?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The present investigation is mainly concerned with the rapid development of extracellular xylanase assay conditions by using Taguchi methodology. The extracellular xylanase was produced from Aspergillus niger (KP874102.1), a new strain isolated from a soil sample of the Baramura forest, Tripura West, India. Four physical parameters including temperature, pH, buffer concentration and incubation time were considered as key factors for xylanase activity and were optimized using Taguchi robust design methodology for enhanced xylanase activity. The main effect, interaction effects and optimal levels of the process factors were determined using signal-to-noise (S/N) ratio. The Taguchi method recommends the use of S/N ratio to measure quality characteristics. Based on analysis of the S/N ratio, optimal levels of the process factors were determined. Analysis of variance (ANOVA) was performed to evaluate statistically significant process factors. ANOVA results showed that temperature contributed the maximum impact (62.58%) on xylanase activity, followed by pH (22.69%), buffer concentration (9.55%) and incubation time (5.16%). Predicted results showed that enhanced xylanase activity (81.47%) can be achieved with pH 2, temperature 50°C, buffer concentration 50 Mm and incubation time 10 min.
Introduction
Xylanase (EC.3.2.1.8) is capable of hydrolysing the 1,4-β-D-xylopyranosyl linkages of the l,4-β-D-xylans. They are classified as exo-or endo-type. Among all xylanolytic enzymes endo-β 1, 4-xylanase can randomly cleave the β-1, 4 glycosidic bonds in xylan backbones and significantly reduce the degree of polymerization of the substrate.Citation1,2 Xylanase gains the most attention and plays a crucial role in the pulp and paper, animal feed, leather and baking industries.Citation3,4 The enzymes also play a key role in many biotechnological processes such as bleaching pulp and paper,Citation5,6 clarification of fruit juice, beer and wine,Citation7,8 and improving poultry feed digestibility. In addition, the production of biofuel from lignocellulosic biomass is considered one of the important applications of xylanase.Citation9
A large number of microorganisms such as bacteria, fungi and yeast are capable of xylanase production. However, xylanase produced from filamentous fungi is most important from an industrial point of view. The main advantage of filamentous fungi in industrial applications is the fact they secrete much higher amounts of xylanolytic enzymes into the medium than other microorganisms like bacteria or yeast.Citation10,11 Advanced areas of xylanase applications require the search for novel enzymes and new microbial producers with higher specific activities and higher productivity. Over the last few decades, a large number of xylanases has been reported from different microbial sources, however due to lack of standard protocol, it is very difficult to explore proper utilization. Additionally, different laboratories follow different procedures for xylanase activity estimation. Standardization of xylanase activity protocol is very laborious, time consuming and costly, yet it is vital for the proper exploitation of xylan, which may be useful in sustainable energy development. In the present study, we endeavor to establish a generalized protocol for the standardization of xylanase activity.
Process parameters like temperature, pH, substrate concentration, incubation time and buffer concentration play important roles in enzyme activity.Citation12 Conventionally, a large number of experiments have to be carried out to optimize all the physical parameters by correlating all the parameters to find out the best possible conditions. This method of optimization can study one variable at a time, which is time consuming, requires more experimental data and cannot provide interaction between these physical parameters. On the other side, traditional experimental design mainly concentrates on an average process performance of characterization.Citation13 Recent literature reviews reveal that application of Taguchi methodology has been used to optimize reaction variables in biochemical processes. This method involves a given set of independent variables, which are controllable and uncontrollable over a specific region of interest.Citation14,15 It is also important that small scale experiments are valid over an entire experimental region.Citation16 Furthermore, ANOVA can be used to analyze the experimental data to find out the statistically significant outcomes at optimum levels. In this study, Taguchi's method was applied to optimize process parameters for quick development of xylanase assay conditions. The experiments were designed for 4 factors at 3 levels with OA layout of L9(34).
Results and discussion
Optimization of enzyme assay conditions
Effects of individual factors
The effect of each factor at the assigned level used for determination of xylanase assay conditions has been listed in . It has been found that xylanase activity is mainly dependent on selected process factors. Individually pH, temperature and buffer concentration are very influential at Level 1, Level 2 and Level 3 respectively among all the selected process parameters. However, the relative effect of the factors is considered from the magnitude of difference between the average effects (L2-L1). The larger magnitude of difference between the average effects is much stronger in influence.Citation17 It is evident from that temperature shows a stronger influence on xylanase activity than all the other factors, followed by buffer concentration, incubation time and pH. It is also revealed from the above sets of experiments that xylanase activity is strongly influenced by the selected process parameters.
Table 1. Main effects of selected factors.
From , it is observed that higher xylanase activity is found with a subsequent increase of pH concentration up to Level 2. With a further increase of pH (Level 3), the xylanase activity is then decreased. Such variation of activity may be due to changes in the 3 dimensional structure of protein at different pH levels which leads to denaturation of enzymes.Citation18 While in the case of temperature, the higher xylanase activity is found with an increase in temperature up to Level 2. However, xylanase activity decreases with further increases in temperature. Such variations of xylanase activity may be due to different orientations of enzyme structures at various temperatures.Citation18-21 Xylanase activity increases with increased buffer concentration up to Level 2. With a further increase in buffer concentration (Level 3), it is observed that xylanase activity decreases, which may be due to the exposure of different autolysis sites on the enzyme surface.Citation22 The xylanase activity increases with an increase in incubation up to Level 2. However, xylanase activity decreases with further increases in incubation time. It is obviously true that reaction time plays a significant role in enzyme substrate reaction.
Effect of factors interaction
The severity index (SI) was evaluated from Taguchi DOE that represents the influence of 2 individual factors at various levels of interaction (.). The columns in the represent the locations of the interacting factors to which they are assigned. 100% SI is indicated by 90° angle between the factors with 0% SI for parallel lines. Reserved columns show that it should be reserved during interaction studies. Levels indicate the level of factors desirable for the optimum conditions.Citation23 It is evident from the highest interaction (SI 59.97%) is observed between pH and incubation time, followed by buffer concentration and incubation time (SI 59.22%), temperature and buffer concentration (SI 29.25%), pH and temperature (SI 22.66%), temperature and incubation time (SI 22.4%), pH and buffer concentration (SI 20.63%). ().
Table 2. Estimated interaction of severity index.
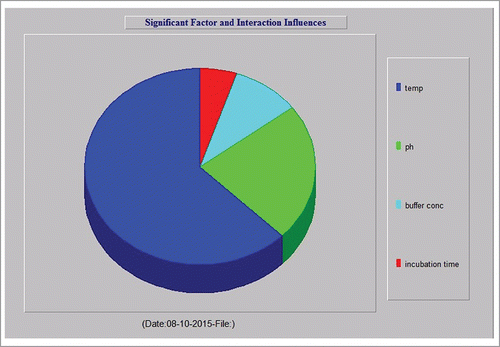
illustrates that temperature is the maximum impact factor followed by buffer concentration (high impact factor), incubation time (moderate impact factor) and pH (least impact factor) for improved xylanase activity. It was interesting to note that pH and incubation time were moderate and the lowest impact factors respectively in , however, they show highest in combination in the severity index. Incubation time is a moderate impact factor, but shows a high severity index in combination with buffer concentration. pH shows the least impact factor in isolation but showed a comparatively high severity index in combination with temperature. Buffer concentration and incubation time show moderate impact factors but a better severity index is found when they are combined. It is evident from the results shown in that yield is quite independent of individual influences but dependent on the interaction of factors.
Analysis of variance
Analysis of variance (ANOVA) was used to analyze the experimental data and to determine the variation of the result due to each factor. ANOVA reports along with the percentage of contribution of each factor are shown in . Results show that temperature contributed the maximum impact (62.58%) on xylanase activity followed by pH (22.69%), buffer concentration (9.55%) and incubation time (5.16%). shows the significant contribution of each factor in xylanase activity. Individually each factor influenced the enzyme activity at a certain level. However, this significant factor gives maximum yield when these factors act collectively, which may be due to the interactive effect of different factors.
Table 3. Analysis of variance.
Optimum process parameters
Optimum conditions of significant factors and their performance in terms of contribution for achieving high xylanase activity are shown in . It is evident from that temperature plays the maximum role in xylanase activity followed by pH, buffer concentration and incubation time. The results have found that a higher level of xylanase activity can be achieved with pH 2, temperature 50°C, buffer concentration 50 mM and incubation time 10 mins. The optimum conditions for expected xylanase activity are found where S/N ratio is 49.87 (total contribution from all the factors being found 4.892 with grand average performance of 61.199). The estimated xylanase activity from the S/N ratio is 2016.51 U [by Eq. (Equation3(3)
(3) )].
Table 4. Optimum condition and performance.
Validation experiments
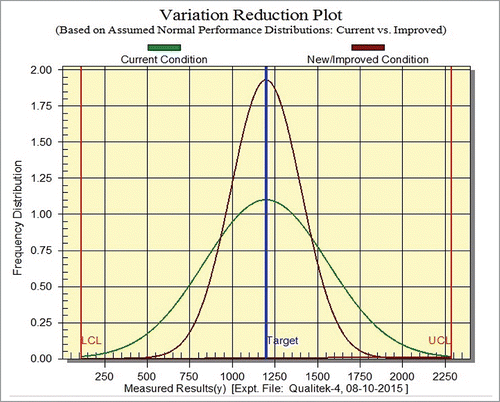
shows the frequency distribution of current conditions along with improved conditions. It is evident from the that xylanase activity may be increased from 1148.02 U (S/N ratio is 61.199) to 2016.51 U (S/N ratio is 66.092). Overall 75.65% enhancement in the xylanase activity may be attained. Further, to validate the proposed experimental methodology, experiments employing the obtained optimized reaction conditions were performed. The experimental data shows that optimized reaction conditions enhanced the xylanase activity up to 2083.33 U (81.47%). The overall deviation of predicted results from experiment results was found to be 5.82% which was within range.Citation24
Materials and method
Materials
Beech wood xylan as well as xylose were purchased from Sigma (Sigma Aldrich Co. Ltd., USA). DNS reagent, sodium sulphite, magnesium sulfate and copper sulfate was from Merck India. Sodium potassium tartrate, sodium hydroxide, tri sodium citrate, potato dextrose and ferric chloride agar was sourced from SRL, India. Bovine serum albumin and Bradford reagent (from Himedia, India) were also used in this study. All other chemicals were of analytical grade commercially available in India.
Microorganism
A new fungal strain of Aspergillus niger (KP874102.1) isolated from a soil sample (Baramura forest, Tripura West, India) was used in this investigation. The isolated strain was grown on PDA media for 5 d at 28°C. After incubation the slants were stored at 4°C for further experiments.
Seed culture
A 250 ml Erlenmeyer flask containing 50 ml medium was inoculated with 107 spores per ml, which and incubated at 28°C in a rotary shaker at 120 rpm for 24 hours. Inoculum was prepared by suspending the spores from a PDA slant by adding sterile distilled water. The spore suspension was then filtered and again suspended in sterile deionized water to give a final spore count of 1×107 spores/ml counted through a haemocytometer.Citation25 The seed medium used was composed of (g/l, w/v): xylan, 1; KNO3, 10; MgSO4, 7H2O, 5; NaCl, 5 and a 1000X trace element solution, 1 ml/l (pH 7). The trace element solution contained (g/l, w/v) ZnSO4,7H20, 20; H3BO3, 10; MnCl2, 7H2O, 5; FeSO4, 7H2O, 5; CoCl2, 5H2O, 1.5; CuSO4, 5H2O, 1.5; Na2MoO4, 4H2O, 1).
Xylanase production
The experiments were carried out at 28°C in a rotary shaker at 120 rpm for 7 d in a 250 ml shake flask containing 50 ml of working volume. A 5% of seed culture was inoculated in production media, containing (g/l) of xylan, 1; KNO3, 10; MgSO4, 7H2O, 5; NaCl, 5 and a 1000X trace element solution. One ml/l (pH 7). Of trace element solution contains (g/l, w/v) ZnSO4,7H20, 20; H3BO3, 10; MnCl2, 7H2O, 5; FeSO4, 7H2O, 5; CoCl2, 5H2O, 1.5; CuSO4, 5H2O, 1.5; Na2MoO4, 4H2O, 1).The culture was centrifuged at 15,000×g for 10 mins at 4°C and the supernatant was used for assaying xylanase activity.
Optimization of enzyme assay condition
Optimization of enzyme activity by Taguchi methodology
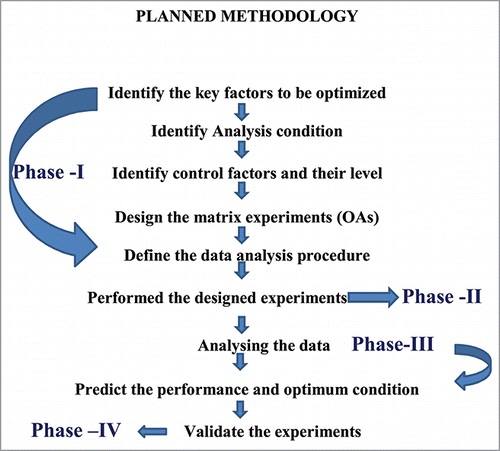
The Taguchi method involves establishing different experimental situations through orthogonal arrays (OA) which reduce experimental errors and enhance the efficiency and reproducibility of experiments. Robust design has been considered in this study because it helps minimize noise in the optimization process and leads to a dynamic or robust experiment design.Citation26 Planning, conducting, analysis and validation are 4 phases of Taguchi methodology. The schematic representation of the planned methodology is shown in . Each phase is separated with distinct objectives and sequences are connected to achieve the overall optimization process.
Design of experiments (Phase I)
In Phase I, the various factors were determined. These parameters have a critical effect on enzyme assay conditions. All the variables within feasible range were investigated and variations inherent in the process do not mask the factor effect. In this study, pH, temperature, buffer concentration (Britton–Robinson buffer) and incubation time were considered for their effect on xylanase activity. All the variables, decided from previous unreported work, were investigated at 3 widely spaced levels and are shown in . In this study, substrate concentration was kept at a higher range; therefore, it is considered that substrate concentration would not interfere in enzyme activity. In the next step, a matrix was designed with the appropriate OAs for the selected parameters and their levels. Taguchi provides many standard OAs and corresponding linear graphs for this purpose.Citation17 In the present study, 3 levels of 4 factors () were considered and the size of experimentation was represented by a symbolic array of L-9 (which indicated 9 experimental trials).
Table 5. Experimental range of four physiochemical factors studied using Taguchi methodology.
Analysis of xylanase activity with selected factors and levels (Phase II)
Xylanase activity was determined according to modified Baileys et al (1992) methodology and reducing sugar was measured using the 3,5-dinitrosalicylic acid.Citation27 Briefly, 200 µl (10.29 µg protein) of fermentation supernatant was added in the reaction mixture containing 1.8 ml of 1% (w/v) beech wood xylan. The mixture was incubated at different incubation conditions, according to the design matrix (). The amount of reducing sugar liberated was determined by the 3,5-dinitrosalicylic acid (DNS) method using xylose as a standard.Citation28 One unit of xylanase activity was defined as the amount of enzyme that catalyzes the release of 1µ mol of xylose equivalent in one minute under the standard assay conditions. Protein concentration was determined by the Bradford method using bovine serum albumin (BSA) as the standard.Citation29 All experiments were done in triplicate.
Table 6. L9 OA (34) of design experiments for 4 variables for optimum xylanase activity.
Data analysis and prediction of performance (Phase III)
The experimental data was processed using Qualitek-4 software (Nutek Inc., MI, USA) to evaluate the influence of individual factors, multiple interaction of the selected factors, determination of optimum conditions and the process performance on xylanase activity. In the present study, S/N analysis was employed with bigger-is-better performance characteristics for all the experimental cases. In the Taguchi method, the term ‘signal’ represents the desirable value (mean) and the term ‘noise’ represents the undesirable value (SD) for the output characteristic.Citation30 Therefore, the signal-to-noise (S/N) ratio is the ratio of the mean to the SD. Taguchi used the S/N ratio to measure quality characteristics deviating from the desired value. A loss function [L(y)] is developed for the deviationCitation31 as represented by L(y)=k×(y−m)2, where k denotes the proportionality constant, m represents the target value and y is the experimental value obtained for each trial. In the case of bigger and better quality characteristics the loss function can be written as L(y) = k×(1/y2) and the expected loss function can be represented by(1)
(1) Where E(1/y2) can be estimated from a sample of n as
(2)
(2)
Taguchi used the S/N ratio as a performance measurement of a dynamic system to evaluate the robustness of the overall process.Citation32 The mathematical expression for the S/N ratio for the “bigger is better” case for the performance statistics that measure deviation from the target, called as mean square deviation (MSD) was given by(3)
(3)
Validation of the experimental model (Phase IV)
In order to validate the methodology, assay experiments were further performed for xylanase activity using the predicted optimized assay conditions.
In conclusion, it may be stated that the present investigation reports an in depth study to optimize process factors for rapid development of xylanase assay conditions using Taguchi methodology. The investigation was verified experimentally. The above methodology predicts the optimum level of process factors through 9 experimental trials. The main effect, interaction effects and the optimal levels of the process factors could be determined using S/N ratio.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Compliance with Ethical Standards
This article does not contain any studies with human participants or animals performed by any of the authors.
Funding
This material is based on work supported by the National Institute of Technology, Agartala, India. The authors would like to acknowledge the National Institute of Technology, Agartala, Ministry of Human Resource and Development, Government of India for Fellowship 0000-0003-4637-991X.
References
- Battan B, Sharma J, Dhiman SS, Kuhad RC. Enhanced production of cellulase-free thermostable xylanase by Bacillus pumilus ASH and its potential application in paper industry. Enzyme Microbial Technol 2007; 41:733-9; http://dx.doi.org/10.1016/j.enzmictec.2007.06.006
- Lu F, Lu M, Lu Z, Bie X, Zhao H, Wang Y. Purification and characterization of xylanase from Aspergillus ficuum AF-98. Bioresour Technol 2008; 99:5938-41; PMID:18068974; http://dx.doi.org/10.1016/j.biortech.2007.10.051
- Beg Q, Kapoor M, Mahajan L, Hoondal G. Microbial xylanases and their industrial applications: a review. Appl Microbiol Biotechnol 2001; 56:326-38; PMID:11548999; http://dx.doi.org/10.1007/s002530100704
- Courtin C, Delcour JA. Arabinoxylans and endoxylanases in wheat flour bread-making. J Cereal Sci 2002; 35:225-43; http://dx.doi.org/10.1006/jcrs.2001.0433
- Li X, Jiang Z, Li L, Yang S, Feng W, Fan J, Kusakabe I. Characterization of a cellulase-free, neutral xylanase from Thermomyces lanuginosus CBS 288.54 and its biobleaching effect on wheat straw pulp. Bioresour Technol 2005; 96:1370-9; PMID:15792585; http://dx.doi.org/10.1016/j.biortech.2004.11.006
- Viikari L, Ranua M, Kantelinen A, Sundquist J, Linko M. Bleaching with enzymes. Proceedings of 3rd International Conference on Biotechnology in the Pulp and Paper Industry, STFI, Stockholm, 1986:67-9.
- Maheswari MU, Chandra T. Production and potential applications of a xylanase from a new strain of Streptomyces cuspidosporus. World J Microbiol Biotechnol 2000; 16:257-63; http://dx.doi.org/10.1023/A:1008945931108
- Saha BC. Hemicellulose bioconversion. J Ind Microbiol Biotechnol 2003; 30:279-91; PMID:12698321; http://dx.doi.org/10.1007/s10295-003-0049-x
- Uday USP, Choudhury P, Bandyopadhyay TK, Bhunia B. Classification, mode of action and production strategy of xylanase and its application for biofuel production from water hyacinth. Int J Biol Macromol 2016; 82:1041-54; PMID:26529189; http://dx.doi.org/10.1016/j.ijbiomac.2015.10.086
- Dobrev GT, Pishtiyski IG, Stanchev VS, Mircheva R. Optimization of nutrient medium containing agricultural wastes for xylanase production by Aspergillus niger B03 using optimal composite experimental design. Bioresour Technol 2007; 98:2671-8; PMID:17092711; http://dx.doi.org/10.1016/j.biortech.2006.09.022
- Polizeli M, Rizzatti A, Monti R, Terenzi H, Jorge J, Amorim D. Xylanases from fungi: properties and industrial applications. Appl Microbiol Biotechnol 2005; 67:577-91; PMID:15944805; http://dx.doi.org/10.1007/s00253-005-1904-7
- Bhunia B, Basak B, Mandal T, Bhattacharya P, Dey A. Effect of pH and temperature on stability and kinetics of novel extracellular serine alkaline protease (70kDa). Int J Biol Macromol 2013; 54:1-8; PMID:23219732; http://dx.doi.org/10.1016/j.ijbiomac.2012.11.024
- Beg QK, Sahai V, Gupta R. Statistical media optimization and alkaline protease production from Bacillus mojavensis in a bioreactor. Process Biochem 2003; 39:203-9; http://dx.doi.org/10.1016/S0032-9592(03)00064-5
- Basak B, Bhunia B, Mukherjee S, Dey A. Optimization of physicochemical parameters for phenol biodegradation by Candida tropicalis PHB5 using Taguchi Methodology. Desalination and Water Treatment 2013; 51:6846-62; http://dx.doi.org/10.1080/19443994.2013.770638
- Basak B, Bhunia B, Dutta S, Dey A. Enhanced biodegradation of 4-chlorophenol by Candida tropicalis PHB5 via optimization of physicochemical parameters using Taguchi orthogonal array approach. Int Biodeterioration Biodegradation 2013; 78:17-23; http://dx.doi.org/10.1016/j.ibiod.2012.12.005
- Phadke MS. Quality Engineering Using Robust Design, PTR Prentice-Hall. Inc, Englewood Cliffs, NJ 1989.
- Taguchi G. Introduction to Quality Engineering. Asian Productivity Organization. Dearborn, MI: American supplier institute Inc., 1986.
- Sadana A, Henley JP. Influence of pH on enzyme stabilization:an analysis using a series-type mechanism. J Biotechnol 1988; 7:95-112; http://dx.doi.org/10.1016/0168-1656(88)90057-0
- Dixon M, Webb EC. Enzymes. New York: Academic Press, 1979.
- Tanford C. Protein denaturation. Adv Prot Chem 1968; 23:121-282; http://dx.doi.org/10.1016/S0065-3233(08)60401-5
- Sode K, Ito K, Witarto AB, Watanabe K, Yoshida H, Postma P. Increased production of recombinant pyrroloquinoline quinone (PQQ) glucose dehydrogenase by metabolically engineered Escherichia coli strain capable of PQQ biosynthesis. J Biotechnol 1996; 49:239-43; PMID:8879174; http://dx.doi.org/10.1016/0168-1656(96)01540-4
- Chen X-L, Sun C-Y, Zhang Y-Z, Gao P-J. Effects of different buffers on the thermostability and autolysis of a cold-adapted protease MCP-01. J Protein Chem 2002; 21:523-7; PMID:12638654; http://dx.doi.org/10.1023/A:1022425621742
- Adnania A, Basria M, Maleka EA, Sallehb AB, Rahmana MBA, Chaibakhsha N, et al. Optimization of lipase-catalyzed synthesis of xylitol ester by Taguchi robust design method. Industrial Crops Products 2010; 31:350-6; http://dx.doi.org/10.1016/j.indcrop.2009.12.001
- Bhunia B, Dutta D, Chaudhuri S. Extracellular alkaline protease from Bacillus licheniformis NCIM-2042: Improving enzyme activity assay and characterization. Engineering Life Sci 2011; 11:207-15; http://dx.doi.org/10.1002/elsc.201000020
- Anderson J, Smith J. The effects of elevated temperatures on spore swelling and germination in Aspergillus niger. Canadian J Microbiol 1972; 18:289-97; PMID:4560486; http://dx.doi.org/10.1139/m72-045
- Dehnad K. Quality control, robust design, and the Taguchi method. CA: Wadsworth and Brooks: Pacific Grove, 1989.
- Bailey MJ, Biely P, Poutanen K. Interlaboratory testing of methods for assay of xylanase activity. J Biotechnol 1992; 23:257-70; http://dx.doi.org/10.1016/0168-1656(92)90074-J
- Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chem 1959; 31:426-8; http://dx.doi.org/10.1021/ac60147a030
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72:248-54; PMID:942051; http://dx.doi.org/10.1016/0003-2697(76)90527-3
- Zhou J, Wu D, Guo D. Optimization of the production of thiocarbohydrazide using the Taguchi method. J Chem Technol Biotechnol 2010; 85:1402-6; http://dx.doi.org/10.1002/jctb.2446
- Mitra A. Fundamentals of Quality Control and Improvement. Delhi: Pearson Educational Asia, 1998.
- Tong L, Wang C, Chen C, Chen C. Dynamic multiple responses by ideal solution analysis. Euro J Operational Res 2004; 156:433-44; http://dx.doi.org/10.1016/S0377-2217(03)00017-1