http://dx.doi.org/10.1371/journal.pbio.1001877
- Whelan AI, Lema MA. Regulatory framework for gene editing and other New breeding techniques (NBTs) in Argentina. GM Crops Food 2015; PMID:26552666; http://dx.doi.org/10.1080/21645698.2015.1114698
- Woo JW, Kim J, Kwon SI, Corvalan C, Cho SW, Kim H, Kim SG, Kim ST, Choe S, Kim JS. DNA-free genome editing in plants with preassembled CRISPR-Cas9 ribonucleoproteins. Nat Biotechnol 2015; 33:1162-4; PMID:26479191; http://dx.doi.org/10.1038/nbt.3389
- Wunderlich S, Gatto KA. Consumer perception of genetically modified organisms and sources of information. Adv Nutr 2015; 6:842-51; PMID:26567205; http://dx.doi.org/10.3945/an.115.008870
- Zilberman D, Kaplan S, Kim E, Hochman G, Graff G. Continents divided: understanding differences between Europe and North America in acceptance of GM crops. GM Crops Food 2013; 4:202-8; PMID:24281195; http://dx.doi.org/10.4161/gmcr.26981
