Abstract
Insecticidal Cry proteins from Bacillus thuringiensis (Bt) have been exploited in the development of genetically modified (GM) crops for pest control. However, several pests are still difficult to control such as the coleopteran boll weevil Anthonomus grandis. By applying in vitro molecular evolution to the cry8Ka1 gene sequence, variants were generated with improved activity against A. grandis. Among them, Cry8Ka5 mutant protein showed coleoptericidal activity 3-fold higher (LC50 2.83 μg/mL) than that of the original protein (Cry8Ka1). Cry8Ka5 has been used in breeding programs in order to obtain coleopteran-resistant cotton plants. Nevertheless, there is some concern in relation to the food safety of transgenic crops, especially to the heterologously expressed proteins. In this context, our research group has performed risk assessment studies on Cry8Ka5, using the tests recommended by Codex as well as tests that we proposed as alternative and/or complementary approaches. Our results on the risk analysis of Cry8Ka5 taken together with those of other Cry proteins, point out that there is a high degree of certainty on their food safety. It is reasonable to emphasize that most safety studies on Cry proteins have essentially used the Codex approach. However, other methodologies would potentially provide additional information such as studies on the effects of Cry proteins and derived peptides on the indigenous gastrointestinal microbiota and on intestinal epithelial cells of humans. Additionally, emerging technologies such as toxicogenomics potentially will offer sensitive alternatives for some current approaches or methods.
Introduction
Bacillus thuringiensis (Bt) is a Gram-positive bacterium that naturally occurs in the soil and is characterized by its ability to produce crystal-containing parasporal inclusions during sporulation.Citation1,2 These crystals are composed of one or more proteins called Cry (Crystal proteins) and Cyt (Cytolitic proteins), also called δ-endotoxins.Citation3 The main property of the δ-endotoxins is their selective entomotoxic activity. The Cry proteins are specifically toxic to insects of the orders Lepidoptera, Coleoptera, Hymenoptera, and Diptera as well as to nematodes. On the other hand, Cyt proteins are primarily toxic to insects of the order Diptera, but also exhibit cytolytic activity against a wide variety of cell types, including vertebrate cells.Citation4,5 The latter limits the biotechnological use of Cyt proteins since they present risks to human health and other non-target organisms. Therefore, the focus of pest management research has been directed toward Cry toxins.
Since the discovery of their insecticidal properties, Cry proteins have been the subject of numerous studies on their diversity, structure, evolution, mode of action and use in the production of insect-resistant genetically modified (GM) plants, the so-called Bt plants.Citation1,3 The development of transgenic plants expressing Cry toxins has facilitated the replacement of chemical insecticides for alternatives less harmful to the environment.Citation1
The present overview will focus on Bt cotton, since it is the main transgenic crop studied in our group. As early as 1995, the Environmental Protection Agency of United States (US EPA) approved the first Bt plants (potato, corn and cotton). Monsanto was the first company to commercialize Bt crops and among them were the cotton varieties Bollgard and Ingard (events 531, 757 and 1076), expressing a modified Cry1Ac toxin that confers resistance to the tobacco budworm (Heliothis virescens), cotton bollworm (Helicoverpa armigera) and pink bollworm (Pectinophora gossypiella).Citation1,6,7 The adoption of the first Bt cotton varieties is regarded as a landmark in agricultural biotechnology.Citation5
Although Bt cotton varieties expressing a single cry gene showed excellent results in controlling lepidopterans, a new generation of cotton plants carrying 2 Bt genes in the same plant entered the market in 2002. In WideStrike® cotton developed by Dow Agrosciences and Bollgard II® cotton launched by Monsanto Company, the Cry1Ac toxin was co-expresesed with Cry1F and Cry2Ab2, respectively. Both varieties are active against a larger number of lepidopteran pests as compared to crops expressing a single Cry toxin.Citation5,7 In fact, one of the aims of Bt gene stacking is to increase plant resistance against a greater number of pests.Citation5
The main threat to the longevity of the use of Bt toxins in agriculture is the evolution of insect pest resistance.Citation8,9 There are numerous reports on the resistance of several target insect species to Bt plants.Citation10-16 Among these are the pests H. armigera and P. gossypiella, for which resistance to Bt cotton has been observed in different regions of China.Citation17 On the other hand, transgenic cotton events carrying 2 or more Bt genes have been shown to be quite effective and so far there are no reports on the occurrence of resistance to these new cultivars.Citation18,19
Apart from the evolution of insect pest resistance to Cry proteins expressed in commercialized Bt crops, there are several pests that still are difficult to control. Among them is the boll weevil (Anthonomus grandis), which is an insect belonging to the order Coleoptera and is the main pest of cotton culture in Brazil. Efforts have been made to search for Bt strains producing Cry3, Cry8, Cry1B and Cry1I toxins, known to be active against Coleoptera and therefore potential candidates for the development of a boll weevil-resistant transgenic cotton.Citation20-23 In that search, Bt strain S811, carrier of cry1I and cry8 genes, appeared to have activity against A. grandis.Citation22 Subsequently, the cry8Ka1 gene was isolated and heterologously expressed in E. coli. However, the Cry8Ka1 recombinant protein proved to be just moderately toxic against beetle larvae and this would not be sufficient to obtain a transgenic cotton capable to control this insect pest.Citation24,25 By using in vitro molecular evolution techniques (DNA shuffling coupled to phage display), Oliveira et al.Citation26 generated a combinatorial library containing 105 variants of the cry8Ka1 gene (2001 bp) and then variants were selected with improved activity against A. grandis. The Cry8Ka5 mutant protein, which was among the most effective variants, was subcloned into a vector for expression in E.coli. The purified Cry8Ka5 protein showed an entomotoxic activity 3-fold higher (LC50 2.83μg/mL) than that of the original protein (Cry8Ka1).Citation26
The Cry8Ka5 mutant entomotoxin has been used in breeding programs in order to obtain cotton plants resistant to A. grandis.Citation27 In addition, other crops of economic importance which are vulnerable to beetle pest attack are being transformed with the Cry8Ka5 protein to obtain coleopteran-resistant Bt plants. Also other Cry proteins are continuously being improved or discovered to control pests by developing Bt crops, either in single or stacked events.Citation28-30 However, the development of GM plants is accompanied by a concern related to bioethics and biosafety. To address the latter issue, food safety assessment studies are indispensable before launching GM crops in the market.
An important component of the safety assessment of agricultural products generated by recombinant DNA technology is the food safety evaluation of the exogenous proteins. In this context, the most widely accepted approach is that presented in the Codex Alimentarius,Citation31 which is a compilation of WHO and FAO guidelines. The following factors are considered in that approach: the source of the gene; the similarity of amino acid sequence of the test protein compared to that of known toxic and/or allergenic proteins; the stability of the protein to pepsin digestion; and, where appropriate, a search for antibodies (IgE) against the test protein in human serum banks and/or tests using animal models. Moreover, no end-point by itself can predict the toxic and/or allergenic potential of a novel protein.Citation31,32
Alternatively, the International Life Sciences Institute (ILSI) proposed a 2-tiered approach to assess the safety of transgenic proteins.Citation33 In fact, the suggested tests are similar to those proposed by the Codex,Citation31 differing only in the manner they are applied which is more flexible and takes into account all the data in a holistic manner. Ideally, the predictive value of each piece of evidence must be understood in order to give certain data more “weight” than others during the evaluation, thus leading to greater confidence in the overall assessment. The first step (Tier I) in the ILSI approach focuses on the “hazard identification” by determining the history of safe use (HOSU); comparing the primary amino acid sequence of the test protein to known toxic and/or allergenic proteins in databases; determining the mode of action and specificity; and analyzing susceptibility to in vitro digestion and heat treatment. The second step (Tier II), “hazard characterization,” which is done case-by-case, however for insecticidal proteins an acute oral toxicity test in mice is recommended. Repeated-dose (28 days) oral toxicity tests in rodents are also recommended by some regulatory agencies.
The safety of several Cry proteins has been analyzed by using the set of tests reviewed by Hammond and Koch.Citation34 and more recently by Koch et al.Citation35 So far, the outcome of these studies point toward the safety of these proteins, either in isolated form or expressed in GM plants. However, we suggest that the safety assessment of Cry proteins can go beyond the proposed official methods in order to focus on less investigated aspects, such as the effects of these proteins on human gastrointestinal microbiota as well as the use of more sensitive and specific approaches such as toxicogenomics, particularly when applied to in vitro cultured human cell lines. Certainly, a progressive accumulation of information on the safety of Cry proteins and the use of in vitro systems are aspects that should be welcomed as it may converge to a growing confidence about the safety of these biotech tools and contributes to the use of 3R alternatives for animal testing.
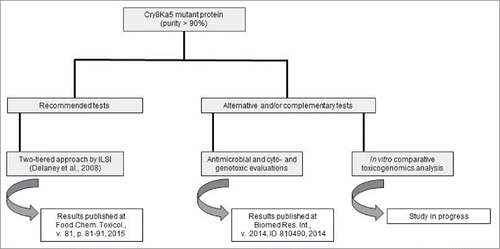
In this context, our research group has performed studies on the risk assessment of the Cry8Ka5 mutant protein, using not only tests recommended by Codex, but also tests proposed by us as alternative and/or complementary approaches. The food safety study of the Cry8Ka5 would ensure the safety use of the edible oil extracted from the cottonseed as well as of the meal, a by-product of the oil extraction process, which is an important source of protein in cattle and sheep diet.Citation36 Thus, our group created a flowchart with the experiments planned for the risk analysis of Cry8Ka5 (). Part of these experiments was concluded, and the results obtained will be briefly discussed here. Later on, we will introduce in few lines the safety tests that are currently being done with Cry8Ka5.
As shown on the left side of , Cry8Ka5 was subjected to tests that are recommend by ILSI in its 2-tiered approach for the food safety assessment of recombinant proteins.Citation33 In our study, the Cry1Ac protein was used as an experimental control since ample safety data are available. Regarding the Tier I step, it has been demonstrated that Cry proteins have a long and favorable history of safe use (in the context of Bt sprays). As to the occurrence of similarity with toxic and/or allergenic proteins, the complete sequence of Cry8Ka5 showed no significant identity in any of the performed analyzes. With respect to the mode of action of Cry proteins, it is well documented that the specificity of these toxins is restricted to insects. In our Tier I study we also showed that Cry8Ka5 is susceptible to degradation by pepsin. In Tier II, an acute oral toxicity test (single dose) in mice was performed and did not reveal any relevant adverse effects of Cry8Ka5 at a dose of 5,000 mg/kg body weight. For Cry1Ac (used as a Bt control protein) similar results were observed. This study was recently published by our research group.Citation37 In general, the results for the Cry8Ka5 protein were similar to those described for other Cry proteins, such as Cry1Ab/Ac, Cry1C, Cry1A.105 and Cry2Ab2.Citation38-40
In the right side of the flowchart in are the tests that we proposed as alternative and/or complementary food safety analysis for Cry8Ka5 protein. Cry8Ka5 and the Bt reference protein Cry1Ac were subjected to cyto- and genotoxicity testing and to antimicrobial activity assay. These tests were performed to address the following questions: (1) Do Cry proteins, in particular, Cry8Ka5 and Cry1Ac, show deleterious effects in human primary cells?; (2) Do Cry8Ka5 and Cry1Ac proteins have negative effects on the growth of fungi and bacteria in the human gastrointestinal microbiota? The results of our study showed that Cry8Ka5 and Cry1Ac did not promote cyto- or genotoxic effects (IC50 > 1,000 µg/mL) in human lymphocytes and did not cause hemolysis or any significant damage (IC50 > 1,000 µg/mL) to mammalian red blood cells. The latter was examined through analysis of membrane topography using atomic force microscopy. Furthermore, Cry8Ka5 and Cry1Ac did not inhibit the growth of any yeast and bacteria species of the human gastrointestinal microbiota (MIC > 1,000 µg/mL). These results were also recently published by our group.Citation41
The reason for performing such tests is related to studies by others who showed genotoxic effects of moderate doses (25-150 μg/mL) of Cry1Aa, Cry1Ab, Cry1Ac and Cry2A, tested separately or in combinations, in embryos and larvae of zebrafish.Citation42 It has been also demonstrated that Cry1Ab, Cry1D and Cry3A toxins have antimicrobial effects against aerobic and anaerobic bacteria (MIC ranging from 45 to 150 µg/mL).Citation43 Thus, further studies on the in vitro and in vivo antimicrobial activity of Cry proteins as well as of peptide mixtures generated by in vitro digestion of these proteins will contribute to evaluate the usefulness of these tests in the context of food safety assessment of Cry proteins. This can also be extended to the search of cyto- and genotoxic effects of Cry proteins by using different cell strains, particularly gut cells.
The last step planned by our research group for assessing food safety of the Cry8Ka5 protein concerns an in vitro toxicogenomics analysis (). The aim of this study, which is currently in progress, is to analyze the effects of the Cry8Ka5 protein and its peptide pool (obtained upon in vitro sequential digestion) on whole genome gene expression of a human intestine cell line when compared to the treatment with known toxic peptides and proteins.
In conclusion, our results on the risk analysis of Cry8Ka5 taken together with those of other Cry proteins, point out that there is a high degree of certainty on the food safety of these proteins. It is reasonable to emphasize that most safety studies on Cry proteins have essentially used the approach and tests recommended by the Codex Alimentarius (FAO/WHO). However, other approaches would potentially provide additional information on the safety of these important biotechnological tools. These approaches include the analysis of the effects of the Cry proteins and their derived peptides on the indigenous gastrointestinal microbiota and on human intestinal cell models. In addition, emerging technologies such as toxicogenomics potentially will offer sensitive alternatives for some current approaches or methods.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
Research in the area of risk and effect analysis of biotech products in our group is funded by Brazilian National Council for Scientific and Technological Development (CNPq) (grant number 461182/2014-9) and Coordination for the Improvement of Higher Education Personnel (CAPES, Brazilian Ministry of Education) (grant number A006_2013).
References
- Sanahuja G, Banakar R, Twyman RM, Capell T, Christou P. Bacillus thuringiensis: a century of research, development and commercial applications. Plant Biotech J 2011; 9:283-300; http://dx.doi.org/10.1111/j.1467-7652.2011.00595.x
- Vachon V, Laprade R, Schwartz JL. Current models of the mode of action of Bacillus thuringiensis insecticidal crystal proteins: a critical review. J Invertebr Pathol 2012; 111:1-12; PMID:22617276; http://dx.doi.org/10.1016/j.jip.2012.05.001
- Bravo A, Gill SS, Soberón M. Mode of action of Bacillus thuringiensis: Cry and Cyt toxins and their potential for insect control. Toxicon 2007; 49:423-35; PMID:17198720; http://dx.doi.org/10.1016/j.toxicon.2006.11.022
- Soberón M, Fernández LE, Pérez C, Gill SS, Bravo A. Mode of action of mosquitocidal Bacillus thuringiensis toxins. Toxicon v. 49, n. 5, p. 2007; 597-600; http://dx.doi.org/10.1016/j.toxicon.2006.11.008
- Kumar S, Chandra A, Pandey KC. Bacillus thuringiensis (Bt) transgenic crop: an environment friendly insect-pest management strategy. J Environ Biol 2008; 29:641-53; PMID:19295059
- Mendelsohn M, Kough J, Vaituzis Z, Matthews K. Are Bt crops safe? Nat Biotechnol 2003; 21:1003-9; PMID:12949561; http://dx.doi.org/10.1038/nbt0903-1003
- Gatehouse AM, Ferry N, Edwards MG, Bell HA. Insect-resistant biotech crops and their impacts on beneficial arthropods. Philos Trans R Soc Lond B Biol Sci 2011; 12:1438-52; http://dx.doi.org/10.1098/rstb.2010.0330
- Tabashnik BE. Evolution of resistance to Bacillus thuringiensis. Annu Rev Entomol 1994; 39:47-79; http://dx.doi.org/10.1146/annurev.en.39.010194.000403
- Gould F. Sustainability of transgenic insecticidal cultivars: integrating pest genetics and ecology. Ann Rev Entomol 1998; 43:701-26; http://dx.doi.org/10.1146/annurev.ento.43.1.701
- Bagla P. Hardy cotton-munching pests are latest blow to GM crops. Science 2010; 327:14-39
- Carriere Y, Crowder DW, Tabashnik BE. Evolutionary ecology of insect adaptation to Bt crops. Evol Appl 2010; 3:561-73; PMID:25567947; http://dx.doi.org/10.1111/j.1752-4571.2010.00129.x
- Storer NP, Babcock JM, Schlenz M, Meade T, Thompson GD, Bing JW, Huckaba RM. Discovery and characterization of field resistance to Bt maize: Spodoptera frugiperda (Lepidoptera: Noctuidae) in Puerto Rico. J Econ Entomol 2010; 103:1031-8; PMID:20857709; http://dx.doi.org/10.1603/EC10040
- Alcantara E, Estrada A, Alpuerto V, Head G. Monitoring Cry1Ab susceptibility in Asian corn borer (Lepidoptera: Crambidae) on Bt corn in the Philippines. Crop Prot 2011; 30:554-9; http://dx.doi.org/10.1016/j.cropro.2010.12.019
- Gassmann AJ, Petzold-Maxwell JL, Keweshan RS, Dunbar MW. Field evolved resistance to Bt maize by western corn rootworm. PLoS One 2011; 6:e22629; PMID:21829470; http://dx.doi.org/10.1371/journal.pone.0022629
- Zhang H, Yin W, Zhao J, Jin L, Yang Y, Wu S, Tabashnik BE, Wu Y. Early warning of cotton bollworm resistance associated with intensive planting of Bt cotton in China. PLoS One 2011; 6:e22874; PMID:21857961; http://dx.doi.org/10.1371/journal.pone.0022874
- Wan P, Huang Y, Wu H, Huang M, Cong S, Tabashnik BE, Wu K. Increased frequency of pink bollworm resistance to Bt toxin Cry1Ac in China. PLoS ONE 2012; 7:e29975; PMID:22238687; http://dx.doi.org/10.1371/journal.pone.0029975
- Tabashnik BE, Wu K, Wu Y. Early detection of field-evolved resistance to Bt cotton in China: cotton bollworm and pink bollworm. J Invertebr Pathol 2012; 110:301-6; PMID:22537835; http://dx.doi.org/10.1016/j.jip.2012.04.008
- Brévault T, Prudent P, Vaissayre M, Carrière Y. Susceptibility of Helicoverpa armigera (Lepidoptera: Noctuidae) to Cry1Ac and Cry2Ab2 insecticidal proteins in four countries of the West African cotton belt. J Econ Entomol 2009; 102:2301-9; http://dx.doi.org/10.1603/029.102.0636
- An J, Gao Y, Wu K, Gould F, Gao J, Shen Z, Lei C. Vip3Aa tolerance response of Helicoverpa armigera populations from a Cry1Ac cotton planting region. J Econ Entomol 2010; 103:2169-73; PMID:21309241; http://dx.doi.org/10.1603/EC10105
- Marquez AM, Dias JM, Ribeiro BM. Screening and characterization of Bacillus thuringiensis isolates from Brazil for the presence of coleoptera-specific cry genes. Microbiol Res 2000; 154:355-62; PMID:10772158; http://dx.doi.org/10.1016/S0944-5013(00)80010-5
- Grossi-de-Sa MF, Quezado de Magalhaes M, Silva MS, Silva SM, Dias SC, Nakasu EY, Brunetta PS, Oliveira GR, Neto OB, Sampaio de Oliveira R et al. Susceptibility of Anthonomus grandis (cotton boll weevil) and Spodoptera frugiperda (fall armyworm) to a cry1Ia-type toxin from a Brazilian Bacillus thuringiensis strain. J Biochem Mol Biol 2007; 30:773-82; http://dx.doi.org/10.5483/BMBRep.2007.40.5.773
- Martins EL, Praça LB, Dumas VF, Silva-Werneck JO, Sone EH, Waga IC, Berry C, Monnerat RG. Characterization of Bacillus thuringiensis isolates toxic to cotton boll weevil (Anthonomus grandis). Biol Control 2007: 40:65-68; http://dx.doi.org/10.1016/j.biocontrol.2006.09.009
- Martins ES, Aguiar RW, Martins NF, Melatti VM, Falcão R, Gomes AC, Ribeiro BM, Monnerat RG. Recombinant Cry1Ia protein is highly toxic to cotton boll weevil (Anthonomus grandis Boheman) and fall armyworm (Spodoptera frugiperda). J Appl Microbiol 2008; 104:1363-71; PMID:18248369; http://dx.doi.org/10.1111/j.1365-2672.2007.03665.x
- Nakasu EY, Firmino AA, Dias SC, Rocha TL, Ramos HB, Oliveira GR, Lucena W, Carlini CR, Grossi-de-Sá MF. Analysis of Cry8Ka5-binding proteins from Anthonomus grandis (Coleoptera: Curculionidae) midgut. J Invertebr Pathol 2010; 104:227-30; PMID:20144614; http://dx.doi.org/10.1016/j.jip.2010.01.012
- Lucena WA, Pelegrini PB, Martins-de-Sa D, Fonseca FC, Gomes JE, Jr, de Macedo LL, da Silva MC, Oliveira RS, Grossi-de-Sa MF. Molecular approaches to improve the insecticidal activity of Bacillus thuringiensis Cry toxins. Toxins (Basel) 2014; 6:2393-423; PMID:25123558; http://dx.doi.org/10.3390/toxins6082393
- Oliveira GR, Silva MC, Lucena WA, Nakasu EY, Firmino AA, Beneventi MA, Souza DS, Gomes JE, Jr, de Souza JD Jr, Rigden DJ et al. Improving Cry8Ka toxin activity towards the cotton boll weevil (Anthonomus grandis). BMC Biotechnol 2011; 9:85; http://dx.doi.org/10.1186/1472-6750-11-85
- Oliveira GR. In vitro evolution of Cry molecules active against Anthonomus grandis and Spodoptera frugiperda [dissertation in Portuguese]. [Porto Alegre]: Federal University of Rio Grande do Sul; 2008. 97p
- Lira J, Beringer J, Burton S, Griffin S, Sheets J, Tan SY, Woosley A, Worden S, Narva KE. Insecticidal activity of Bacillus thuringiensis Cry1Bh1 against Ostrinia nubilalis (Hubner) (Lepidoptera: Crambidae) and other lepidopteran pests. Appl Environ Microbiol 2013; 79:7590-7; PMID:24077715; http://dx.doi.org/10.1128/AEM.01979-13
- Palma L, Muñoz D, Berry C, Murillo J, de Escudero IR, Caballero P. Molecular and insecticidal characterization of a novel Cry-related protein from Bacillus thuringiensis toxic against Myzus persicae. Toxins (Basel) 2014; 6:3144-56; PMID:25384108; http://dx.doi.org/10.3390/toxins6113144
- Zhao C, Jurat-Fuentes JL, Abdelgaffar HM, Pan H, Song F, Zhang J. Identification of a new cry1I-type gene as a candidate for gene Pyramiding in corn to control Ostrinia species larvae. Appl Environ Microbiol 2015: 81:3699-705; PMID:25795679; http://dx.doi.org/10.1128/AEM.00379-15
- Codex Alimentarius. Foods derived from modern biotechnology [Internet]. Rome, Italy, Codex Alimentarius Commission, Joint FAO/WHO Food Standards Programme, Food and Agriculture Organization of the United Nations. Second Edition. 2009. [cited 2015 Jul 10] Available from: http://www.fao.org/docrep/011/a1554e/a1554e00.htm
- Goodman RE, Hefle SL, Taylor SL, van Ree R. Assessing genetically modified crops to minimize the risk of increased food allergy: a review. Int Arch Allergy Immunol 2005; 137:153-66; PMID:15947471; http://dx.doi.org/10.1159/000086314
- Delaney B, Astwood JD, Cunny H, Conn RE, Herouet-Guicheney C, Macintosh S, Meyer LS, Privalle L, Gao Y, Mattsson J, et al. Evaluation of protein safety in the context of agricultural biotechnology. Food Chem Toxicol 2008; 46:S71-97; PMID:18348900; http://dx.doi.org/10.1016/j.fct.2008.01.045
- Hammond BG, Koch MS. Bacillus thuringiensis biotechnology. 1st ed. Dordrecht: Springer; 2012. Chapter 16, A review of the food safety of Bt crops; p. 305-25
- Koch MS, Ward JM, Levine SL, Baum JA, Vicini JL, Hammond BG. The food and environmental safety of Bt crops. Front Plant Sci 2015; 6:283; PMID:25972882; http://dx.doi.org/10.3389/fpls.2015.00283
- Martins WFS, Ayres CFJ, Lucena WA. Genetic diversity of Brazilian natural populations of Anthonomus grandis Boheman (Coleoptera: Curculionidae), the major cottonpest in the New World. Genet Mol Res 2007, 6:23-32; PMID:17299893
- Farias DF, Viana MP, Oliveira GR, Santos VO, Pinto CE, Viana DA, Vasconcelos IM, Grossi-de-Sa MF, Carvalho AF. Food safety assessment of Cry8Ka5 mutant protein using Cry1Ac as a control Bt protein. Food Chem Toxicol 2015; 81:81-91; PMID:25890087; http://dx.doi.org/10.1016/j.fct.2015.04.008
- Xu W, Cao S, He X, Luo Y, Guo X, Yuan Y, Huang K. Safety assessment of Cry1Ab/Ac fusion protein. Food Chem Toxicol 2009; 47:59-65
- CAO S, He X, Xu W, Ran W, Liang L, Luo Y, Yuan Y, Zhang N, Zhou X, Huang K. Safety assessment of Cry1C protein from genetically modified rice according to the national standards of PR China for a new food resource. Regul Toxicol Pharmacol 2010; 58:474-81; PMID:20801181; http://dx.doi.org/10.1016/j.yrtph.2010.08.018
- EPA. Biopesticide Registration Action Document. Bacillus thuringiensis Cry1A.105 and Cry2Ab2 Insecticidal Proteins and the Genetic Material Necessary for Their Production in Corn [PC Codes 006515 (Cry2Ab2), 006514 (Cry1A.105)]. Washington, DC: US. Environmental Protection Agency, Office of Pesticide Programs, Biopesticides and Pollution Prevention Division. 2010 [cited 2015 Jul 10]. Available from: http://www.epa.gov/oppbppd1/biopesticides/pips/mon-89034-brad.pdf
- Farias DF, Viana MP, de Oliveira GR, Beneventi MA, Soares BM, Pessoa C, Pessoa IP, Silva LP, Vasconcelos IM, de Sá MF et al. Evaluation of cytotoxic and antimicrobial effects of two Bt Cry proteins on a GMO safety perspective. Biomed Res Int. 2014; 2014:810490; PMID:25165717; http://dx.doi.org/10.1155/2014/810490
- Grisolia CK, Oliveira R, Domingues I, Oliveira-Filho EC, Monerat RG, Soares AM. Genotoxic evaluation of different delta-endotoxins from Bacillus thuringiensis on zebrafish adults and development in early life stages. Mutat Res 2009; 31:119-23; http://dx.doi.org/10.1016/j.mrgentox.2008.10.017
- Yudina TG, Brioukhanov AL, Zalunin IA, Revina LP, Shestakov AI, Voyushina NE, Chestukhina GG, Netrusov AI. Antimicrobial activity of different proteins and their fragments from Bacillus thuringiensis parasporal crystals against clostridia and archaea. Anaerobe 2007; 13:6-13; PMID:17126041; http://dx.doi.org/10.1016/j.anaerobe.2006.09.006