?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
A convenient and effective soft chemical method is presented for the synthesis of nano-scaled calcium citrate sheets. The preparation involved the precipitation of nano-calcium citrate by adding ethanol to reach the super saturation state of a solution containing calcium and citrate salts. The obtained nano-calcium citrate formed nanosheets, with the following dimensions: width of about 50∼500 nm and thickness of about 8∼30 nm. The results of the XRD analysis confirmed that the obtained sample is calcium citrate tetrahydrate, and the crystal degree decreased with an increase quantity of ethanol added. Animal experiments showed that the calcium citrate can promote the formation of new bone.
Introduction
Calcium citrate is an organic calcium salt, often used for calcium supplements and can be also used as a food additive. In recent years, calcium citrate has been used in biomaterials such as calcium citrate and rhBMP-2 that showed a beneficial effect on osteoinduction and osteogenesis.Citation1 Calcium citrate affects the early stages of bone defects healing mechanism because it can resorb faster than calcium phosphate, hydroxyapatite, and other biomaterials as reported in the case of Japanese white rabbits when the defect is not too large.Citation2 Tao et al.Citation3 also demonstrated that injectable alginate-gelatin-calcium citrate can significantly enhance tendon-bone healing at an early stage. Furthermore, the nano-calcium citrate is more bioavailable than the micro-form as shown in an ICR mice tests. The experiments showed that the variation of serum calcium concentrations influenced the bone mineral density and that calcium citrate exhibited better effects compared to calcium carbonate.Citation4
Traditionally, there are 2 calcium citrate preparation methods: on the one hand, it be can obtained directly from the reaction of citric acid and calcium carbonate; On the other hand, it can be produced from citric acid and calcium hydroxide by acid-base reaction. Eggshells, shells and other biological waste materials contain high contents of calcium carbonate and are often used as the raw material. The protocol of Zeng X'sCitation5 consists in cleaning the egg shell, grinding it, separating the shell membrane, dissolving with organic acids (acetic acid, lactic acid etc.) to generate organic calcium, then adding citric acid which induces a replacement reaction to finally obtain calcium citrate with a purity of 99%. Wang WB et al.Citation6 dissolved crushed egg shells with diluted hydrochloric acid until the solution stopped bubbling, then separated the supernatant after filtration, and finally obtained a relatively pure calcium citrate by successively adding sodium hydroxide and citric acid. In the Chen SY et al.'s Citation7 method, diluted hydrochloric acid is added to broken shells, the calcium carbonate precipitate is produced when sodium carbonate is added into the clear filtrate, then the calcium oxide was obtained by calcining the precipitate, and finally, a citric acid solution was added to afford pure calcium citrate.
The above-mentioned methods pay more attention to the yield of calcium citrate, rather than the concern about the size and shape of calcium citrate. The crystal structure of the material, size and morphology have significant impact on the performance of materials. Nano-calcium citrate is nowadays generally prepared by physical techniques. Huang et al.Citation4 rely on a pulsed jet mill to nanocrystallize calcium citrate, however the weakness of this method is significant, which is energy-consuming and particles are hardly obtained and screening is thus required in order to obtain a calcium citrate at a nanoscale. Herdtweck et al.Citation8 prepared calcium citrate by a hydrothermal method utilizing a needle-like calcium citrate to control the morphology; however this method is hard to industrialize due to the complex process of the hydrothermal method, equipment requirements, and long time required. In this study, a simple method is introduced yielding nanoscale sheets of calcium citrate by direct chemical synthesis, and the effect of graft substitutes with the synthesized nanoscale calcium citrate is studied by animal experiments.
Materials and methods
Anhydrous calcium chloride (CaCl2, analytical grade), trisodiumcitrate (Na3C6H5O7, analytical grade) and ethanol (CH3CH2OH, AR, 95%) were purchased at Cheng Du Ke Long Chemical Co., Ltd. (Chengdu, China).
Calcium citrate was prepared by a method involving neutralization and precipitation. We have selected ethanol as the precipitant because it can change the free energy of the reaction and allows supersaturation of the solution.Citation9 A solution of sodium citrate (0.12mol·L−1) was mixed to a solution of calcium chloride (0.18mol·L−1) at a molar ratio of calcium chloride: sodium citrate of exactly 3:2. The obtained mixture was stirred at 25°C, and ethanol was added until the production of a white slurry. The slurry was then centrifuged and washed and collected as a pure calcium citrate slurry. In order to obtain the slurry, we applied a volume ratio of alcohol: water between 1:2 and 2:1. Finally, the nano-calcium citrate was obtained after freeze drying of the slurry (LGJ-10, Beijing Songyuan Huaxing Technology Development Co., Ltd, China).
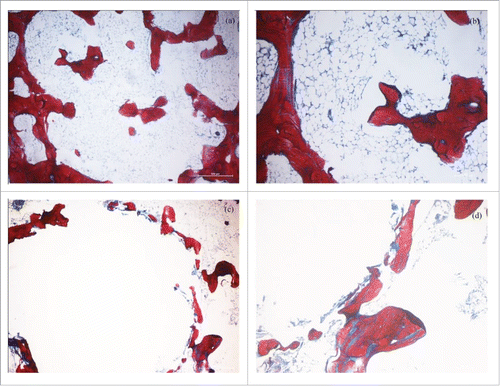
The analysis of the phases and the determination of the crystallite sizes and lattice parameters were undertaken by X-ray diffraction (XRD) (Dandong Fangyuan Instrument Co., Ltd., China) using Cu-Kα radiations. A scanning electron microscope (SEM; JMS-5900lv, Hitachi Ltd., Japan) was used to observe the microstructures of the samples. Further analytical experiments were performed on a Fourier IR spectrometer (Tensor 27, Bruker Corp., Germany), confocal Raman microscope (Renishaw plc, United Kingdom) with a 514.5 nm laser. Thermoanalysis was performed with a STA 409 PC/PG device (Netzsch Group, Germany), and it was carried out between 50–500°C at the rate of 10°C·min−1. The effect of nano-calcium citrate on bone regeneration was studied on bone defects of rabbits from New Zealand. The bone defects were 3 mm-long and 5 mm-deep and created in their left and right femoral condyles respectively. A correct amount of nano-calcium citrate powder was implanted into the left bone defect (Experimental), while the right femur (control) remained empty. The muscle and skin were sutured with absorbable sutures. The microanatomy of the bone tissues (implanted and normal) was observed 12 weeks after implantation with a light microscope (Nikon TE2000) and after Masson staining in order to compare the experimental group with the normal.
Results and discussions
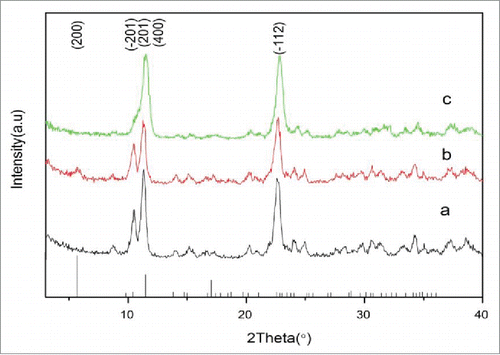
illustrates the XRD patterns of the samples after adding different volume of alcohol and freeze drying. The results show that the XRD patterns of the samples are similar with the ones of JCPDS No. 28-2003 [Ca3(C6H5O7)2·4H2O], confirming the preparation of calcium citrate tetrahydrate. The diffraction peaks of the planes (201) at 11° and (12) at 22° are high and can be easily distinguished, however, compared with the JCPDS No. 28-2003 patterns, the (200) and (400) crystal planes diffraction peaks at 5° and 11° are relatively low. This feature can be explained by the adsorption of ethanol on the surface of the crystal and therefore the change of the growth velocity of each crystal planeCitation9 and calcium citrate forming slightly different crystals. In addition, the peaks of the planes (
01) at 9° and 11° decrease and broaden as the ratio of alcohol: water increases (). Particularly, one notices the only broad diffraction peak between 9–11° when the ration of alcohol: water is at highest (). The decrease of the peaks intensity suggests that the ratio of alcohol: water is critical for the preparation of pure calcium citrate. Furthermore, the absence of the characteristic peaks of sodiumchloride and other reaction residues indicates that the synthesized sample is pure calcium citrate.
Figure 1. XRD pattern of calcium citrate precipitated at different volume ratio of alcohol: water: (A) 1:2, (B) 1:1, (C) 2:1.
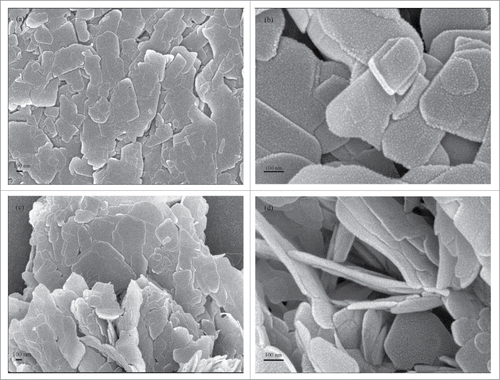
The SEM micrographs () reveal the shape of the nano-calcium citrate prepared at different ratio of alcohol: water. As shown in the image, the calcium citrate crystals produced by a 2:1 () and a 1:2 () ratio of alcohol: water grew forming a 2-dimensional surface and formed a sheet that measures approximately 50–500 nm × 50–200 nm × 8–30 nm. The sheet is thicker, relatively smooth and its edges and corners are not clear when the ratio of alcohol: water is 2:1. This observation may be explained by the lower crystallinity of the obtained calcium citrate. While the volume ratio is reduced to 1:2, the nano-calcium citrate formed a thinner sheet with a regular morphology and exhibits some hexagonal lamellar structures (). In addition, because of the smaller thickness and larger width of the sample, the calcium citrate particles had a tendency to agglomerate, which implies that the nano-calcium citrate prepared by the 1:2 ratio of alcohol: water had a higher surface energy and potentially better biological activities. Based on the results of XRD and SEM experiments, the calcium citrate nanosheet prepared by the 1:2 ratio of alcohol: water is chosen for the rest of the study.
Figure 2. SEM micrographs of nano-calcium citrate powders produced at different ratio of alcohol: water: (A) alcohol: water=2:1, ×30000 (B) alcohol: water = 2:1, ×100000 (C) alcohol: water=1:2, ×30000 (D) alcohol: water=1:2, ×100000.
shows Fourier transform IR spectroscopy (FTIR) and Raman spectra of the samples prepared with the 1:2 ratio of alcohol: water. In the FTIR curve (), the 2 main absorption bands located at 3479cm−1 and 3176cm−1 are attributed to the O–H bonds in crystal water or other moieties. In calcium citrate, because the carboxy group can be coordinated to the calcium ion which forms carboxylate, many strong characteristic absorption peaks can be detected between 1578–1443 cm−1.Citation10 Moreover, the FTIR characteristic absorption bands of the 3 carboxylate ions in calcium citrate, are split into 2 peaks due to the strong coupling effect of the antisymmetric stretching vibration (1578cm−1) whose infrared activity is very high, and the symmetric stretching vibration (1443cm−1) that has relatively low infrared activity. The absorption bands between 1257–1221 cm−1 might be assigned to the stretching vibration of the C–O bond.
Figure 3. FTIR and Raman spectra of the nano-calcium citrate (A) Raman spectra of the nano-calcium citrate, (B) FTIR spectra of the nano-calcium citrate.
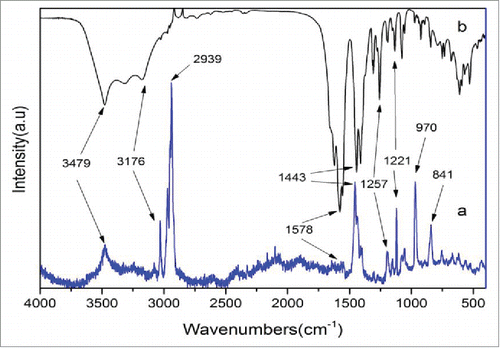
Similar to the FTIR spectra, the vibration peaks of the O–H bonds at 3479cm−1 and 3176cm−1 dominate the Raman spectra. However, the characteristic peak of the carboxylate at 1578cm−1 is intense in the FTIR spectra in opposite to the ones in the Raman spectra at 1443cm−1. These behaviors can only be attributed to the different mechanism of FTIR and Raman spectroscopies. The wave numbers at 970cm−1 and 841cm−1 are possibly due to the rocking vibrations of the −CH2 bond. The major difference with the FTIR is the stronger peaks nearby 2939cm−1, which should be assigned to the stretching vibrations and the in-plane bending vibrations of –CH2. In addition, numerous split peaks can be observed in this region because of the vibronic coupling of multiple CH2 groups. These observations are consistent with the XRD measurements mentioned above and support the synthesis of calcium citrate.
The TG and DTG experiments were performed in order to investigate the thermal behavior of calcium citrate synthesized using the 1:2 ratio of alcohol: water. As seen from the curves on there are 4 regions of weight loss. The loss of 8.25% up to 75°C (process I) corresponds to the removal of surface-adsorbed water molecules and a part of the crystal water molecules:
Figure 4. Thermal gravimetric (TG) and derivative thermogravimetric (DTG) curves of the nano-calcium citrate.
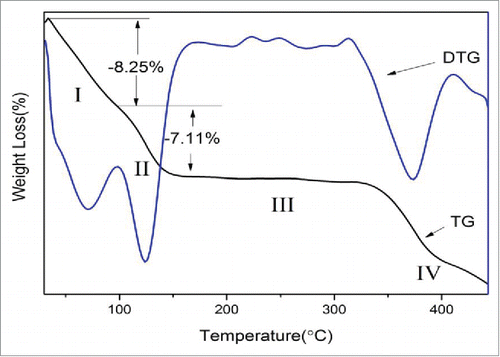
Two lower cohesion water molecules are released form [Ca3(C6H5O7)2(H2O)2]·2H2O and the calculated theoretical mass loss is 6.3%.Citation11 The difference between the theoretical and experimental values maybe attributed to the size of the prepared sample. The nano-calcium citrate tends to absorb more water due to its higher hydrophilicity.
The second weight loss of 7.11% appears between 75–125°C (process II) refers to the subsequent removal of 2 other water molecules of Ca3(C6H5O7)2·2H2O transformed into Ca3(C6H5O7)2:Citation11
The experimental value is in very good agreement with the theoretical value of 6.74%.
There was hardly any mass loss observed between 125–350°C (process III) which corresponds to the melting of calciumcitrate.
Beyond 340°C (process IV), the calcium citrate decomposes into CaCO3:Citation11
Micrographs from the control group () exhibit plenty of red woven bones around the margin indicating that the edge of the defect is a normal bone tissue, meanwhile a small amount of scattered new bone formation in the defect. The micrographs of the specimen which had the spaces filled with nano-calcium citrate, exhibit plenty of newly formed bones that appear dense around the circular defect (), which illustrates the power of calcium citrate to induce no inflammatory reaction and biological compatibility while the forming new bone. We presume that the nano-scale calcium citrate flakes has the ability to release more calcium ions in a short time therefore allowing a high activity, and a high concentration of calcium ions that could stimulate bone formation. As with commercially pure calcium citrate powder affects the early periods of bone defect healing mechanism in Japanese white rabbits positively when the defect was less than or equal to 2 mm.Citation2 The regenerated new bones () formed around the circular defect filled with nano-calcium citrate when the defect was larger (3 mm-long and 5 mm-deep), and the filling material would be replaced by the new bones with its degradation.
Conclusion
Nano-sized sheets were obtained by adding ethanol to a solution containing calcium chloride and sodium citrate. We obtained calcium citrate tetrahydrate [Ca3(C6H5O7)2·4H2O] that could well crystallized when a 1:2 (v: v) ethanol/water was used. The length of the particles is between 50 nm and 500 nm, the width is between 50 nm and 200 nm, and the thickness is between 8 nm and 30 nm. Moreover, experiments on animal bone revealed that calcium citrate could affect positively the healing of a fracture of rabbits from New Zealand. The ability to stimulate new bone formation makes the synthesized nano-calcium citrate a good candidate for bone graft substitutes. Thus new applications of nano-calcium citrate should be investigated in the future.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Wang LM, Wang W, Li XC, Peng L, Lin ZQ, Xu HZ. Calcium citrate: a new biomaterial that can enhance bone formation in situ. Asian Pac J Trop Med 2012; 15:291-6.
- Zhang W, Wang W, Chen QY, Lin ZQ, Cheng SW, Kou DQ, Ying XZ, Shen Y, Cheng XJ, Nie PF, et al. Effect of calcium citrate on bone integration in a rabbit femur defect model. Asian Pac J Trop Medi 2012; 5:310-4; PMID:22449524 http://dx.doi.org/10.1016/S1995-7645(12)60045-5
- An T, Chen QY, Li S, Zhou YY, Zhao ZR, Peng L. Bone-tendon healing enhancement using injectable gelatin-sodium alginate-calcium citrate in rabbits. J Trauma Surg 2015; 17:247-51.
- Huang S, Chen JC, Hsu CW, Chang WH. Effects of nano calcium carbonate and nano calcium citrate on toxicity in ICR mice and on bone mineral density in an ovariectomized mice model. Nanotechnology 2009; 20:375102; PMID:19706952 http://dx.doi.org/10.1088/0957-4484/20/37/375102
- Zeng X, Ma MH. Technologic Research on Transforming Calcium Carbonate in Eggshell to Calcium Citrate. Scientia Agricultura Sinica 2010; 43:1031-40.
- Wang WB, Zhao YQ, Sun H. Study on preparation of calcium citrate from egg shell. App Chem Indus 2012; 41:557-8
- Chen SY, Wang LC, Liu R, Ji J, Wu H, Wu QH. Study on the technology of calcium citrate prepared with the shell of Mactra veneriformis Reeve. Chin J Mar Drugs 2011; 30:18-22.
- Herdtweck E, Kornprobst T, Sieber R, Straver L, Plank J. Crystalstructure, synthesis, and properties of tri-calcium di-citrate tetra-hydrate [Ca3(C6H5O7)2(H2O)2]·2H2O. Z. anorg. allg. Chem 2011; 637:655-9
- Zhong LZ, Li JF, Gao Y, Cao WQ, Zhang PC, Lai XF. Preparation and characterisation of calcium citrate wires. Micro Nano Lett 2015; 10:419-21; http://dx.doi.org/10.1049/mnl.2015.0090
- Ding XM, Peng L, Wen F, Tan ZW, Mu ZL. Simulated body fluid immersion method for assessing biological characteristics of calcium citrate. Chinese JTissue Engineer Res 2013; 17:6811-6
- Mansour SAA. Thermal decomposition of calcium citrate tetrahydrate. Thermochimica Acta 1994; 233:243-56; http://dx.doi.org/10.1016/0040-6031(94)85118-2