Abstract
Three-dimensional (3D) tumor models generated in vitro using methods of tissue engineering are just starting to show potential for predictive studies of therapeutic targets and screening of anticancer drugs. By mimicking some of the key features of the in vivo tumor environment, these models allow us to grow physiologically relevant tumors and study the initiation, progression and metastasis. Using a recent report on how to engineer bone tumors, we comment on the state-of-the-art in bioengineered bone tumors, with focus on the components required for recapitulating the in vivo milieu of bone tumor development.
Introduction
For many cancers, there is a real need for more effective therapy. Realistic in vitro models of human tumors would be transformative to cancer research and the development of new therapeutic options to many patients in need. The ability of an in vitro assay to produce reliable biomedical information is essential for drug development. Although many drugs show promising results in vitro (in cell monolayers)Citation1 and in vivo (in animal models),Citation2 most of them fail in clinical trials. This is because cancer is a complex disease in which the cell microenvironment plays important roles.Citation3,4 Therefore, creating biologically relevant tumor models requires the inclusion of the tumor microenvironment.Citation5
In recent years, many groups have made significant progress in creating advanced in vitro models that recapitulate some of the key factors of the native tumor microenvironment, with the aid of tissue engineering.Citation6 Our lab is applying the knowledge and techniques acquired over years in generating in vitro human bone tissueCitation7-10 to build specific niches for developing bone tumors.Citation11
Building Tissue-Engineered Models of Human Tumors
We believe that the first step in tumor modeling should be to identify the roles of key elements implicated in a specific tumor niche. Understanding of the tumor hallmarks and the specifics of the tumor microenvironment is the first and necessary step in modeling tumors. The next step should be to engineer a controllable biomimetic system that resembles a set of particular properties of interest rather than developing complex models trying to recapitulate the whole landscape of the tumor. We think that such a “minimally functional unit” capturing the key aspects of the tumor complexity will provide a platform for addressing specific questions and thereby advance cancer research.
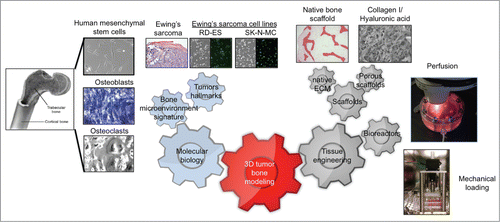
Based on our previous studies on the bone niche,Citation7-10 we consider that—in addition to cancer cells—the most important component for building a bone tumor is the bone tissue context, with the bone cells and extracellular matrix. Together, these 2 components can be mimicked using the tissue engineering tools, such as scaffolds and bioreactors (). It seems self-evident that cancer bioengineering requires collaborative expertise from many diverse backgrounds. Multidisciplinary teams are the future of cancer research and the molecular biologists, engineers and clinicians are now synergizing their efforts to provide a global perspective of the disease.
As an example, our multidisciplinary group has recently engineered a tissue model of Ewing's sarcomaCitation11 that incorporates (i) A cellular compartment of the bone derived from mesenchymal stem cells; (ii) The non-cellular compartment of the bone comprised of the organic extracellular matrix (ECM) and the mineral phase; and (iii) The tumor compartment with Ewing's sarcoma cells. Basically, we co-cultured tumor cell aggregates with human mesenchymal stem cells (hMSC) differentiated into osteogenic lineages within a native decellularized bone used as a scaffold. This innovative model allows a cross-talk between cancer cells, bone cells and bone matrix, with the tumor residing within its native bone niche.
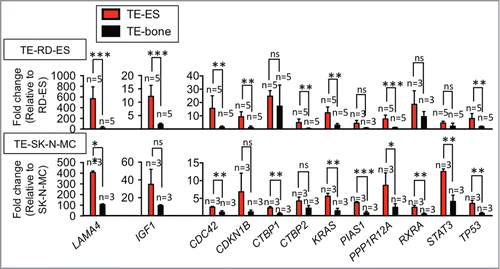
A large body of work has demonstrated that cell lines cultured in 2D lose many of their transcriptional profiles and down-regulate genes implicated in cell-cell and cell-ECM interactions.Citation12,13 Our studies show that Ewing's sarcoma cells in monolayer culture down-regulate ∼600 genes implied in cancer and expressed in fresh tumors obtained from patients (). Importantly, we observed re-expression of at least 12 genes related to focal adhesion genes and pathways in cancer and the re-establishment of the tumor phenotype when Ewing's sarcoma cell lines were incorporated into the tissue-engineered bone (). These results indicate that tissue-engineered models of human tumors could have utility for identification and characterization of differentially expressed genes which then could be investigated as potential therapeutic targets.
Figure 2. Numerous genes expressed in native Ewing sarcoma tumors are down-regulated in tumor cell lines cultured in monolayers. The fold change in gene expression was normalized to actin levels in the individual samples and then to the corresponding levels in cells cultured in monolayer. Data are shown as Average ± SD (n = 3–5). Two-tailed Student´s T-test was used to determine statistical significance.*P < 0.05; **P < 0.01, ***P < 0.001; ns, not significant (Reproduced with permission from Villasante et al. Biomaterials 35: 5785-5794, 2014).
The primary and metastatic bone tumors modify their phenotype by trying to resemble bone cells and express bone matrix proteins (such as osteopontin, bone sialoprotein and osteocalcin), alkaline phosphatase, and molecules regulating the osteoblast/osteoclast cross-talk.Citation14 For example, metastatic breast cancer cells express bone sialoprotein.Citation15 This ability of cancer cells to acquire a bone cell phenotype is known as osteomimicry and it is an adaptive advantage that gives tumor cells a better chance to survive and proliferate in the bone tissue.Citation14 We observed that the Ewing's sarcoma cell lines cultured in monolayers loose in part the ability for osteomimicry by downregulating bone matrix proteins. Notably, these proteins are re-expressed when cultured within a bone-engineered niche ().
Figure 3. Tissue-engineered bone models express osteomimicry. (a) Tissue-engineered (TE) model of Ewing's sarcoma. Bone markers BSP, OPN and OCN are re-expressed in the TE-Ewing's sarcoma 3D model, as shown histologically (hematoxylin and eosin) and by immunostains (Ewing's sarcoma marker CD99; bone markers BSP, OPN and OCN) after 6 weeks of culture. Negative control without primary antibody is shown for comparison. Counterstaining was performed with hematoxylin QS (blue). (b) Marker expression after 4 weeks in co-culture. Data are shown for bone pellets in co-culture with Ewing's sarcoma aggregates. Immunohistochemical stainings of the aggregates model for Ewing's sarcoma marker CD99 and bone marker OCN at week 4 in co-culture with osteoblasts-derived from hMSC. Negative control without primary antibody is shown for comparison. OB = Osteoblasts; ES = Ewing's sarcoma cells. (Reproduced with permission from Villasante et al. Biomaterials 2014; 35: 5785-94).
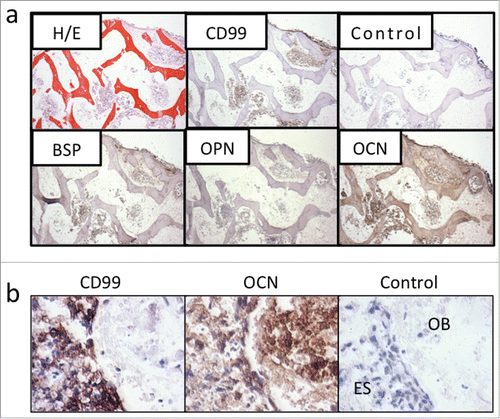
Another interesting observation from our ongoing studies is that the Ewing's sarcoma cell lines upregulate only osteocalcin—but not bone sialoprotein or osteopontin—in co-culture with aggregates of osteoblasts derived from human mesenchymal stem cells (). This is an important finding that supports the need for incorporating the non-cellular compartment into the bioengineered tumor systems. Decellularized bone matrix preserves the architecture (the macro- and micro- structural features), bioactive molecules (the mineral phase, growth factors), and mechanical properties of native bone, which are all critical for the vicious cycle of tumor survival and development.Citation16
Challenges
Despite all advances made in generating bone tumor models, there is a need to more adequately introduce the essential components of the tumor microenvironment for clinical utility. Incorporation of osteoclasts is a must for modeling osteolytic tumors and gaining insights into how cancer cells regulate the bone osteoclasts and osteoblasts.Citation17 Vasculature is another important element of tumor development and metastasis, due to its role in supplying cells with nutrients, oxygen and systemic factors, and in the maintenance of cellular homeostasis. However only a few tumor models incorporate blood vessels and none of them is suitable for studying bone malignancies. Our group has developed a robust protocol to engineer vascularized bone that could be useful for generating vascularized bone tumors.Citation10 Another reachable goal in bone tumor modeling is the introduction of the components of the immune system, such as T and B lymphocytes, which have major roles in bone remodeling, and regulation of bone tumors through osteoclast activation.Citation18 In summary, the implementation of tissue engineering tools allows us to “build better tumors” and investigate tumor biology and new drugs in settings that more closely mimic the clinical situation.Citation19
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the National Institutes of Health (grant EB002520).
References
- Hutchinson L, Kirk R. High drug attrition rates–where are we going wrong? Nat Rev Clin Oncol 2011; 8:189-90; PMID:21448176; http://dx.doi.org/10.1038/nrclinonc.2011.34
- van der Worp HB, Howells DW, Sena ES, Porritt MJ, Rewell S, O'Collins V, Macleod MR. Can animal models of disease reliably inform human studies? PLoS Med 2010; 7:e1000245; PMID:20361020; http://dx.doi.org/10.1371/journal.pmed.1000245
- Bissell MJ, Hines WC. Why don't we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med 2011; 17:320-9; PMID:21383745; http://dx.doi.org/10.1038/nm.2328
- Correia AL, Bissell MJ. The tumor microenvironment is a dominant force in multidrug resistance. Drug Resist Updat 2012; 15:39-49; PMID:22335920; http://dx.doi.org/10.1016/j.drup.2012.01.006
- Goodbye, flat biology? Nature 2003; 424:861; PMID:12931146
- Kimlin LC, Casagrande G, Virador VM. In vitro three-dimensional (3D) models in cancer research: an update. Mol Carcinog 2013; 52:167-82; PMID:22162252; http://dx.doi.org/10.1002/mc.21844
- Grayson WL, Frohlich M, Yeager K, Bhumiratana S, Chan ME, Cannizzaro C, Wan LQ, Liu XS, Guo XE, Vunjak-Novakovic G. Engineering anatomically shaped human bone grafts. Proc Natl Acad Sci U S A 2010; 107:3299-304; PMID:19820164; http://dx.doi.org/10.1073/pnas.0905439106
- Marolt D, Campos IM, Bhumiratana S, Koren A, Petridis P, Zhang G, Spitalnik PF, Grayson WL, Vunjak-Novakovic G. Engineering bone tissue from human embryonic stem cells. Proc Natl Acad Sci U S A 2012; 109:8705-9; PMID:22586099; http://dx.doi.org/10.1073/pnas.1201830109
- de Peppo GM, Marcos-Campos I, Kahler DJ, Alsalman D, Shang L, Vunjak- Novakovic G, Marolt D. Engineering bone tissue substitutes from human induced pluripotent stem cells. Proc Natl Acad Sci U S A 2013; 110:8680-5; PMID:23653480; http://dx.doi.org/10.1073/pnas.1301190110
- Correia C, Grayson WL, Park M, Hutton D, Zhou B, Guo XE, Niklason L, Sousa RA, Reis RL, Vunjak-Novakovic G. In vitro model of vascularized bone: synergizing vascular development and osteogenesis. PLoS One 2011; 6:e28352; PMID:22164277; http://dx.doi.org/10.1371/journal.pone.0028352
- Villasante A, Marturano-Kruik A, Vunjak-Novakovic G. Bioengineered human tumor within a bone niche. Biomaterials 2014; 35:5785-94; PMID:24746967; http://dx.doi.org/10.1016/j.biomaterials.2014.03.081
- Smalley KS, Lioni M, Herlyn M. Life isn't flat: taking cancer biology to the next dimension. In Vitro Cell Dev Biol Anim 2006; 42:242-7; PMID:17163781; http://dx.doi.org/10.1290/0604027.1
- Chitcholtan K, Asselin E, Parent S, Sykes PH, Evans JJ. Differences in growth properties of endometrial cancer in three dimensional (3D) culture and 2D cell monolayer. Exp Cell Res 2013; 319:75-87; PMID:23022396; http://dx.doi.org/10.1016/j.yexcr.2012.09.012
- Rucci N, Teti A. Osteomimicry: how tumor cells try to deceive the bone. Front Biosci (Schol Ed) 2010; 2:907-15; PMID:20515833; http://dx.doi.org/10.2741/S110
- Barnes GL, Javed A, Waller SM, Kamal MH, Hebert KE, Hassan MQ, Bellahcene A, Van Wijnen AJ, Young MF, Lian JB, et al. Osteoblast-related transcription factors Runx2 (Cbfa1/AML3) and MSX2 mediate the expression of bone sialoprotein in human metastatic breast cancer cells. Cancer Res 2003; 63:2631-7; PMID:12750290
- Guelcher SA, Sterling JA. Contribution of bone tissue modulus to breast cancer metastasis to bone. Cancer Microenviron 2011; 4:247-59; PMID:21789687; http://dx.doi.org/10.1007/s12307-011-0078-3
- Olechnowicz SW, Edwards CM. Contributions of the host microenvironment to cancer-induced bone disease. Cancer Res 2014; 74:1625-31; PMID:24599133; http://dx.doi.org/10.1158/0008-5472.CAN-13-2645
- Mori G, D'Amelio P, Faccio R, Brunetti G. The Interplay between the bone and the immune system. Clin Dev Immunol 2013; 2013:720504; PMID:23935650; http://dx.doi.org/10.1155/2013/720504
- Spiller K. Bioengineering disease models: How to build a better bone tumor. Sci Transl Med 2014; 6:235.