ABSTRACT
The synthesis of renewable bioproducts using photosynthetic microorganisms holds great promise. Sustainable industrial applications, however, are still scarce and the true limits of phototrophic production remain unknown. One of the limitations of further progress is our insufficient understanding of the quantitative changes in photoautotrophic metabolism that occur during growth in dynamic environments. We argue that a proper evaluation of the intra- and extracellular factors that limit phototrophic production requires the use of highly-controlled cultivation in photobioreactors, coupled to real-time analysis of production parameters and their evaluation by predictive computational models. In this addendum, we discuss the importance and challenges of systems biology approaches for the optimization of renewable biofuels production. As a case study, we present the utilization of a state-of-the-art experimental setup together with a stoichiometric computational model of cyanobacterial metabolism for quantitative evaluation of ethylene production by a recombinant cyanobacterium Synechocystis sp. PCC 6803.
Introduction
In our recent work, we characterized the impact of different light intensities on ethylene production by a recombinant cyanobacterium Synechocystis sp. PCC 6803. Ethylene is among the most widely used compounds in chemical industry and is currently mainly derived from fossil resources. A technology for its renewable production is therefore highly desirable. In addition to the formation in plants, ethylene is produced naturally by various microorganisms either by oxidation of 2-keto-4-methylthiobutyric acid or by utilization of α-ketoglutarate and arginine as substrates in a reaction catalyzed by the ethylene-forming enzyme (EFE).Citation1 The EFE was previously expressed in Escherichia coli, Saccharomyces cerevisiae, Trichoderma viride and Trichoderma reesei.Citation2 A promising alternative to heterotrophic microbial synthesis is the light-driven conversion of atmospheric CO2 into ethylene by photosynthetic microorganisms. The EFE has been introduced in the cyanobacterial strains Synechococcus elongatus sp. PCC 7942 and in Synechocystis sp. PCC 6803,Citation2 resulting in stable production rates of approximately 200 nL (C2H4) mLculture−1 h−1 OD730/750−1 with the highest reported productivity of 171 mg (C2H4) Lculture−1 d−1 achieved using dense cultures.Citation3
To facilitate sustainable and economically viable production, however, further improvements and novel strategies with respect to yield, genetic stability, and robustness of production strains are urgently needed. In this Addendum, we argue that overcoming the current challenges with respect to cyanobacteria as a resource for bioproducts requires a better integration of quantitative measurements (using highly-controlled bioreactor setups) together with computational analysis and quantitative models at both single-cell and culture levels. As yet, systematic attempts to identify and quantify possible intra- and extracellular limitations of cellular production are still in their infancy and computational frameworks allowing identification and ranking of suitable modifications for phototrophic production improvements are still very rare.Citation4,5
A quantitative evaluation of ethylene production
We recently evaluated the impact of light on ethylene production in 2 recombinant strains of Synechocystis sp. PCC 6803.Citation3,6 To this end, we have taken advantage of a previously developed platform for a detailed characterization of growth of photosynthetic microorganisms,Citation7 based on utilization of laboratory-scale flat-panel photobioreactors.Citation8 The cultivation and monitoring units allow the characterization of growth during batch,Citation9 quasi-continuousCitation7 or continuous cultivation under highly controlled conditions, including regulation of key cultivation parameters such as temperature, light, pH and concentration of input CO2.Citation10 Moreover, the photobioreactors are designed to non-invasively and in real time estimate physiological parameters such as photosynthetic performance (based on O2 production, CO2 uptake or pigment fluorescence), as well as growth rates based on optical density monitoring.
Ethylene production was monitored by a membrane-inlet mass spectrometer (MIMS), model Gas-MS-100 (Photon Systems Instruments, spol. s r.o., Brno, CZ). The membrane inlet was placed directly in the photobioreactor cuvette, which enabled high resolution online monitoring of dynamics in oxygen and ethylene production, as well as in carbon dioxide uptake. This setup represented a significant improvement over previously reported ethylene quantification methodology based on offline measurements of sample aliquots in separate vial flasks. The combination of MIMS and a photobioreactor allowed the quantification of ethylene production under a wide range of light intensities as well as the derivation of biotechnologically relevant production parameters such as the photochemical conversion efficiency or oxygen evolution accompanying the production of ethylene.
Analysis of the resulting experimental data was supported by a computational metabolic model of Synechocystis sp. PCC 6803.Citation11 The genome-scale metabolic reconstruction allows to interconnect relevant exchange fluxes, in particular light uptake, O2 release, growth and product formation, and thereby allows to evaluate the energetic consistency of the experimental data. Importantly, such a model-based evaluation requires only knowledge of the stoichiometry of the underlying network of reactions, making the analysis robust against unknown regulatory interactions and unknown kinetic parameters. The computational metabolic model of Synechocystis sp. PCC 6803Citation11 was parametrized using measured cellular dry weight, growth rate, oxygen evolution and ethylene production rates. Given the measured data, the model was over-parametrized, that is, one of the measured values was a function of the remaining data. It was therefore possible to test the model by comparing the measured values of ethylene production rates with predicted model-derived rates, exhibiting excellent agreement for low light intensities (100 μmol(photons) m−2 s−1) and increasing deviations for high light intensities (200 μmol(photons) m−2 s−1). The deviations at higher light intensities correspond to the fact that the highest (relative) conversion rates were obtained under low light intensities and the model itself only provided upper bounds on the maximal production rates (for details, see chapter 3.4 in Zavřel et al. (2015)Citation12).
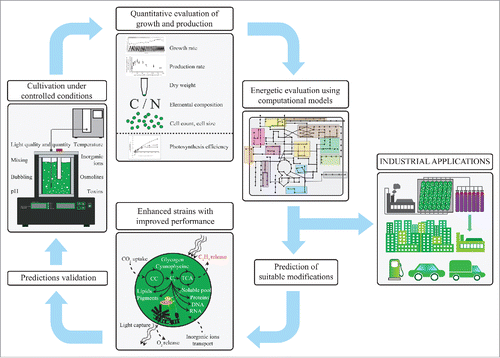
Given the good overall agreement between measured and model-derived values, the model was further utilized to estimate hidden parameters of ethylene synthesis that were not directly accessible experimentally, such as carbon partitioning, predicted de novo synthesis of α-ketoglutarate as well as maximal ethylene production rate in hypothetical zero-growth phenotype in which all resources are redirected to ethylene. An overview of our workflow together with the main factors affecting the production rates is provided in .
Figure 1. Schematic representation of the main parameters that affect growth and formation of bioproducts by photoautotrophic cells on both intra- and extracellular levels. The measured values of the observed exchange fluxes (in context of this work represented by growth, O2-evolution and ethylene formation) represent inputs for mathematical models of photoautotrophic metabolism. The models provide additional information of potential limitations and guide strains optimization. Only with the production rates measured in conjunction with other metabolic rates under varying environmental conditions, the production setup can be truly optimized. Abbreviations: CC – Calvin cycle, TCA cycle – tricarboxylic acid cycle.
Current challenges and perspectives of systems biology approaches to cyanobacterial bioproduction
Although impressive progress has been achieved in algal and cyanobacterial biotechnology in the recent years,Citation13,14 significant gaps in our understanding of cyanobacterial and algal growth still remain. So far, a broad range of possible bioproducts has been introduced into various cyanobacteria as prototype production strains.Citation15 However, often the production rate and conditions have not been optimized and the true production limits remain unknown.Citation16 Besides other major challenges, such as genetic instability,Citation17 a significant shortcoming is the scarcity of quantitative descriptions of metabolic changes during production phases under highly controlled conditions in dynamically changing environment.Citation18
The reported production yields of target compounds by algae or cyanobacteria are typically evaluated using batch cultures in common labware of different geometry, such as flasks, vials or plates. Such cultivation systems are inexpensive, easy to operate, and can be standardized to some extent. However, it remains challenging to reliably identify factors that significantly impact the final production rates and thereby to systematically improve strain performance. Furthermore, production rates (or yields in closed batch cultures) are often reported only relative to other production parameters, such as culture volume, chlorophyll concentration, or optical density - again making cross-experimental comparisons challenging.
A more suitable approach for systematic optimization is the use of dedicated photobioreactors with precisely controlled cultivation conditions. Among the various designs of photobioreactors described in the literature,Citation19 the most advantageous are flat-panel designs, because they allow detailed light capture quantification and the energy balance evaluation. Advanced photobioreactors usually also offer options for additional non-invasive monitoring of culture physiological parameters such as growth rate, photosynthetic efficiency or evaluation of culture carbon balance.Citation10 In addition to parameters monitored on the culture level, biochemical properties of the cells need to be quantified for identification of production limits on the intracellular level. At the best, the evaluation includes quantification of cellular composition (elemental as well as biochemical – proteins, RNA, DNA, lipids, pigments and storage compounds), photosynthetic activity (both oxygen production and carbon fixation), light capture, growth rate, cell count and dry weight, as well as production rate of the target compound. As shown in studies of intracellular changes of Synechocystis sp. PCC 6803 during ethyleneCitation20 or isoprene production,Citation21 recent approaches increasingly incorporate quantitative analysis of cellular parameters.
Genome-scale models as a tool for strain engineering
To obtain further insights into the limits of production, experimental coverage can be complemented with computational methods to identify and rank suitable modifications for the improvement of phototrophic production strains. In particular, the construction of genome-scale metabolic models over the past 2 decades has become a useful tool for applications in industrial biotechnology.Citation22 Similar approaches have been recently also applied to cyanobacterial product synthesis.Citation23-25 A number of genome-scale reconstructions of several cyanobacterial strains are now available (see Baroukh et al., (2015)Citation5 for an overview).
Genome-scale metabolic reconstructions aim to provide a comprehensive overview of all stoichiometric conversions of small molecules (metabolites) inside a living cell. Reconstructions are typically based on the annotated genome sequence of an organism, augmented with extensive manual curation as needed for completing the synthesis routes for all known cellular constituents. The current reconstructions of cyanobacterial metabolism are increasingly evaluated utilizing quantitative and comprehensive metabolic data.Citation26
Genome-scale reconstructions are particularly suited for the identification of optimal conversions routes inside large stoichiometric networks and their respective stoichiometric yields. Estimation of a putative flux distribution is based on optimality principles: given a certain input (in terms of photon or carbon flux), what is the optimal distribution of cellular fluxes that synthesize all known cellular components in the observed stoichiometric ratio? The optimization procedure requires knowledge of the biomass objective function (BOF), summarizing the observed or assumed ratios of cellular constituents, but does not require extensive knowledge of kinetic parameters or regulatory interactions.
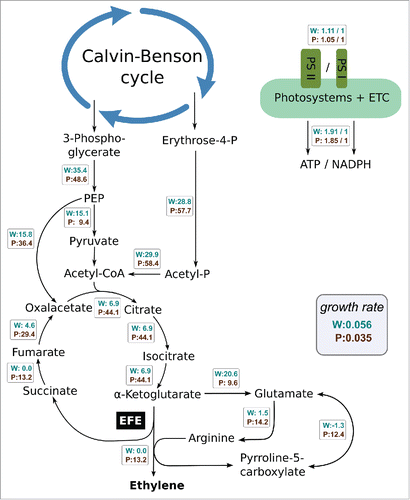
Once established, a genome-scale reconstruction and its (possibly condition-dependent) BOF can serve a multitude of purposes such as probing potential production strains using in silico mutants. In the context of ethylene production, we were primarily interested in estimation of the maximal production yields, in the trade-offs between cellular growth and product synthesis as well as in the identification of reactions that may decelerate or limit ethylene synthesis. To this end, we considered the maximal rate of ethylene synthesis, given the measured O2 evolution and growth rate, with excellent agreement between estimated maximal rates and observed values for low light intensities. More general, we could implement a hypothetical in silico production phenotype which channels a given amount of carbon into ethylene, and evaluate the changes in resulting fluxes compared to the WT phenotype. As shown in , the introduction of the EFE cause significant changes in the estimated flux distribution. We observe a remodeling of the fluxes of the TCA cycle, in particular a substantially increased flux through phosphoenolpyruvate carboxylase (PEPC), increased flux toward α-ketoglutarate, and increased synthesis of succinate, which is in good agreement with experimental findings.Citation20 As expected, differences between FBA predictions and experimentally obtained values are observed for cyclic pathways, such as the measured increased flux through the malic enzyme.Citation20 In general, the in silico analysis of metabolism remodeling due to synthetic reactions helps to identify potential production bottlenecks. Predicted changes in flux values may not only correspond to the synthetic reactions themselves, but may also involve seemingly distant reaction fluxes which are required for recycling of co-factors and precursors synthesis.Citation25
Figure 2. Changes within the metabolic flux distributions leading toward synthesis of ethylene via the ethylene forming enzyme (EFE), as predicted by a metabolic model of Synechocystis sp. PCC 6803.Citation12 The fluxes from a computational wild type growth scenario (W) and producer scenario assuming cellular growth with additional synthesis of ethylene (P) are given in rates of 10−2 mmol h−1 gDW−1. In addition, ratio between activity of both photosystems (PSII and PSI) and the resulting ratio of ATP to NADPH synthesis via the electron transport chain (ETC) is shown. The growth rates of both W and P simulations are shown in units of h−1. The simulation is based on an experimental evaluation of ethylene production by the recombinant strain 2x Sy-efe adapted to 100 μmol(photons) m−2s−1 of red light.Citation12 For the ethylene production scenario all of the reactions within and adjoining the TCA cycle carry a higher flux when compared to the wild type solution, whereas the ratio between ATP and NADPH recycling through the photosystems and ETC shows only a small difference. The wrapped reaction between glutamate and pyrroline-5-carboxylate (P5C) features a flux toward P5C for the WT growth scenario (negative flux) whereas for the ethylene producing scenario the reactions runs in direction toward glutamate (positive flux). Within the WT growth scenario more pyruvate is used for competing pathways and biomass synthesis, resulting in a high relative flux of pyruvate synthesis from 3-phosphoglycerate and overall higher growth rate. Both simulations show no flux for the reaction of the pyruvate dehydrogenase due to the costly loss of carbon dioxide within this reaction. PEP, phosphoenolpyruvate.
Conclusions: Toward systematic strain improvements
The challenges of the 21st century, including mitigation of climate change, food and fresh water supply security or sustainable energy development make the domestication of cyanobacteria as a human resource of high importance. Large-scale biotechnological applications using photosynthetic prokaryotes, however, are still in their infancy. We argue that further integration of metabolic modeling with high-quality measurements under controlled conditions will result in significant advances in our understanding of phototrophic production limits. Beyond phenomenological analysis, the combination of experimental data with theoretical modeling allows to identify key principles of rational metabolic engineering strategies - as shown in the recent study aiming at identification and characterization of suitable strain design strategies for cyanobacterial bioproducts synthesis.Citation24
In case of ethylene bioproduction, significant improvement of the strains productivity has been reported.Citation20 However, the ethylene yields are still significantly lower when compared to heterotrophic cultivationsCitation2 as well as when compared to yields of other bioproducts such as ethanol or lactic acid.Citation16 It also remains unknown to what extent industrial production is feasible, since reliable data for large-scale cultivation are not available. The only preliminary study evaluating large-scale ethylene bioproduction only pointed out the need of usage of sea water and coastal areas for operation of production plants to achieve positive environmental impact.Citation27 While many challenges remain, we conjecture that theoretical modeling, in conjunction with experimental data, will ultimately result in rational strain design strategies that consider the host cell and its environment as interacting system - and thereby enable green biotechnology for the 21st century.
Abbreviations
| BOF | = | biomass objective function |
| EFE | = | ethylene forming enzyme |
| FBA | = | flux balance analysis |
| MIMS | = | membrane-inlet mass spectrometry |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
T.Z. and J.Č. were supported by the Ministry of Education, Youth and Sports of the Czech Republic within the National Sustainability Program I (NPU I), grant number LO1415 and within Center for Systems Biology (C4Sys), grant number LM2015055. J.Č. was also supported by GA ČR, grant number 15-17367S. R.S. and H.K. are funded by the German Federal Ministry of Education and Research as part of the “e:Bio – Innovationswettbewerb Systembiologie” [e:Bio – systems biology innovation competition] initiative (reference: FKZ 0316192).
References
- Fukuda H, Ogawa T, Tanase S. Ethylene production by micro-organisms. Adv Microb Physiol 1993; 35:275-306. http://www.ncbi.nlm.nih.gov/pubmed/9892551; PMID:8310882; http://dx.doi.org/10.1016/S0065-2911(08)60101-0
- Eckert C, Xu W, Xiong W, Lynch S, Ungerer J, Tao L, Gill R, Maness PC, Yu J. Ethylene-forming enzyme and bioethylene production. Biotechnol Bio Fuels 2014; 7:1-11. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3946592&tool=pmcentrez&rendertype=abstract; PMID:24387051; http://dx.doi.org/10.1186/1754-6834-7-33
- Ungerer J, Tao L, Davis M, Ghirardi M, Maness PC, Yu J. Sustained photosynthetic conversion of CO2 to ethylene in recombinant cyanobacterium Synechocystis 6803. Energy Environ Sci 2012; 5:8998-9006. http://xlink.rsc.org/?DOI=c2ee22555g; http://dx.doi.org/10.1039/c2ee22555g
- Klamt S, Mahadevan R. On the feasibility of growth-coupled product synthesis in microbial strains. Metab Eng 2015; 30:166-78. http://www.ncbi.nlm.nih.gov/pubmed/26112955; PMID:26112955; http://dx.doi.org/10.1016/j.ymben.2015.05.006
- Baroukh C, Muñoz-Tamayo R, Steyer JP, Bernard O. A state of the art of metabolic networks of unicellular microalgae and cyanobacteria for biofuel production. Metab Eng 2015; 30:49-60; PMID:25916794; http://dx.doi.org/10.1016/j.ymben.2015.03.019
- Guerrero F, Carbonell V, Cossu M, Correddu D, Jones PR. Ethylene synthesis and regulated expression of recombinant protein in Synechocystis sp PCC 6803. PLoS One 2012; 7:e50470. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3503970&tool=pmcentrez&rendertype=abstract; PMID:23185630; http://dx.doi.org/10.1371/journal.pone.0050470
- Zavřel T, Sinetova MA, Búzová D, Literáková P, Červený J. Characterization of a model cyanobacterium Synechocystis sp. PCC 6803 autotrophic growth in a flat-panel photobioreactor. Eng Life Sci 2015; 15:122-32; http://doi.wiley.com/10.1002/elsc.201300165; http://dx.doi.org/10.1002/elsc.201300165
- Nedbal L, Trtílek M, Cervený J, Komárek O, Pakrasi HB. A photobioreactor system for precision cultivation of photoautotrophic microorganisms and for high-content analysis of suspension dynamics. Biotechnol Bioeng 2008; 100:902-10; http://www.ncbi.nlm.nih.gov/pubmed/18383143; PMID:18383143; http://dx.doi.org/10.1002/bit.21833
- Sinetova MA, Červený J, Zavřel T, Nedbal L. On the dynamics and constraints of batch culture growth of the cyanobacterium Cyanothece sp. ATCC 51142. J Biotechnol 2012; 162:148-55. http://www.ncbi.nlm.nih.gov/pubmed/22575787; PMID:22575787; http://dx.doi.org/10.1016/j.jbiotec.2012.04.009
- Červený J, Šetlík I, Trtílek M, Nedbal L. Photobioreactor for cultivation and real-time, in-situ measurement of O2 and CO2 exchange rates, growth dynamics, and of chlorophyll fluorescence emission of photoautotrophic microorganisms. Eng Life Sci 2009; 9:247-53. http://doi.wiley.com/10.1002/elsc.200800123; http://dx.doi.org/10.1002/elsc.200800123
- Knoop H, Zilliges Y, Lockau W, Steuer R. The metabolic network of Synechocystis sp. PCC 6803: systemic properties of autotrophic growth. Plant Physiol 2010; 154:410-22. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2938163&tool=pmcentrez&rendertype=abstract; PMID:20616194; http://dx.doi.org/10.1104/pp.110.157198
- Zavřel T, Knoop H, Steuer R, Jones PR, Červený J, Trtílek M. A quantitative evaluation of ethylene production in the recombinant cyanobacterium Synechocystis sp. PCC 6803 harboring the ethylene-forming enzyme by membrane inlet mass spectrometry. Bio Resour Technol 2016; 202:142-51; PMID:26708481; http://dx.doi.org/10.1016/j.biortech.2015.11.062
- Wijffels RH, Kruse O, Hellingwerf KJ. Potential of industrial biotechnology with cyanobacteria and eukaryotic microalgae. Curr Opin Biotechnol 2013; 24:405-13. http://www.ncbi.nlm.nih.gov/pubmed/23647970; PMID:23647970; http://dx.doi.org/10.1016/j.copbio.2013.04.004
- Sarsekeyeva F, Zayadan BK, Usserbaeva A, Bedbenov VS, Sinetova MA, Los DA. Cyanofuels: Biofuels from cyanobacteria. Reality and perspectives. Photosynth Res 2015; 125:329-40; PMID:25702086; http://dx.doi.org/10.1007/s11120-015-0103-3
- Gudmundsson S, Nogales J. Cyanobacteria as photosynthetic biocatalysts: a systems biology perspective. Mol Bio Syst 2015; 11:60-70. http://xlink.rsc.org/?DOI=C4MB00335G; PMID:25382198; http://dx.doi.org/10.1039/C4MB00335G
- Angermayr SA, Gorchs Rovira A, Hellingwerf KJ. Metabolic engineering of cyanobacteria for the synthesis of commodity products. Trends Biotechnol 2015; 33(6):352-61. http://linkinghub.elsevier.com/retrieve/pii/S0167779915000712
- Jones PR. Genetic instability in cyanobacteria – an elephant in the room? Synth Biol 2014; 2:12. http://journal.frontiersin.org/article/10.3389/fbioe.2014.00012/full\nhttp://journal.frontiersin.org/article/10.3389/fbioe.2014.00012/pdf
- Berla BM, Saha R, Immethun CM, Maranas CD, Moon TS, Pakrasi HB. Synthetic biology of cyanobacteria: Unique challenges and opportunities. Front Microbiol 2013; 4:1-14; PMID:23346082; http://dx.doi.org/10.3389/fmicb.2013.00246
- Fernandes BD, Mota A, Teixeira JA, Vicente AA. Continuous cultivation of photosynthetic microorganisms: Approaches, applications and future trends. Biotechnol Adv 2015; 33(6 Pt 2):1228-45; PMID:25777495
- Xiong W, Morgan JA, Ungerer J, Wang B, Maness P, Yu J. The plasticity of cyanobacterial metabolism supports direct CO2 conversion to ethylene. Nat Plants 2015; 1:1-6
- Pade N, Erdmann S, Enke H, Dethloff F, Dühring U, Georg J, Wambutt J, Kopka J, Hess WR, Zimmermann R, et al. Insights into isoprene production using the cyanobacterium Synechocystis sp. PCC 6803. Biotechnol Biofuels 2016; 9:89. http://biotechnologyforbiofuels.biomedcentral.com/articles/10.1186/s13068-016-0503-4; PMID:27096007; http://dx.doi.org/10.1186/s13068-016-0503-4
- King ZA, Lloyd CJ, Feist AM, Palsson BO. Next-generation genome-scale models for metabolic engineering. Curr Opin Biotechnol 2015; 35:23-9; PMID:25575024; http://dx.doi.org/10.1016/j.copbio.2014.12.016
- Nogales J, Gudmundsson S, Thiele I. Toward systems metabolic engineering in cyanobacteria: opportunities and bottlenecks. Bioengineered 2013; 4:158-63. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3669157&tool=pmcentrez&rendertype=abstract; PMID:23138691; http://dx.doi.org/10.4161/bioe.22792
- Erdrich P, Knoop H, Steuer R, Klamt S. Cyanobacterial biofuels: new insights and strain design strategies revealed by computational modeling. Microb Cell Fact 2014; 13:128. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4180434&tool=pmcentrez&rendertype=abstract; PMID:25323065; http://dx.doi.org/10.1186/s12934-014-0128-x
- Knoop H, Steuer R. A Computational Analysis of stoichiometric constraints and trade-offs in cyanobacterial biofuel production. Front Bioeng Biotechnol 2015; 3:1-15. http://www.frontiersin.org/Synthetic_Biology/10.3389/fbioe.2015.00047/abstract; PMID:25654078; http://dx.doi.org/10.3389/fbioe.2015.00047
- Saha R, Liu D, Hoynes-O'Connor A, Liberton M, Yu J, Bhattacharyya-Pakrasi M, Balassy A, Zhang F, Moon TS, Maranas CD, et al. Diurnal regulation of cellular processes in the cyanobacterium Synechocystis sp. strain PCC 6803: Insights from transcriptomic, fluxomic, and physiological analyses. MBio 2016; 7:e00464-16. http://mbio.asm.org/lookup/doi/10.1128/mBio.00464-16; PMID:27143387; http://dx.doi.org/10.1128/mBio.00464-16
- Final Report Summary - DIRECTFUEL (Direct biological conversion of solar energy to volatile hydrocarbon fuels by engineered cyanobacteria). Collaborative STREP project EC grant agreement 256808 [Internet]. 2014. http://cordis.europa.eu/project/rcn/95914_en.html
