ABSTRACT
Mexico is a center of origin for several economically important plants including maize, cotton, and cocoa. Maize represents more than a food crop, has been declared a biological, cultural, agricultural and economic patrimony, and is linked to the national identity of Mexicans. In this review, we describe the historic and current use of genetically modified plants in Mexico and factors that contributed to the development of the biosafety regulation. We developed a database containing all permit applications received by the government to release genetically modified plants. A temporal and geographical analysis identified the plant species that have been authorized for experimental purposes, pilot programs, or commercial production, the geographic areas where they have been released, and the traits that have been introduced. Results show that Mexico has faced a dual challenge: accepting the benefits of genetically modified plants and their products, while protecting native plant biodiversity.
INTRODUCTION
Animals, plants, and microorganisms including bacteria, fungi, and viruses have been genetically modified through a variety of methods including chemical mutagenesis, breeding, transgenesis or gene editing. Plants have been genetically modified for a growing number of purposes including enhanced nutritional value, prolonged shelf life, disease or pest resistance, herbicide tolerance, male sterility, production of vaccines against human and livestock diseases, recombinant proteins for industrial purposes, antibodies, therapeutic enzymes and virus-like particles, and enhanced biofuel potential (Lomonossoff and D’Aoust Citation2016; Bleotu et al. Citation2018). Benefits of using genetically modified plants to grow food or fiber include improvement in yield and reliability of the food supply in conditions that include changing climate and reduction in farmland (Brookes and Barfoot Citation2016; Sharp and Leshner Citation2016; Raman Citation2017; Taheri et al. Citation2017). Mainly trhough gene editing approaches, currently plants are being developed to enhance nutrient utilization and to tolerate drought, high salt content in the soil, and for environmental remediation (Jez et al. Citation2016; Ma et al. Citation2018). Furthermore, technology is being developed to program novel metabolic pathways in plants for their use as chemical feedstock at industrial levels (Fesenko and Edwards Citation2014; Jez et al. Citation2016; Lomonossoff and D’Aoust Citation2016; Ma et al. Citation2018), and to engineer disease resistance for pathogen-plant combinations for which natural resistance has not been identified (Romay and Bragard Citation2017; Ma et al. Citation2018).
To introduce genetic modifications in plants, genes are moved across species, or plant genomes are specifically edited using materials and knowledge that are proprietary (Bleotu et al. Citation2018). Thus, commercial production of genetically modified plants (transgenic or genome-edited) has the potential to impact both the environment and society in multiple ways (The_National_Academy_of_Sciences Citation2010; Bonny Citation2017; Raman Citation2017; Whelan and Lema Citation2017; Van Rijssen and Morris Citation2018). Concerns about the use of genetically modified plants include potential harm to the environment, biodiversity, and human health in combination with socioeconomic, political, and ethical consequences (Hug Citation2008; Bonny Citation2017; Raman Citation2017; Van Rijssen and Morris Citation2018). Accordingly, the use of genetically modified plants has been controversial and caused opposing reactions among scientists, consumers, and the public. While some concerns have been labeled as myths, others have scientific support (Parrott Citation2010; Buiatti et al. Citation2013; Rastogi Verma Citation2013; Panchin and Tuzhikov Citation2017).
The Cartagena Protocol on Biosafety established the basis to regulate the release and international trade of living genetically modified organisms. According to this protocol, a genetically modified organism is defined as “any living organism that possesses a novel combination of genetic material obtained using modern biotechnology” (CBD Citation2000). This definition is based on the transformation process, and includes transgenic and non-transgenic organisms (CBD Citation2000; Sprink et al. Citation2016). A transgenic organism has been altered by the addition of genetic material from a different species (Bleotu et al. Citation2018), such as a plant expressing a gene from a bacterium. Non-transgenic organisms have been genetically modified without the addition of genetic material from any other or the same species (Bleotu et al. Citation2018). Organisms that result from gene editing tools such as the CRISPR/Cas system are non-transgenic genetically modified organisms, unless they carry a gene insertion. Accordingly, approaches to regulate genetically modified plants have been based on the tranformation process or features of the product (Kuzma Citation2016; Georges and Ray 2017). Although approaches have been proposed (Huang et al. Citation2016; Sprink et al. Citation2016; Smyth Citation2017), genetic modification through gene editing represents a regulatory challenge that remains to be addressed.
Transgenic plants have been used in commercial agriculture since the mid 1990’s, after being released for the first time in the United States, China, Argentina, Australia and Canada (Nap et al. Citation2003; Fernandez-Cornejo et al. Citation2014; Taheri et al. Citation2017). Additionally, since 2010, 12 plant species including appless and potatoes, resulting from gene editing have been authorized in the United States and Canada without going through the biosafety regulation stablished for transgenic plants (Kuzma Citation2016; Smyth Citation2017). Similarly, in 2015, mushrooms genetically modified through gene editing were declared excempt from biosafety regulation in the United States (Gene-Edited Citation2016; Waltz Citation2016).
Production and consumption of genetically modified plants has followed contrasting patterns. While some countries, like the United States and Canada, grow and consume them openly, others have banned the production and reject their consumption (Fernandez-Cornejo et al. Citation2014; Smyth Citation2014; James Citation2015; Brookes and Barfoot Citation2016; Aldemita and Hautea Citation2018). Adoption of genetically modified plants by producers has been rapid in some developed and in some developing countries (Aldemita and Hautea Citation2018). In 2016, genetically modified plants were grown in twenty-six countries with notable increments in Latin America, Africa, and Asia (Aldemita and Hautea Citation2018). World-wide, the most commonly produced genetically modified plants are soybean (Glycine max L.), maize (Zea mays subesp. mays L.), cotton (Gossypium hirsutum L.), and canola (Brassica rapa subsp. oleifera) (James Citation2010, Citation2015; Marinho et al. Citation2014; Aldemita et al. Citation2015; Aldemita and Hautea Citation2018). The most frequent traits introduced into these species are herbicide tolerance (53%), insect resistance (14%), and a combination of both (33%) (James Citation2015). The top growers of genetically modified plants are the United States, Brazil, Argentina, Canada and India (Aldemita and Hautea Citation2018).
Based on the extend of the area used to grow genetically modified plants, in 2016 Mexico was ranked number seventeen (Aldemita and Hautea Citation2018). However, there is little information about the features and geographic distribution of genetically modified plants authorized in Mexico, the biosafaty regulation and the factors shaping it. In Mexico, a legal framework and an approval process was establisheded in 2005 through the biosafety law (DOF Citation2005). However, records of permit applications and authorizations to release genetically modified plants exist since 1995. Here, we developed a database containing all permit applications and authorizations to release genetically modified plants from 1995 to 2017. A temporal and geographical analysis identified the plant species that have been authorized for experimental purposes, pilot programs, or commercial production, their geographic distribution and traits. Results provide a profile of genetically modified plants authorized in Mexico.
BIOSAFETY REGULATORY BODY
Import into Mexico, export, and release of genetically modified organisms into confined spaces (laboratory, greenhouse, and processing plants) or to the environment for agricultural production, bioremediation, industrialization, public health, or any other purpose is regulated by the biosafety law of genetically modified organisms published in 2005 (DOF Citation2005) and updated in 2009 (DOF Citation2009) (). This law and its implementation rules provide a framework to regulate genetically modified organisms and describe an approval process. Both pieces of regulation were designed to document, evaluate, and minimize the risk of possible negative effects on human, animal, and plant health, the environment and biodiversity, derived from the release of genetically modified organisms into the environment and from the use of their products for human consumption, animal feed, or medicinal purposes (DOF Citation2009).
FIGURE 1. Organization and roles of the Intersecretarial Commission on Biosafety of Genetically Modified Organisms in Mexico (CIBIOGEM). The Spanish acronym is provided for each ministry or organization.CIBIOGEM regulates import, consumption and release of genetically modified organisms for all purposes into confined spaces and to the environment (DOF, 2005).
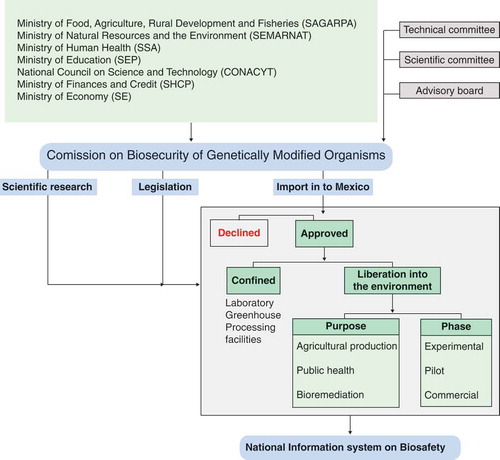
The biosafety law supports the creation of the Inter-Ministerial Commission for Biosafety of Genetically Modified Organisms (CIBIOGEM, for the Spanish acronym), and the National Information System on Biosafety (DOF Citation2005). CIBIOGEM is integrated by sixs secretariats and the Director of the National Council on Science and Technology, is responsible for the regulatory process in all aspects of biotechnology and all organisms (), and is not responsible for law enforcement. The National Information System on Biosafety contains descriptive information regarding all permit applications to release genetically modified organisms and is maintained by CIBIOGEM (DOF Citation2009).
APPROVAL PROCESS
The approval process initiates with a written application for every transformation event and consists of three sequential phases: experimental, pilot program, and commercial release (DOF Citation2005) (). On a case-by-case basis, CIBIOGEM evaluates the potential risks of releasing genetically modified organisms or consuming products containing them. Part of the risk analysis is based on information provided by the interested party, which could have been generated in the country of origin (). The advantages of using genetically modified plants over alternative technologies are part of the criteria that integrate the risk analysis ().
FIGURE 2. Schematic representation of the regulatory process and risk analysis for the release of genetically modified organisms based on the Mexico biosafety law. A) The approval process consists of three sequential phases: experimental purposes, pilot programs and commercial production. On a case-by-case basis, each phase requires a permit application, risk analysis, and is subjected to compliance monitoring. B) Core information for risk analysis based on scientific information generated by the applicant and may include information generated in the country of origin.
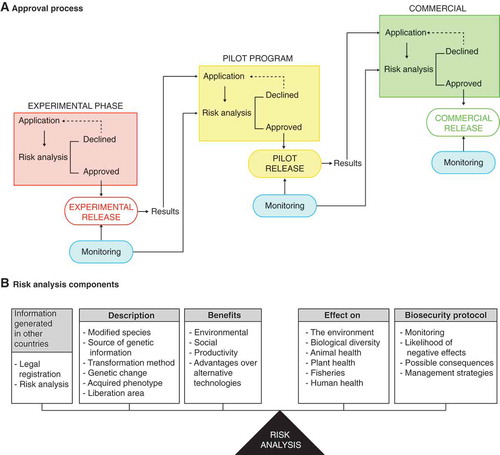
Release for experimental purposes requires the use of physical, chemical, and biological barriers, or their combination, for the confinement of genetically modified organisms and to limit contact with people and other organisms in the environment. In pilot programs, genetically modified organisms may be released with or without physical, chemical, or biological barriers to limit contact with people and other organisms in the environment. Commercial release of genetically modified organisms does not require the establishment of physical, chemical, or biological barriers (DOF Citation2005).
GENETICALLY MODIFIED PLANTS THROUGH THE APPROVAL PROCESS
From 1995 to 2017 a total of 893 permit applications were received by CIBIOGEM. Five applications were withdrawn, and 141 applications were declined after risk analysis or due to lack of information to carry out a risk analysis. This represents a rejection rate of 15.8%. At the time of this analysis, 122 applications were in process, twenty-six of them since 2012 and the rest from 2013 to 2017 (). The number of permit applications authorized to release genetically modified plants to the environment reached 625: 492 for experimental purposes, 118 for pilot programs and 16 for commercial production ().
FIGURE 3. Number of permits or applications in progress to release genetically modified plants by regulatory phase and proprietary from 1995 to 2017. Records from 1995 to 2004 were pooled. Maps show geographic distribution of areas (shaded in green to the municipal level) for which at least one permit has been approved for any category. A) Cumulative number of permits by experimental phase. Lines and dots indicate permits per category and are plotted on the left Y axis. Vertical bars indicate applications in risk analysis per category, and are plotted on the right Y axis. On the map, color-coded digits indicate the number of permits issued by regulatory phase and per state. B) Number of permits by proprietary, per year, including all three phases (experimental, pilot, commercial). On the map, color-coded digits indicate the number of permits per state.
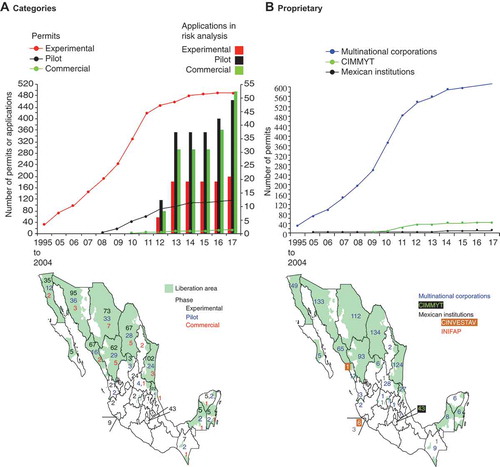
Permits granted to release genetically modified plants have been issued for 12 plant species (). Species with the most number of permits for experimental purposes and pilot programs are cotton (Gossypium hirsutum L.), maize (Zea mays subesp mays L.), wheat (Triticum aestivum), and soybean (Glycine max L.) (). Species with smaller number of permits include tomato (Solanum lycopersicum L.), canola (Brassica rapa subsp. oleifera), potato (Solanum tuberosum L.), common bean (Phaseolus vulgaris), mexican lime (Citrus aurantifoli), sweet orange (Citrus sinensis), alfalfa (Medicago sativa) and sugar beet (Beta vulgaris) (). Although experimental trails were authorized since 1995, officially cotton and soybean were not authorized for commercial purposes until 2010 and 2012, respectively ( and ). All transformation events authorized until 2017 were the result of transgenic approaches.
TABLE 1. Genetically modified organisms authorized for commercial production in Mexico.
FIGURE 4. Number of permits issued to release genetically modified plants by regulatory phase, state, and species from 1995 to 2017. Records from 1995 to 2004 were pooled. A) Number of permits per year and by regulatory phase for top four plants: Cotton (Gossypium hirsutum L.), maize (Zea mays L.), soybean (Glycine max [L.] Merr.), and wheat (Triticum aestivum). B) Number of permits per year and by regulatory phase for other species: alfalfa (Medicago sativa L.), potato (Solanum tuberosum), mexican lime (Citrus aurantifolia), sweet orange (Citrus sinensis), tomato (S. lycopersicum), canola (Brassica napus L.), bean (Phaseolus vulgaris) and sugar beet (Beta vulgaris). C) Geographic distribution per plant species and areas with at least one permit for experimental purposes and pilot programs. Release area is as in . Color-coded digits indicate the cumulative number of permits by regulatory phase and per state. D) Geographic distribution per plant species of areas with at least one permit for commercial release. Release area is shaded in green. Color-coded digits indicate the cumulative number of permits per state.
![FIGURE 4. Number of permits issued to release genetically modified plants by regulatory phase, state, and species from 1995 to 2017. Records from 1995 to 2004 were pooled. A) Number of permits per year and by regulatory phase for top four plants: Cotton (Gossypium hirsutum L.), maize (Zea mays L.), soybean (Glycine max [L.] Merr.), and wheat (Triticum aestivum). B) Number of permits per year and by regulatory phase for other species: alfalfa (Medicago sativa L.), potato (Solanum tuberosum), mexican lime (Citrus aurantifolia), sweet orange (Citrus sinensis), tomato (S. lycopersicum), canola (Brassica napus L.), bean (Phaseolus vulgaris) and sugar beet (Beta vulgaris). C) Geographic distribution per plant species and areas with at least one permit for experimental purposes and pilot programs. Release area is as in Fig. 3. Color-coded digits indicate the cumulative number of permits by regulatory phase and per state. D) Geographic distribution per plant species of areas with at least one permit for commercial release. Release area is shaded in green. Color-coded digits indicate the cumulative number of permits per state.](/cms/asset/13688aea-1049-4485-8eaf-69edef2a57ce/kgmc_a_1507601_f0004_c.jpg)
Until May of 2018 no applications were received for plants resulting from gene editing. Gene editing is powerfull technology to ingeneer traits in plants. This approach is faster than transgenic approaches, does not requiere antibiotic or herbicide selection, and the engineered mutations are similar to natural mutations (Zhang et al. Citation2017; Ma et al. Citation2018; Zhe et al. Citation2018). Thus, it is safe to predict that transgenic plants currenlty going through the approval process will be replaced in the near future by plants developed through gene editing. Furthermore, plants that have not been modified by transgenic approaches, such as sugarcane, bananas, plantains, coffe, or cocoa, are being modified by gene editing (Ma et al. Citation2018) and are expected to enter the approval process soon.
Transgenic plants have been released for experimental purposes in 24 of the 31 Mexican states (). In most of the states, transgenic plants have been liberated for experimental purposes and pilot programs (). Permits issued for commercial production cover 14 states, 10 located in the north, and four states located at or near the Yucatan peninsula ().
Soybean, cotton, and maize have been genetically engineered to tolerate herbicides, resist insect pests, or both (). In these plants, the most frequent trait is resistance to the herbicide glyphosate alone or in combination with resistance to insects in the orders Coleoptera or Lepidoptera, or tolerance to herbicides glufosinate ammonium, dicamba, acetolactate synthase (ALS) inhibitors, or sulfonylurea (). Other traits that have been introduced into plants include drought and cold tolerance, high lysine, high oleic acid, or reduced lignin content, or resistance to pathogens ().
FIGURE 5. Cumulative number of permits issued from 2004 to 2017 to release genetically modified plants by species and acquired phenotype after genetic modification. a) Number of permits by species: Cotton (Gossypium hirsutum L.), maize (Zea mays L.), soybean (Glycine max [L.] Merr.), wheat (Triticum aestivum), alfalfa (Medicago sativa L.), potato (Solanum tuberosum), mexican lime (Citrus aurantifolia), tomato (S. lycopersicum), canola (Brassica napus L.), and sugar beet (Beta vulgaris). b) For the most common species, number of permits by acquired phenotype. Below the pie charts, acquired phenotypes are color coded to indicate single and combination of traits.
![FIGURE 5. Cumulative number of permits issued from 2004 to 2017 to release genetically modified plants by species and acquired phenotype after genetic modification. a) Number of permits by species: Cotton (Gossypium hirsutum L.), maize (Zea mays L.), soybean (Glycine max [L.] Merr.), wheat (Triticum aestivum), alfalfa (Medicago sativa L.), potato (Solanum tuberosum), mexican lime (Citrus aurantifolia), tomato (S. lycopersicum), canola (Brassica napus L.), and sugar beet (Beta vulgaris). b) For the most common species, number of permits by acquired phenotype. Below the pie charts, acquired phenotypes are color coded to indicate single and combination of traits.](/cms/asset/2b79a80c-328e-4a84-9a59-5b3bb089dcd9/kgmc_a_1507601_f0005_c.jpg)
MEXICO IS A NOT A DEVELOPER OF TRANSGENIC PLANTS
Of the total number (625) of permits granted, 97.6% have been obtained by multinational corporations (). Two Mexican institutions (CINVESTAV and INIFAP) have obtained 12 (2.4%) permits for experimental purposes and the corresponding transformation events have not progressed into pilot programs or commercial production. Transgenic cotton and soybean authorized for commercial production in Mexico belong to the same multinational corporation (). These numbers show that Mexico is not a developer of transgenic plants.
TRANSGENIC SOYBEAN
Transgenic soybean was the second species (2012) authorized for commercial release in Mexico (). It was authorized in four southern states (Chiapas, Campeche, Yucatan and Quintana Roo), and three in the norteast (Tamailipas, Veracruz and San Luis Potosi) (). Only one transformation event was authorized for commercial production (). However, several other transformation events are in experimental trails or pilot programs in other parts of the country (). The most abundant trait introduced to soybean is tolerance to the herbicide glyphosate, which has been combined with tolerance to other herbicides or resistance to insects ().
The permit for commercial production of transgenic soybean in Mexico was revoked on September 17, 2017 due to pressure from Maya farmers and honey producers in the Yucatan peninsula. Farmers and honey producers from 30 Maya communities and environmental organizations formed a coalition and filed a law suit before the National Supreme Court against the Ministry of Agriculture, Food, Rural Development and Fisheries (SAGARPA), one of the components of CIBIOGEM. The coalition argued that permits were granted without farmers approval, that trangenic soybean has been illigally grown in areas not authorized, and that pollen from trangenic soybean contaminates honey for export to Europe (Bacalar Citation2017).
TRANSGENIC COTTON
Transgenic cotton was the first genetically modified plant authorized for commercial production in Mexico, is authorized in eight states in the north (), and the cumulative area requested reached 744,500 hectares (). Transgenic cotton was authorized for experimental purposes in 1995 and for commercial production officially in 2010 (). Four transformation events have been authorized for commercial release, and the most abundant trait is tolerance to the herbicide glyphosate alone or in combination with resistance to insects in the order Lepidoptera (). For experimental purposes and pilot programs, permits have been authorized for cotton with a combination of traits that include tolerance to the herbicides glyphosate, glufosinate ammonium, or both, and resistance to insects in the order Coleoptera, Lepidoptera, or both ().
Mexico is a center of origin and diversity of Gossypium hirsutum L. Other cotton species originated in tropical and subtropical parts of Africa and Asia. However, over 95% of the cultivated cotton in the world is G. hirsutum (Wegier et al. Citation2011). In Mexico, eight metapopulations of wild cotton have been identified and are mainly distributed along the west and east costs (Wegier et al. Citation2011). The areas authorized for commercial production () do not overlap the distribution of wild cotton metapopulations. Cotton is self-pollinated, cross pollination rarely occurs and is dependent on wind, and pollinator birds and insects (Heuberger et al. Citation2010). Thus, transgene contamination in cotton is largely dependent on pollinators and the distance between plants (Heuberger et al. Citation2010; Yan et al. Citation2015). However, transgene contamination has been detected in four out of eight wild cotton metapopulations (Wegier et al. Citation2011).
MAIZE BIODIVERSITY IN MEXICO
As a fundamental part of their people’s diet and national identity, maize is of major economic and cultural importance to Mexico (Kato Yamakake et al. Citation2009; Alvarez-Buylla and Piñeyro Citation2013). Additionally, Mexico harbors the greatest maize diversity in the world, including genetically defined landraces, and maize wild relatives teosinte (Zea spp., except Z. mays) and gamagrass (Tripsacum spp.) (Kato Yamakake et al. Citation2009).
Subsistence and small farmers account for approximately 86% of the maize cultivated area in Mexico, and select, conserve, and exchange seed (Xolocotzi Citation1985; Pressoir and Berthaud Citation2004; Acevedo et al. Citation2011; Burgeff et al. Citation2014; Orozco-Ramírez et al. Citation2016; Orozco-Ramirez et al. Citation2016). This practice is the main force driving maize evolution and diversification in Mexico (Dyer and Taylor Citation2008), and has been active for thousands of years. Permanent diversification and selection has led to adaptation of maize to a wide variety of environments, growth habits, culinary, and cultural purposes. Accordingly, in Mexico, there are landraces adapted to grow from sea level, to high altitudes, and in between; in cold or warm climates, in the tropics, and in the desert (Kato Yamakake et al. Citation2009; Alvarez-Buylla and Piñeyro Citation2013; Arteaga et al. Citation2016; Orozco-Ramirez et al. Citation2016).
In 2006, following the biosafety law, a “Global project on native maize of Mexico” (http://www.biodiversidad.gob.mx/genes/proyectoMaices.html) was initiated to survey and update information on the genetic diversity of maiz, teosinte and gamagrass. Results of this project recognized sixty maize landraces, and three teosinte species, two subspecies, and four landraces (DOF Citation2012). Areas rich in native maize biodiversity with defined landraces have been identified in all states of Mexico (Burgeff et al. Citation2014; Orozco-Ramírez et al. Citation2016).
DECREE TO PROTECT MAIZE BIODIVERSITY
To protect native maize populations and its wild relatives teosinte and gamagrass, in 2012 CIBIOGEM published a decree to establish maize centers of origin and centers of biodiversity (DOF Citation2012). This decree declares native Mexican maize a biological, cultural, agricultural and economic patrimony of Mexico and declares centers of origin of maize all geographical areas were genetically distinct native land races have been identified, which includes parts of all states in Mexico, except Baja California (Orozco-Ramírez et al. Citation2016). Additionally, this decree declares centers of maize biodiversity areas in the states of Baja California, Baja California Sur, Chihuahua, Coahuila, Nuevo Leon, Tamaulipas, Sinaloa, and Sonora (DOF Citation2012). Combined, areas declared centers of origin or centers of biodiversity cover the entire country.
The decree establishes that centers of origen and centers of biodiversity must be maintained free of genetically modified maize (DOF Citation2012), and does not ban the release of genetically modified maize. Instead, it provides a framework to release genetically modified maize (DOF Citation2012). However, in the same areas, commercial production of cultivars or hybrids developed by conventional breeding is allowed. Due to cross-pollination in maize, the effects on maize biodiversity is not greater for genetically modified maize than for cultivars or hybrids developed by conventional breeding (Carpenter Citation2011; Dyer et al. Citation2014).
TRANSGENIC MAIZE IS NOT AUTHORIZED
Until 2013, transgenic maize was authorized for experimental purposes (178 permits) and pilot programs (25 permits) (). These permits were for maize engineered to tolerate glyphosate, alone or in combination with tolerance to other herbicides and/or resistance to insects (). The transgene that confers glyphosate tolerance in maize is the same that confers glyphosate tolerance in cotton and soybean. However, to date, commercial release of genetically modified maize has not been authorized (). Furthermore, no permits have been issued to release genetically modified maize for experimental purposes or pilot programs since 2013 (). This was in response to public pressure and a legal case against the Mexican government. In October of 2013, a federal judge ordered the Mexican government to “suspend all activities involving the planting of transgenic maize in the country and end the granting of permits for experimental and pilot plantings” because the release of transgenic maize is an “imminent harm to the environment” (Peña Citation2013).
CONTRASTING REGULATION BETWEEN MAIZE AND COTTON
Mexico is a center of origin of both maize and cotton and landraces or metapopulations have been identified for both species (). Transgene contamination has been demonstrated both in native maize (Dyer et al. Citation2009; Pineyro-Nelson et al. Citation2009) and native cotton (Wegier et al. Citation2011). Transgenic cotton has been authorized for commercial release since 2010, while release of transgenic maize has been banned since 2013. Strikingly, centers of origin and centers of biodiversity have been declared for maize (DOF Citation2012), but not for cotton.
TABLE 2. Maize, cotton and soybean features that impact biosafety and regulation.
Related to biosafety, there are several differences between maize and cotton (). Maize is a fundamental part of mexican diets, while cotton is not for human consumption. This difference does not explain the current regulation, because Mexico allows the import of products and whole kernels for food or feed containing genetically modified maize from the United States (Kaiser Citation2005; Brandt Citation2014; Burgeff et al. Citation2014). While maize is openly pollinated, cotton is self-pollinated. Additionally, local farmers select and conserve maize but not cotton seed. However, these differences do not explain the current regulation, because transgene contamination has been demonstrated both in native maize (Dyer et al. Citation2009; Pineyro-Nelson et al. Citation2009) and in native cotton (Wegier et al. Citation2011).
Transgenic cotton provides an increment in yield and profit over non-trangenic cultivars due to modifications that reduce damage caused by weeds and pests (Brookes and Barfoot Citation2014, Citation2016). This represents an economic benefit both for producers and consumers (The National Academy of Sciences Citation2010). In 2017 and compared to other countries in the world, Mexico was ranked 43th in maize yield per hectar and 6th in total maize production. In the same year Mexico imported 37.4% of the maize it consumed (https://apps.fas.usda.gov). Genetically modified, maize could provide an increment in yield, total production and profit benefiting both producers and consumers (Brookes and Barfoot Citation2016).
FACTORS SHAPING THE BIOSAFETY REGULATION
Import of transgenic maize kernels for consumption and maize seed for experimentation were authorized before publication of the biosafety law (). These activities were blamed as the source of transgene contamination in native maize populations (Snow Citation2009). This captured the attention of the general public, led to demonstrations against genetically modified organisms in general and maize in particular (Alvarez-Buylla and Piñeyro Citation2013). These events provided a motivation to establish the biosafety law (DOF Citation2005), the “Global project on native maize of Mexico” (Acevedo et al. Citation2011), and the decree to establish maize centers of origin and centers of biodiversity (DOF Citation2012). Despite the establishment of regulation to protect native maize populations (DOF Citation2012), the release of transgenic maize into the environment was banned in 2013 as a result from a legal case against the Mexican government (Peña Citation2013).
Maize production in Mexico is 37.4% below consumption, thus forcing import, mainly form the United States (https://apps.fas.usda.gov). Furthermore, under the North American Free trade agreement, Mexico could not ban the import of genetically modified maize. This sequence of events reflects willingness by the Mexican government to allow production and consumption of genetically modified maize, and a strong opposition from the general public (Alvarez-Buylla and Piñeyro Citation2013).
In a similarly scenario, five years after authorization of commercial production, the ban on transgenic soybean establised in 2017 was the result of pressure from Maya farmers and honey producers in the Yucatan peninsula (Bacalar Citation2017).
CONCLUSION
Regulation and authorizations to release genetically modified plants in Mexico reflect both acceptance and rejection. The main focus of the biosafety regulation is the protection of native maize biodiversity. Mexico allows import and consumption of genetically modified maize kernels or maize products (Kaiser Citation2005; Brandt Citation2014; Burgeff et al. Citation2014). In contrast, genetically modified maize has been banned since 2013 for all purposes (). The Mexico biosafety law is process-based and was established at the time of controversial reports of maize landrace contamination in response to intense public pressure. Following the approval process, cotton and soybean were authorized for commercial production and maize was authorized for experimental and pilot programs. Due to presure from honey producers, environmental organizations, and the public, transgenic soybean and maize are currently banned.
Scientic advances have improved our understanding of genetically modified crops and their impact on human health, biodiversity and the environment. It is now known that gene flow and the risk of replacement of native plant populations by genetically modified plants is not greater than for hybrids or cultivars developed by conventinal breeding (The_ National_ Academy_ of_ Sciences Citation2010; Carpenter Citation2011; Dyer et al. Citation2014). Furthermore, gene editing technologies are being used to improve many plants of agricultural importance, including maize and all plants currently only authorized for experimental purpuses or pilot programs (). Under this scenario, the current biosafety law is no longer scientifically sound, and hampers further scientific advances and economic development, as illustrated by the contrasting differences in regulation between cotton and maize, and between genetically modified maize and maize hybrids.
The advantages of using genetically modified plants over alternative technologies are part of the criteria that integrate the risk analysis during the approval process (). Genetically modified plants resulting from genome editing have potential to provide an increment in yield, production and profit, benefiting both producers and consumers. Through gene editing, plants can be modified and not carry transgenes (Zilberman et al. Citation2018), and their genetic structure is similar to mutations that arise naturally. Thus, it is predicted that their consumption will not have an impact on human health (Ma et al. Citation2018). What is their impact on biodiversity? The answer to this question has potential to determine the fate of gene-edited maize in Mexico.
Factors with potential to re-shape the biosafety regulation in the near future include the economic need to feed an increasing population with less farmland, the imperative need to regulate plants modified using gene editing technology, education, and unbiased disemination of current advances on the nature, features, benefits and negative effects of genetically modified plants.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Author Contributions
H.G.R. conceived and designed the study; A.N.K and H.G.R designed the database. M.T.G.R, and H.G.R. populated the database and analyzed the data; M.T.G.R, and H.G.R. wrote the paper.
Acknowledgments
This research was supported by the Nebraska Agricultural Experiment Station with funding from the Hatch Act (Accession Number 1007272) through the USDA National Institute of Food and Agriculture and by a First Award from the Nebraska EPSCoR to HGR. Open access costs were provided by the same grant. Aaron N. Knapp was supported by the UCARE program and by a fellowship from the Institute of Agriculture and Natural Resources. We thank Esteban Betancur for assistance in generating the digital map of Mexico.
Additional information
Funding
References
- Acevedo F, Huerta E, Burgeff C, Koleff P, Sarukhan J. 2011. Is transgenic maize what Mexico really needs? Nat Biotechnol. 29:23–24.
- Aldemita RR, Hautea RA. 2018. Biotech crop planting resumes high adoption in 2016. GM Crops & Food. 9:1–12.
- Aldemita RR, Reaño IME, Solis RO, Hautea RA. 2015. Trends in global approvals of biotech crops(1992–2014). GM Crops & Food. 6:150–166.
- Alvarez-Buylla ER, Piñeyro NA. 2013. El Maíz enPeligro ante los Transgénicos: un análisis integralsobre el caso de México. UNAM, Centro de Investigaciones Interdisciplinarias en Ciencias y Humanidades. Union de Cientificos comprometidosc on la sociedad.
- Arteaga MC, Moreno-Letelier A, Mastretta-Yanes A, Vazquez-Lobo A, Brena-Ochoa A, Moreno-Estrada A, Eguiarte LE, Pinero D. 2016. Genomic variation inrecently collected maize landraces from Mexico.Genom Data. 7: 38–45.Q81265
- Bacalar CRIMD. 2017. Lucha de mayas frena a Monsanto y su soya transgénica. Boletín de prensa 2017 diciembre 8. http://www.greenpeace.org/mexico/es/Prensa1/2017/Diciembre/Lucha-de-mayasfrena-a-Monsanto-y-su-soya-transgenica/.
- Bleotu, C, et al. 2018. In: Grumezescu AM, editor. Genetically engineered foods. Academic Press. p. 385-401.
- Bonny S. 2017. Corporate Concentration and Techno-logical Change in the Global Seed Industry.Sustain-ability. 9:1632.
- Brookes G, Barfoot P. 2014. Economic impact of GM crops. GM Crops & Food. 5: 65–75.
- Brookes G, Barfoot P. 2016. Global income and production impacts of using GM crop technology1996-2014. GM Crops Food. 7(38–77). doi:10.1080/21645698.2016.1176817.
- Brandt M. 2014. Zapatista corn: a case study inbiocultural innovation. Soc Stud Sci. 44:874–900.
- Buiatti M, Christou P, Pastore G. 2013. The applicationof GMOs in agriculture and in food productionfor a better nutrition: two different scientific points of view. Genes Nutr. 8: 255–270.
- Burgeff C, Huerta E, Acevedo F, Sarukhan J. 2014 .How much can GMO and non-GMO cultivars coexistin a megadiverse country? AgBioForum. 17:90–101.
- Carpenter JE. 2011. Impact of GM crops on biodi-versity. GM Crops. 2:7–23.
- CBD. 2000. Cartagena protocol on biosafety to theconvention on biological diversity: text and annexes. Montreal: Secretariat of the Convention onBiological Diversity.
- DOF. 2005. Ley de Bioseguridad de organismos genéticamente modificados. Secretaría de Servicios-Parlamentarios. Diario Oficial de la Federación Nueva Ley DOF-18-03-2005.
- DOF. 2009. Reglamento de la Ley de Bioseguridad de organismos genéticamente modificados.
- Dyer GA, Serratos-Hernandez JA, Perales HR, Gepts P, Pineyro-Nelson A, Chavez A, Salinas-Arreortua N, Yunez-Naude A, Taylor JE, Alvarez-Buylla ER. 2009. Dispersal of transgenes through maize seed systems in Mexico. PLoS One. 4:e5734.
- Dyer GA, Lopez-Feldman A, Yunez-Naude A, Taylor JE. 2014. Genetic erosion in maize’s centerof origin. Proc Natl Acad Sci U S A.111: 14094–14099.
- Dyer GA, Taylor JE. 2008. A crop population per- spective on maize seed systems in Mexico. Proc NatlAcad Sci U S A. 105:470–475.
- Fernandez-Cornejo J, Wechsler S, Livingston M, Mitchell L. 2014. Genetically Engineered Crops inthe United States. USDA-ERS Econ Res Rep. 162. doi:10.2139/ssrn.2503388.
- Fesenko E, Edwards R. 2014. Plant synthetic biology: a new platform for industrial biotechnology. JExp Bot. 65:1927–1937.
- Georges F, Ray H. 2017. Genome editing of crops: Arenewed opportunity for food security. GM Crops& Food. 8:1–12.
- Heuberger S, Ellers-Kirk C, Tabashnik BE, Pollen- CY2010. Seed-Mediated Transgene Flow in CommercialCotton Seed Production Fields. PLoS ONE. 5:e14128. doi:10.1371/journal.pone.0014128
- Hug K. 2008. Genetically modified organisms: dothe benefits outweigh the risks? Medicina (Kaunas).44:87–99.
- Huang S, Weigel D, Beachy RN, Li J. 2016. A proposed regulatory framework for genome-editedcrops. Nat Genet. 48:109.
- James C. 2010. A global overview of biotech (GM)crops: adoption, impact and future prospects. GM Crops. 1:8–12. doi:10.4161/gmcr.1.1.9756.
- James C. 2015. 20th Anniversary (1996 to 2015)of the Global Commercialization of Biotech Cropsand Biotech Crop Highlights in 2015. ISAA BriefNo. 51.
- Jez JM, Lee SG, Sherp AM. 2016. The next greenmovement: plant biology for the environment and sustainability. Science. 353:1241–1244.
- Kaiser JB. 2005. Calming fears, no foreign genesfound in Mexico’s maize. Science. 309:1000.
- Kato Yamakake TA, Mapes Sánchez C, Mera OvandoLM, Serratos-Hernandez JA, Bye Boettler RA. 2009. Origen y diversificación del maíz. Una revisión analítica. Mexico: UNAM, Instituto de Biolgia.
- Kuzma J. 2016. Policy: Reboot the debate on genetic engineering. Nature. 531:165–167.
- Lomonossoff GP, D’Aoust MA. 2016. Plant-produced biopharmaceuticals: A case of technical developments driving clinical deployment. Science. 353 :1237–1240. doi: 10.1126/science.aaf6638.
- Ma X, Mau M, Sharbel TF. 2018. Genome Editingfor Global Food Security. Trends Biotechnol.36:123–127.
- Marinho, C. D. et al. 2014. Genetically modifiedcrops: brazilian law and overview. Genet Mol Res.13:5221–5240.
- Nap JP, Metz PL, Escaler M, Conner AJ. 2003. The release of genetically modified crops into the environment.Part I. Overview of current status and regulations.Plant J. 33:1–18.
- Orozco-Ramírez Q, Perales H, Hijmans RJ. 2016. Geographical distribution and diversity of maize (Zea mays L. subsp. mays) races in Mexico. Genet Resour Crop Evol. 1–11. doi:10.1007/s10722-016-0405-0
- Orozco-Ramirez Q, Ross-Ibarra J, Santacruz-VarelaA, Brush S 2016. Maize diversity associated with social origin and environmental variation in Southern Mexico. Heredity (Edinb). 116:477–484.doi:10.1038/hdy.2016.10
- Peña DG. 2013. Mexican judge rules that GMOs areimmiment threat. Food First. http://www.gene.ch/genet/2013/Oct/msg00032.html.
- Panchin AY, Tuzhikov AI. 2017. Published GMO studies find no evidence of harm when corrected formultiple comparisons. Crit Rev Biotechnol. 74537:213–217.
- Parrott W. 2010. Genetically modified myths andrealities. N Biotechnol. 27:545–551.
- Pineyro-Nelson A, Van Heerwaarden J, Perales HR, Serratos-Hernandez JA, Rangel A, Hufford MB, Gepts P, Garay-Arroyo A, Rivera-Bustamante R, Alvarez-Buylla ER. 2009. Resolution of theMexican transgene detection controversy: error sources and scientific practice in commercial and ecological contexts. Mol Ecol. 18:4145–4150.
- Pressoir G, Berthaud J 2004. Patterns of population structure in maize landraces from the Central Valleysof Oaxaca in Mexico. Heredity (Edinb). 92:88–94. doi:10.1038/sj.hdy.6800387
- Raman R. 2017. The impact of Genetically Modified(GM) crops in modern agriculture: A review. GMCrops & Food. 8:195–208. doi:10.1080/21645698.2017.1413522.
- Rastogi Verma S. 2013. Genetically modified plants: publicand scientific perceptions. ISRN Biotechnol. 7352013(820671). doi:10.5402/2013/820671.
- Romay G, Bragard C. 2017. Antiviral Defenses inPlants through Genome Editing. Front Microbiol. 8. doi:10.3389/fmicb.2017.00047
- Sharp PA, Leshner A. 2016. We need a new green revolution. New York Times. 2016 Jan 4.
- Snow A. 2009. Unwanted transgenes re-discovered in Oaxacan maize. Mol Ecol. 18:569–571.
- Sprink T, Eriksson D, Schiemann J, Hartung F. 2016. Regulatory hurdles for genome editing: process-vs. product-based approaches in different regulatorycontexts. Plant Cell Rep. 35:1493–1506.
- Smyth SJ. 2014. The state of genetically modified cropregulation in Canada. GM Crops Food. 5:195–203.
- Smyth SJ. 2017. Canadian regulatory perspectives ongenome engineered crops. GM Crops & Food. 8:35–43.
- Taheri F, Azadi H, D’Haese MA. 2017. World without Hunger: organic or GM Crops? Sustainability.9:580.
- The_National_Academy_of_Sciences. 2010. The Impact of Genetically Engineered Crops on Farm Sustainability in the United States. Report in Brief Washington (DC): The National Academies Press.
- Van Rijssen WJ, Morris EJ. 2018. In: Grumezescu AM, editor. Genetically engineered foods. Academic Press. p. 335–368.
- Waltz E. Gene-edited CRISPR mushroom escapes US regulation. Nature 2016; 532:293.
- Wegier A, Pineyro-Nelson A, Alarcon J, Galvez-Mariscal A, Alvarez-Buylla ER, Pinero D. 2011. Recent long-distance trans-gene flow into wild populations conforms to historical patterns of gene flow in cotton (Gossypium hirsutum) at its centre of origin. Mol Ecol. 20:4182–4194. doi:10.1111/j.1365-294X.2011.05258.x.
- Whelan AI, Lema MA. 2017. A research program forthe socioeconomic impacts of gene editing regula- tion. GM Crops & Food. 8:74–83. doi:10.1080/21645698.2016.1271856.
- Xolocotzi EH 1985. Maize and man in the Greater Southwest. Economic Botany. 39:416–430.doi:10.1007/bf02858749
- Yan S, Zhu J, Zhu W, Li Z, Shelton AM, Luo, J, Cui J, Zhang Q, Liu, X. 2015. Pollen-mediated gene flow from transgenic cotton under greenhouse conditions is dependent on different pollinators. Sci Rep. 5 (15917). doi:10.1038/srep15917.
- Zhang K, Raboanatahiry N, Zhu B, Li M. 2017. Progress in Genome Editing Technology and Its Application in Plants. Front Plant Sci 8. doi:10.3389/fpls.2017.00177
- Zhe N, Qian H, Yong‐Qiang H, Sheng H. 2018. Application of genome‐editing technology in crop improvement. Cereal Chem. 95:35–48.
- Zilberman D, Gordon B, Hochman G, Wesseler J. 2018. Economics of Sustainable Development and the Bioeconomy. Appl Econ Perspect Policy. 40:22–37.
